Marco Floridia1, Giulia Masuelli2, Beatrice Tassis3, Enrica Tamburrini4, Valeria Savasi5, Matilde Sansone6, Arsenio Spinillo7, Giuseppina Liuzzi8, Anna Degli Antoni9, Serena Dalzero10, Laura Franceschetti11, Giuliana Simonazzi12, Gianpaolo Maso13, Daniela Francisci14, Carmela Pinnetti8 and Marina Ravizza10. On behalf of The Italian Group on Surveillance of Antiretroviral Treatment in Pregnancy.
1 National Centre for Global Health, Istituto Superiore di Sanità, Rome, Italy.
2 Department of Obstetrics and Neonatology, Città della Salute e della Scienza Hospital, and University of Turin, Turin, Italy.
3 Obstetrics and Gynecology Unit, Fondazione IRCCS Ospedale Maggiore Policlinico di Milano, Milan, Italy.
4 Department of Infectious Diseases, Catholic University and Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy.
5 Department of Obstetrics and Gynaecology, Luigi Sacco Hospital and University of Milan, Milan, Italy.
6 Department of Neurosciences, Reproductive and Dentistry Science, University Federico II, Naples, Italy.
7 Department of Obstetrics and Gynaecology, IRCCS S. Matteo, Pavia, Italy.
8 I.N.M.I. Lazzaro Spallanzani, Rome, Italy.
9 Department of Infectious Diseases and Hepatology, Azienda Ospedaliera di Parma, Parma, Italy.
10 Department of Obstetrics and Gynaecology, DMSD San Paolo Hospital Medical School, University of Milan, Milan, Italy.
11 Department of Obstetrics and Gynecology, University of Brescia, Brescia, Italy.
12 Department of Medical and Surgical Sciences, Policlinico Sant'Orsola-Malpighi and University of Bologna, Bologna, Italy.
13 Institute for Maternal and Child Health, IRCCS Burlo Garofolo, Trieste, Italy.
14
Clinic of Infectious Diseases, Department of Experimental Medicine and
Biochemical Sciences, University of Perugia, Perugia, Italy.
Correspondence to: Marco Floridia. National Center for Global Health,
Istituto Superiore di Sanità, Viale Regina Elena 299, 00161 Rome,
Italy. Tel: +39 06 4990 3228; Fax: +39 06 4938 7199. E-mail:
marco.floridia@iss.it
Published: September 1, 2019
Received: May 14, 2019
Accepted: August 8, 2019
Mediterr J Hematol Infect Dis 2019, 11(1): e2019050 DOI
10.4084/MJHID.2019.050
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
There is limited information on pregnancy loss in women with HIV, and
it is still debated whether HIV-related markers may play a role.
Objectives:
To explore potential risk factors for pregnancy loss in women with HIV,
with particular reference to modifiable risk factors and markers of HIV
disease.
Methods:
Multicenter observational study of HIV-positive pregnant women. The
main outcome measure was pregnancy loss, including both miscarriage
(<22 weeks) and stillbirth (≥22 weeks). Possible associations of
pregnancy loss were evaluated in univariate and multivariate analyses.
Results:
Among 2696 eligible pregnancies reported between 2001 and 2018, 226
(8.4%) ended in pregnancy loss (miscarriage 198, 7.3%; stillbirth 28,
1.0%). In multivariate analyses, only older age (adjusted odds ratio
[AOR] per additional year of age: 1.079, 95% confidence interval [CI]
1.046-1.113), HIV diagnosis before pregnancy (AOR: 2.533, 95%CI
1.407-4.561) and history of pregnancy loss (AOR: 1.625, 95%CI
1.178-2.243) were significantly associated with pregnancy loss. No
significant association with pregnancy loss was found for parity,
coinfections, sexually transmitted diseases, hypertension, smoking,
alcohol and substance use, CD4 cell count, HIV-RNA viral load, and CDC
HIV stage.
Conclusions:
Older women and those with a previous history of pregnancy loss should
be considered at higher risk of pregnancy loss. The severity of HIV
disease and potentially modifiable risk factors did not increase the
risk of pregnancy loss.
|
Introduction
HIV
infection, when appropriately treated, has currently a much less severe
impact on the quality of life and life expectancy. This more favorable
prognosis, together with the possibility to have healthy and uninfected
children, has determined among women with HIV an increased desire for
pregnancy, a more confident family planning, and increasing use of
fertility treatments and services.[1-8] In this
context, miscarriage and stillbirth represent severe events that may
have adverse consequences on parenting desire and family planning. It
is therefore important to define determinants of pregnancy loss,
identify pregnancies at risk, and implement targeted preventive
measures that may increase the probability of delivering live and
healthy newborns in this particular population. Large multinational
projects,[9] systematic reviews,[10] and smaller regional studies[11-13]
have identified risk factors in the general population, but data in
HIV-infected women are still sparse and often inconsistent,
particularly with respect to the potential predictive role of some
HIV-specific markers such as viral load and CD4+ cell levels.[14-18]
In order to further explore this issue, we used data from a national
study to define determinants of pregnancy loss in a large cohort of
pregnant women with HIV.
Methods
We
studied all miscarriages and stillbirths reported to the National
Program on Surveillance on Antiretroviral Treatment in Pregnancy. This
is a national observational study of pregnant women with HIV
established in Italy in 2001.[19] The study
(currently not funded) was supported in the past by public,
peer-reviewed research grants (ref.: H85E08000200005) from the Italian
Medicines Agency (AIFA), with no role of the funder in study design,
data collection, data analysis, manuscript preparation and/or
publication decision. The study is structured as a prospective cohort,
with reporting recommended before pregnancy outcome. Retrospective
reports are also allowed but represent roughly 20% of total cases in
the project. Laboratory and clinical data are collected from hospital
records of Obstetrics, Infectious Diseases and Paediatrics departments
following women’s consent. Both the study protocol and patient
information sheet were approved by the competent Ethics Committee
(National Institute for Infectious Diseases L. Spallanzani, Rome).
Information on past and recent (less than one year) substance use and
on the HIV status of the current partner is based on the women’s
reports. Status and level of smoking (with no smoking defined by less
than one cigarette per day [CPD], light smoking by one to nine CPD and
moderate to heavy smoking by 10 or more CPD)[20] are
defined at first visit in pregnancy. Hypertension, diabetes, and
alcohol consumption were defined according to national guidelines for
the management of pregnancy.[21] Gestational age is
determined on the basis of the last menstrual period, ultrasound
biometry, or both. Preterm and very preterm delivery are defined as
delivery before 37 and 32 completed weeks of gestation, respectively,
and low and very low birth weight by values below 2500 and 1500 g,
respectively. Cesarean section is considered elective if performed
before the rupture of membranes and the onset of labor, and nonelective
if performed after the rupture of membranes, the onset of labor, or
both.
For the present analysis we considered all the
centres who reported at least one case of pregnancy loss (miscarriage,
before 22 weeks of gestation; stillbirth, at or after 22 weeks) from
December 2001 (study start) to October 2018, and compared the
pregnancies ending in a pregnancy loss with all the pregnancies with a
live birth concurrently reported from the same centres. Voluntary
terminations and cases with a diagnosis of HIV during the third
trimester of pregnancy were excluded. The study period (2001-2018) was
divided into three intervals of six years each (2001-2006, 2007-2012,
2013-2018). The possible role of HIV-related variables was evaluated
considering periconception values of CD4 cell count and plasma HIV-RNA
as potential predictors of pregnancy loss. We considered for this
analysis as periconception values all available CD4 cell counts and
HIV-RNA values with a time distance no greater than 13 weeks before or
after the date of the last menstrual period. HIV-RNA was categorized at
a threshold of 50 copies per ml and CD4 cell levels at two different
thresholds, of 200 cells/mm3 and 500 cells/mm3,
respectively. Quantitative variables were summarized as medians with
interquartile ranges (IQR) and compared using the Mann-Whitney U-test.
Categorical variables were compared using the chi-square test, with
odds ratios (OR) and 95% confidence intervals (CI) calculated. Temporal
trends were analyzed using the chi-square test for trend. In order to
adjust for potential confounders, pregnancy loss was also evaluated as
a dependent variable in multivariable logistic regression analyses, and
sensitivity analyses were conducted individually valuating miscarriage
and stillbirth as dependent variables and introducing other possibly
relevant covariates as independent variables. P values below 0.05 were
considered statistically significant. All analyses were performed using
the SPSS software, version 25.0 (IBM Corp, 2017, Armonk, NY, USA).
Results
As
of October 2018, 2696/4132 pregnancies in the study database (65.2%)
were eligible for analysis. Among those pregnancies, 226 (8.4%) ended
in either miscarriage (198, 7.3%) or stillbirth (28, 1.0%). The rate of
pregnancy loss remained substantially unchanged across the study period
(7.4% in 2001-2006, 10.3% in 2007-2012, 6.8% in 2013-2018; p=0.772,
chi-square for trend). No temporal trends were observed also analyzing
separately miscarriage and stillbirth (data not shown).
The general characteristics of the population studied according to pregnancy loss are shown in Table 1.
The main markers of HIV disease, represented by CD4 cell count, HIV-RNA
viral load, and CDC HIV stage, showed no differences between the two
groups of women with and without pregnancy loss. Additional analyses
conducted on CD4 levels categorized at different thresholds confirmed
this finding: rates of pregnancy loss were 6.8% for CD4<200/mm3 and 8.2% for CD4 ≥200/mm3 (OR 0.822, 95%CI 0.436-1.550, p=0.545), 8.2% for CD4≥500/mm3 and 8.0% for CD4 <500/mm3 (OR 1.018, 95% CI 0.740-1.400, p=0.912).
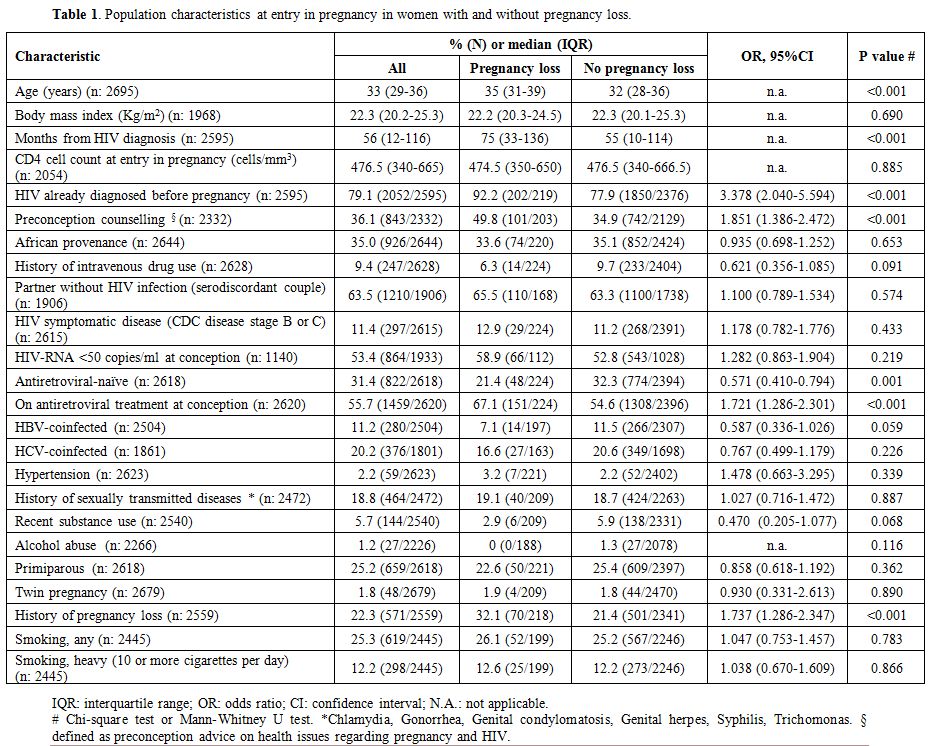 |
Table 1. Population characteristics at entry in pregnancy in women with and without pregnancy loss. |
Women
with pregnancy loss were significantly older, HIV-infected from a
longer time, more frequently diagnosed with HIV and on antiretroviral
treatment before pregnancy, had received more frequently preconception
counseling, and were more likely to have experienced previous pregnancy
losses. No differences were observed between the two groups in other
possible risk factors for pregnancy loss, such as parity, coinfections,
sexually transmitted diseases, hypertension, smoking, alcohol, and
substance use.
The above analyses were also conducted separately
for miscarriage and stillbirth. For miscarriage, the results
substantially overlapped those of the common analysis (data not shown).
For stillbirth, the results showed significant associations with
African nationality (odds ratio [OR]: 2.728, 95%CI 1.261-5.904,
p=0.011) and with twin pregnancy (OR: 4.356, 95%CI 1.004-18.898,
p=0.049).
The associations found in the above univariate analyses
were evaluated in a multivariable logistic regression analysis that
included as dependent variable (outcome) pregnancy loss, and as
independent (predictive) variables age, African provenance, HIV
diagnosis before conception, being on antiretroviral treatment (ART) at
conception, twin pregnancy, and history of pregnancy loss. Other
variables significantly associated in univariate analyses with
pregnancy loss were excluded being considered either redundant compared
to others already included in the model (months since HIV diagnosis,
antiretroviral status at entry in pregnancy) or reflecting spurious
associations (preconception counseling, apparently increasing risk of
pregnancy loss). The results of the multivariable analysis are shown in
Table 2. After adjusting for
covariates, only older age, the timing of HIV diagnosis and history of
pregnancy loss remained significantly associated with pregnancy loss.
Sensitivity analyses that included additional covariates in the model
consistently confirmed the above results (data not shown).
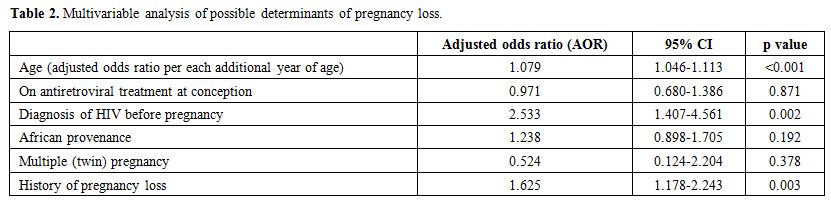 |
Table 2. Multivariable analysis of possible determinants of pregnancy loss. |
Discussion
This
large study explored the determinants of pregnancy loss in a large
series of roughly three thousand pregnancies with HIV. Our sample was
entirely represented by HIV-positive women, and we were, therefore,
unable to assess the role of HIV infection, that was reported as a
significant risk factor in other studies.[9] We were also unable to assess the role of other potentially relevant variables such as socioeconomic and marital status,[9] placental and amniotic status.[12]
As
expected, most of the cases (198/226) of pregnancy loss were
represented by miscarriages. The ratio between pregnancy loss and live
birth remained relatively constant over time, with no significant
change across the study period. In general, the observed rate (around
seven percent of pregnancies) was lower compared to data reported for
the general population in Italy (14% of all pregnancies in 2015),[22] and for pregnant women with HIV by others (15 and 20% in the studies by Hoffman and Stringer, respectively),[18,17]
suggesting underreporting or missed enrolment of women with miscarriage
in this surveillance. This occurrence might be favored by preferential
access of women with early pregnancy loss to other structures, such as
emergency departments. The observed rate of stillbirth (1.0%) is
consistent with other studies, that usually showed rates between 0.8%
and 4%.[17,18,23]
The main
objective of this study was to identify preventable determinants of
pregnancy loss among women with HIV. In this large series, the two
major determinants of pregnancy loss were represented by two
non-modifiable risk factors, represented by older age and history of a
previous pregnancy loss. Both these associations have already been
described.[9-12]
We found an association,
apparently paradoxical, between pregnancy loss and preconception
counseling. Our interpretation is that preconception counseling acted
here as a proxy for the previous pregnancy losses or pregnancy at risk,
with women with such a history more likely to seek preconception
advise. The absence of a positive effect of preconception counseling in
preventing pregnancy loss is nonetheless important, because is
consistent with the absence of modifiable factors among the
determinants found. Importantly, no significant role was found for
smoking, alcohol, and recent substance use. This finding was confirmed
in sensitivity analyses that included such variables in the main
multivariable model (data not shown). We also found no effect of BMI,
another potentially modifiable risk factor for pregnancy loss,[10]
also when the risk was assessed specifically for the presence of
overweight and/or obesity (data not shown). Finally, we found no
significant role of smoking, in discordance with the observations by
Flenady et al in the general population[10] and by Westreich et al in women with HIV.[16]
We also found no association of pregnancy loss with hypertension and
parity, that represented risk factors in larger studies evaluating the
general population.[9,10] In univariate analysis twin pregnancy represented a predictor of stillbirth, as reported by others.[9]
Although this association did not persist in the multivariable
analysis, this lack of significance could be due to the limited number
of stillbirth events, and we think that multiple pregnancy should be
still considered as a potential risk factor for this adverse outcome.
This
study also contributed information to the debate on the potential role
of severity of HIV disease in increasing the risk of pregnancy loss. We
did not find any role for clinical or laboratory markers of HIV,
confirming the findings by Stringer et al. for CD4 and HIV-RNA,[17]
but in discordance with the significant associations between pregnancy
loss and HIV disease indicators (CD4, plasma HIV-RNA levels and
clinical HIV stage) found in a previous study conducted in Zambia,[4]
while another study had found conflicting results, with a small
absolute increase in risk of pregnancy loss for the highest viral load
category compared to the lowest category, and a simultaneous
paradoxical protective effect of increased cumulative viremia against
pregnancy loss.[15] Presence of ART at conception
showed in the present series no association with pregnancy loss in
multivariable analyses, confirming the findings of other studies and
systematic reviews.[17,18, 23,24]
The
interpretation of the study should take into account some limitations.
Study population may have been selected because of different reasons,
that include missing outcome information (the main reason for patient
ineligibility), exclusion of women diagnosed with HIV in late pregnancy
(that might have higher viral load and lower CD4), and referral bias
(with specialized centres more likely to participate in this
surveillance). The patient’s desire of acceptability may also have
influenced the accurate reporting of personal risk factors/behaviors
(e.g., smoking, substance use), and ascertainment of outcomes
(particularly for miscarriage) is usually problematic. The low rate
observed, actually, suggests incomplete coverage or underreporting of
this outcome. Information on periconception HIV-RNA levels was also
missing in a substantial number of cases, and this should prompt
caution in the interpretation of the findings. Such a high rate of
missing information, however, includes more than 500 cases in which HIV
infection was diagnosed during pregnancy, and HIV-RNA analyzed for the
first time at second or third trimester. This occurrence is also likely
to have influenced through selection bias the finding of a higher risk
of pregnancy loss in women diagnosed before current pregnancy, that
should therefore also be considered cautiously.
Conclusions
In
conclusion, despite the above caveats, our findings show that pregnancy
loss is a multifactorial outcome. Older women and those with a previous
history of pregnancy loss should be considered at high risk of
pregnancy loss, and twin pregnancy should be considered a risk factor
for stillbirth. Our data indicated that modifiable factors, such as
excess body weight, smoking, alcohol, and substance abuse, have a
limited role in pregnancy loss. The degree of severity of HIV disease
apparently did not increase the risk of pregnancy loss in general and
of miscarriage in particular. Larger, possibly multinational studies
may be necessary to define more accurately the determinants of
stillbirth in women with HIV, given the low prevalence of this
condition.
The Italian Group on Surveillance of Antiretroviral Treatment in Pregnancy
Project coordinators: M. Floridia, M. Ravizza, E. Tamburrini.
Participants:
M. Ravizza, E. Tamburrini, F. Di Lorenzo, G. Sterrantino, M. Meli, I.
Campolmi, F. Vichi, B. Del Pin, R. Marocco, C. Mastroianni, V.S.
Mercurio, D. Zanaboni, G. Guaraldi, G. Nardini, C. Stentarelli, B.
Beghetto, A.M. Degli Antoni, A. Molinari, M.P. Crisalli, A. Donisi, M.
Piepoli, V. Cerri, G. Zuccotti, V. Giacomet, S. Coletto, F. Di Nello,
C. Madia, G. Placido, P. Milini, F. Savalli, V. Portelli, F. Sabbatini,
D. Francisci, C. Papalini, L. Bernini, P. Grossi, L. Rizzi, M.
Bernardon, G. Maso, E. Rizzante, C. Belcaro, S. Bussolaro, M.
Rabusin, A. Meloni, A. Chiodo, M. Dedoni, F. Ortu, P.
Piano, A. Citernesi, I. Bordoni Vicini, K. Luzi, A. Spinillo, M.
Roccio, A. Vimercati, D. Calabretti, S. Gigante, B. Guerra, F. Cervi,
G. Simonazzi, E. Margarito, M.G. Capretti, C. Marsico, G. Faldella, M.
Sansone, P. Martinelli, A. Agangi, A. Capone, G.M. Maruotti, C.
Tibaldi, L. Trentini, T. Todros, G. Masuelli, V. Frisina, V. Savasi, E.
Cardellicchio, C. Giaquinto, M. Fiscon, E. Rubino, L. Franceschetti, R.
Badolato, M.A. Forleo, B. Tassis, G.C. Tiso, O. Genovese, C. Cafforio,
C. Pinnetti, G. Liuzzi, A.M. Casadei, A.F. Cavaliere, M. Cellini, A.M.
Marconi, S. Dalzero, M. Ierardi, C. Polizzi, A. Mattei, M.F. Pirillo,
R. Amici, C.M. Galluzzo, S. Donnini, S. Baroncelli, M. Floridia.
Advisory Board: A. Cerioli, M. De Martino, F. Parazzini, E. Tamburrini, S. Vella.
SIGO-HIV Group National Coordinators: P. Martinelli, M. Ravizza.
Acknowledgments
We
thank Cosimo Polizzi and Alessandra Mattei of the Istituto Superiore di
Sanità in Rome, Italy, for providing technical secretarial for this
study. We also thank Ernesto Costabile for providing assistance as
documentalist. No compensation was received for these contributions.
References
- Laursen T, Kesmodel US, Højgaard A, Østergaard L,
Ingerslev HJ, Wejse C. Reproductive patterns and fertility wishes among
HIV-infected patients: survey from six outpatient clinics in Denmark.
Int J Infect Dis 2013; 17: e851-6. https://doi.org/10.1016/j.ijid.2013.01.024 PMid:23499182
- Nöstlinger
C, Desjardins F, Dec J, Platteau T, Hasker E; Eurosupport V Study
Group. Child desire in women and men living with HIV attending HIV
outpatient clinics: evidence from a European multicentre study. Eur J
Contracept Reprod Health Care 2013; 18: 251-63. https://doi.org/10.3109/13625187.2013.801072 PMid:23738886
- Berhan Y, Berhan A. Meta-analyses of fertility desires of people living with HIV. BMC Public Health 2013; 13: 409. https://doi.org/10.1186/1471-2458-13-409 PMid:23627965 PMCid:PMC3649930
- Wessman
M, Aho I, Thorsteinsson K, et al. Perception of sexuality and fertility
in women living with HIV: a questionnaire study from two Nordic
countries. J Int AIDS Soc 2015; 18: 19962. https://doi.org/10.7448/IAS.18.1.19962 PMid:26037151 PMCid:PMC4452736
- Hernando
V, Alejos B, Montero M, et al. Reproductive history before and after
HIV diagnosis: A cross-sectional study in HIV-positive women in Spain.
Medicine 2017; 96: e5991. https://doi.org/10.1097/MD.0000000000005991 PMid:28151893 PMCid:PMC5293456
- Ramos
de Souza M, do Amaral WN, Alves Guimarães R, Rezza G, Brunini SM.
Reproductive desire among women living with HIV/AIDS in Central Brazil:
Prevalence and associated factors. PLoS One 2017; 12: e0186267. https://doi.org/10.1371/journal.pone.0186267 PMid:29053712 PMCid:PMC5650151
- Lo
CK, Kennedy VL, Yudin MH, Shapiro HM, Loutfy M. Access to fertility
services in Canada for HIV-positive individuals and couples: a
comparison between 2007 and 2014. AIDS Care 2017; 29: 1433-1436. https://doi.org/10.1080/09540121.2017.1332332 PMid:28553759
- Cohn
SE, Haddad LB, Sheth AN, et al. Parenting Desires Among Individuals
Living With Human Immunodeficiency Virus in the United States. Open
Forum Infect Dis 2018; 5: ofy232. https://doi.org/10.1093/ofid/ofy232
- Hirst
JE, Villar J, Victora CG, et al. The antepartum stillbirth syndrome:
risk factors and pregnancy conditions identified from the
INTERGROWTH-21st Project. BJOG 2018; 125: 1145-1153. https://doi.org/10.1111/1471-0528.14463 PMid:28029221 PMCid:PMC6055673
- Flenady
V, Koopmans L, Middleton P, et al. Major risk factors for stillbirth in
high-income countries: a systematic review and meta-analysis. Lancet
2011; 377: 1331-40. https://doi.org/10.1016/S0140-6736(10)62233-7
- Gutaj
P, Zawiejska A, Wender-Ożegowska E, Brązert J. Maternal factors
predictive of first trimester pregnancy loss in women with
pregestational diabetes. Pol Arch Med Wewn 2013; 123: 21-8. https://doi.org/10.20452/pamw.1585 PMid:23302725
- Poorolajal
J, Cheraghi P, Cheraghi Z, Ghahramani M, Doosti Irani A. Predictors of
miscarriage: a matched case-control study. Epidemiol Health 2014; 36:
e2014031. https://doi.org/10.4178/epih/e2014031 PMid:25420952 PMCid:PMC4282085
- Warr
AJ, Pintye J, Kinuthia J, et al. Sexually transmitted infections during
pregnancy and subsequent risk of stillbirth and infant mortality in
Kenya: a prospective study. Sex Transm Infect 2018. [Epub ahead of
print] https://doi.org/10.1136/sextrans-2018-053597 PMid:30228109 PMCid:PMC6525108
- Kim
HY, Kasonde P, Mwiya M, et al. Pregnancy loss and role of infant HIV
status on perinatal mortality among HIV-infected women. BMC Pediatr
2012; 12: 138. https://doi.org/10.1186/1471-2431-12-138 PMid:22937874 PMCid:PMC3480840
- Cates
JE, Westreich D, Edmonds A, et al. The Effects of Viral Load Burden on
Pregnancy Loss among HIV-Infected Women in the United States. Infect
Dis Obstet Gynecol 2015; 2015: 362357. https://doi.org/10.1155/2015/362357 PMid:26582966 PMCid:PMC4637076
- Westreich D, Cates J, Cohen M, et al. Smoking, HIV, and risk of pregnancy loss. AIDS 2017; 31: 553-560. https://doi.org/10.1097/QAD.0000000000001342 PMid:27902507 PMCid:PMC5263172
- Stringer
EM, Kendall MA, Lockman S, et al. Pregnancy outcomes among HIV-infected
women who conceived on antiretroviral therapy. PLoS One 2018; 13:
e0199555. https://doi.org/10.1371/journal.pone.0199555 PMid:30020964 PMCid:PMC6051581
- Hoffman
RM, Brummel SS, Britto P, et al. Adverse Pregnancy Outcomes among Women
who Conceive on Antiretroviral Therapy. Clin Infect Dis 2018. [Epub
ahead of print] https://doi.org/10.1093/cid/ciy471 PMid:29868833 PMCid:PMC6321847
- Floridia
M, Mastroiacovo P, Tamburrini E, et al.Birth defects in a national
cohort of pregnant women with HIV infection in Italy, 2001-2011. BJOG
2013; 120:1466-75. https://doi.org/10.1111/1471-0528.12285 PMid:23721372
- Kotz
D, Fidler J, West R. Very low rate and light smokers: smoking patterns
and cessation-related behaviour in England, 2006-11. Addiction 2012;
107: 995-1002. https://doi.org/10.1111/j.1360-0443.2011.03739.x PMid:22126678
- Ministero
della Salute - Sistema Nazionale per le linee guida. Linee guida per la
gravidanza fisologica. Aggiornamento 2011. Available at: http://www.epicentro.iss.it/itoss/LineeGuida.asp. Accessed on january 10, 2019.
- ISTAT - Istituto Nazionale di statistica. La salute riproduttiva della donna. Available at https://www.istat.it/it/archivio/2100606. Accessed January 10, 2019.
- Mandelbrot
L, Tubiana R, Le Chenadec J, et al. No perinatal HIV-1 transmission
from women with effective antiretroviral therapy starting before
conception. Clin Infect Dis 2015; 61: 1715-25. https://doi.org/10.1093/cid/civ578 PMid:26197844
- Uthman
OA, Nachega JB, Anderson J, et al. Timing of initiation of
antiretroviral therapy and adverse pregnancy outcomes: a systematic
review and meta-analysis. Lancet HIV 2017; 4: e21-e30. https://doi.org/10.1016/S2352-3018(16)30195-3

