Salam Alkindi1,2#, Nada AL-Umairi1, Sanjay Jaju2 and Anil Pathare1.
1 Department of Haematology, Sultan Qaboos University Hospital, Muscat, Oman.
2 College of Medicine & Health Sciences, Muscat, Oman.
Correspondence to: Dr. Salam Alkindi, BA, MB, BCh, BAO, MSc, FRCP.
Professor in Haematology and Consultant Haematologist, Department of
Haematology, College of Medicine & Health Sciences, Sultan Qaboos
University, P. O. Box 35, Muscat 123, Sultanate of Oman. Tel.:
+96824141182, Fax: +96824144887. E-mail:
sskindi@yahoo.com
Published: November 1, 2019
Received: July 29, 2019
Accepted: September 24, 2019
Mediterr J Hematol Infect Dis 2019, 11(1): e2019058 DOI
10.4084/MJHID.2019.058
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
In Oman, the prevalence of hepatitis B (HBV) infection is 5.8%, with
2.8–7.1% HBV carriers. Hepatitis C (HCV) prevalence among Omanis is
0.41%. A total of 2917 human immunodeficiency virus (HIV) infections
were notified among Omanis by 2017. This study was performed as there
was no data on the prevalence of HIV, HBV and HCV in sickle cell
disease (SCD) patients from Oman.
Study Design and Methods:
In this retrospective, cross-sectional study, medical records of all
SCD patients who attended our hospital between 2011 to 2017 were
retrieved from the hospital information system. Following approval by
the local medical research and ethics committee, data on HIV, HBV, and
HCV exposure were recorded to estimate the prevalence.
Results:
Among a total of 1000 SCD patients (491 males and 509 females),
twenty-three (2.3%) patients showed positive serology for hepatitis B
surface antigen (HbsAg), of whom sixteen (1.6%) were HBV DNA
positive. 126 (12.6%) had anti-HCV antibodies (anti-HCV), of whom
fifty-two (5.2%) were HCV RNA positive. None of the patients had
positive serology for HIV. A normal liver was observed on abdominal
ultrasound in 788 (78.8%) patients, whereas 208 (20.8%) had
hepatomegaly, and 4 (0.4%) had liver cirrhosis. Thirty-six (3.6%)
patients died, but in only two patients, the mortality was due to
cirrhosis of liver.
Conclusions:
This study provides the first comprehensive data on the prevalence of
HBV and HCV infections among Omani SCD patients exposed to blood
transfusions. Reassuringly, no case with HIV was observed.
|
Introduction
Sickle-cell
disease (SCD) is a monogenic disorder characterized by a mutation in
the beta-globin gene, where glutamic acid is replaced by valine,
resulting in the polymerization of Hb and formation of Hb S, with many
devastating clinical manifestations.[1-2] It is not
only affecting red cells but also evolving into multi-system
involvement. Although the mutation of the sickle gene originated in the
African continent, it is now a world-wide disorder.[2,3] SCD is highly prevalent in Oman, with the reported incidence of sickle trait close to 6% of the population.[4-6]
The
most common complications of SCD are recurrent vaso-occlusive crises,
predisposition to significant anemia, acute chest syndrome and
recurrent infections.[7,8] Blood transfusion therapy
is one of the established therapies commonly used in the management of
SCD-related complications, including stroke, ACS, priapism,
pregnancy-related complications, and symptomatic anemia.[9]
Unfortunately, such transfusions increase the risk of exposure to
bloodborne infections like hepatitis B virus (HBV), hepatitis C virus
(HCV) and immune deficiency virus (HIV).
Chronic viral hepatitis
is a major global public health problem because of its association with
increased morbidity and mortality related to chronic hepatitis,
cirrhosis and hepatocellular carcinoma.[10] In 2015,
WHO Global hepatitis report describes the global and regional estimates
of viral hepatitis with an estimated 257 million people living with
chronic HBV infection and 71 million people with chronic HCV infection.[11]
The report also addresses mortality due to these infections, with viral
hepatitis causing 1.34 million deaths in 2015, a number comparable to
deaths caused by tuberculosis, but higher than those caused by HIV.
However, the number of deaths due to viral hepatitis is steadily
increasing over time, while mortality due to tuberculosis and HIV is
declining.[12]
SCD patients are at high risk
for transfusion-associated infections such as HBV, HCV, and HIV. The
prevalence of these infections in SCD has been studied worldwide. In
Mexico, the prevalence of HBV, HCV, HIV in multi-transfused patients
was 7%, 13.7%, and 1.7%, respectively.[13] In Turkey
between 1996 to 2005, HBsAg positivity was found to be 0.79% whereas,
anti-HCV antibody positivity was 4.51%, but no HIV infections were
observed among multi-transfused patients.[14] In
comparison, Oman is a country with an intermediate prevalence of HBV
carriers (2.8–7.1%), reported by a retrospective study conducted in
2010, with a prevalence rate of 5.8% for HBV infection.[15] Further, among the entire resident population in Oman, anti-HCV antibody positivity was reported to be 0.41%.[16]
The WHO classifies Oman as having a low HIV prevalence, with total of
2917 HIV/AIDS infections among Omanis that were notified until end of
2017 with 1606 patients still being alive.[17]
Thus,
despite the high prevalence of SCD in Oman, there is no data on the
prevalence of HBV, HCV, and HIV in these patients. We, therefore,
conducted this retrospective study using electronic medical records to
estimate the prevalence of these infections and study its impact on
morbidity and mortality.
Material and Methods
This
is a retrospective cross-sectional study performed in patients with
SCD, admitted to our hospital between 2011 to 2017, and data is
obtained from the electronic patients' records (EPR). Among a total of
1012 EPR records that were retrieved, twelve patients were excluded
from the final analysis as their data was incomplete.
The
details obtained from the EPR records included: age, diagnosis,
frequency of blood transfusion, laboratory markers for hepatitis B
including surface antibody (anti-HBs), hepatitis B surface antigen
(HbsAg), anti-hepatitis B IgM (anti-HBc IgM), hepatitis B core total
antibodies (total anti-HBc), hepatitis B polymerase chain reaction (HBV
DNA), hepatitis B e-antigen (HBeAg), hepatitis B e-antibody (anti-HBe),
anti-hepatitis C antibody (anti-HCV), hepatitis C polymerase chain
reaction (HCV RNA), hepatitis C genotype (HCV Genotype), HIV.
Radiological data included abdominal liver ultrasound study results to
assess for hepatomegaly and cirrhosis of the liver. The frequency of
blood transfusions was categorized into four groups; never (no blood
transfusion), occasional (less than two times per year), intermittent
(3-6 times per year), and regular (monthly). Information was also
collected on the current status of patients to obtain mortality data.
All
blood samples were tested for HIV (anti-HIV 1/2), HBV HBsAg (if HBsAg
was positive, full markers were performed including HBeAg, and
anti-HBe, and total core IgM) and HCV. In case of HCV (anti-HCV)
serological markers were screened by Architect HCV Ag Test (Architect
HCV Ag Test, Abbot, Germany), and if positive were confirmed by second
confirmatory method, the electrochemiluminescence immunoassay on Cobas
e 411 immunoassay analyzer. Samples that were initially reactive by
ELISA were retested in duplicate, and results were interpreted
according to manufacturers’ instructions.
HCV antigen was tested
by third-generation assays (RIBA HCV 3, Chiron and HCV Blot, Genelab
Diagnostics, Singapore). Sera, reactive in ELISA, were tested by RIBA
according to manufacturers’ recommendations. In these assays specimens
were considered positive if they demonstrated reactivity to two or more
antigen bands at an intensity higher than or equal to the weak positive
control.
HCV ribonucleic acid (RNA) was tested on samples using
the RT-PCR kit COBAS® AMPLICOR HCV Test, Version 2.0 (Roche Molecular
Systems Inc., Pleasanton, California, USA). Since 2009, HCV RNA
was tested using quantitative COBAS® TaqMan® analyzers (Roche Molecular
Systems Inc.) with a linear quantification range between <15 and 100
× 106 IU/mL and a lower limit of detection of 12.6 IU/mL.
HBV
infection was ascertained by assaying for HBeAg and HBsAg using
enzyme-linked immunosorbent assay (Axsym, Abbott Laboratories). The
assay cut-off for HBeAg was ≥1 unit/ml and the assay cut-off for HBsAg
was ≥0.17–0.6 ng/ml; however, the assay cut-off varied according to the
lot number of the kit. HBV DNA level was assessed using plasma with the
platform of Roche COBAS® TaqMan® HBV Test, V1.0, with a linear
quantification range between <20 and 170 × 106 IU/mL.
HIV
(anti-HIV 1/2) was ascertained by an immunometric bridging technique
using the VITROS ECi/ECiQ Immunodiagnostic System. Any positive
serology would be confirmed by Western-Blot assay or HIV DNA PCR assay
using the Cobas AmpliPrep/Cobas TaqMan HIV-1 v2.0, with a linear
quantification range between <20 and 10 × 106 IU/mL.
Statistical Analysis.
The statistical package for social science (IBM SPSS, USA ver.23) was
used to analyze the collected data. Normally distributed data were
characterized as mean with standard deviation, whereas data for
continuous variable and percentage and frequency for categorical
variables. The prevalence was reported as counts and percentages as
appropriate.
Results
Table 1 shows
the data from 1000 SCD patients (491 males and 509 females) with a mean
age of 29.48 ± 10.40 years (range 1-82). 197 (19.7%) never received
blood transfusions, whereas, 607 (60.7%) had occasional transfusions,
148 (14.8%) had intermittent transfusions and 48 (4.8%) were receiving
regular transfusion.
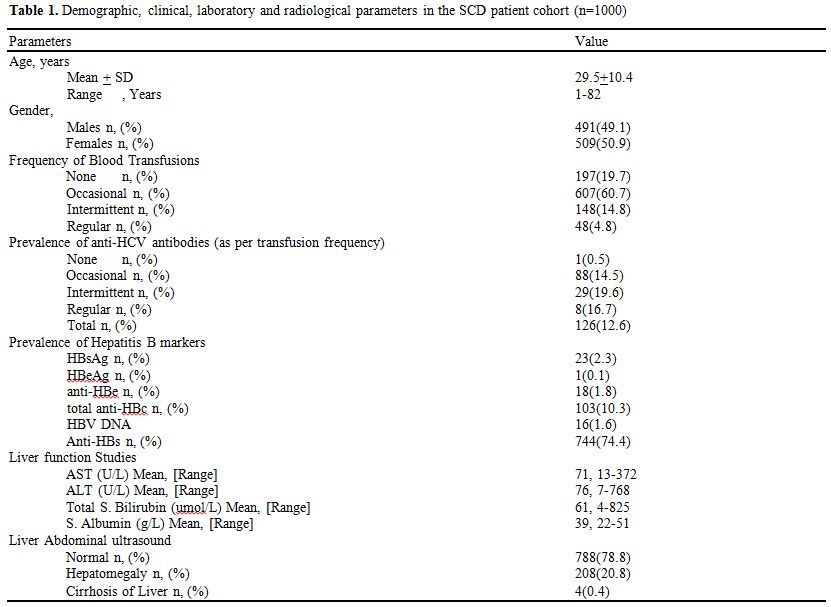 |
Table 1. Demographic, clinical, laboratory and radiological parameters in the SCD patient cohort (n=1000) |
In
this study cohort, twenty-three (2.3%) were positive by serology for
HBsAg, with sixteen (1.6%) being HBV DNA positive as well. Total
anti-HBc, anti-HBe, and HBeAg were positive in 10.3%,1.8%, and 0.1%,
respectively. 74.4% of these SCD patients showed anti-HBs positivity,
indicating adequate immunity against hepatitis B. 126 (12.6%) had
serologically positive anti-HCV antibodies (anti-HCV), with fifty-two
(5.2%) showing HCV RNA positivity. The prevalence of anti-HCV in SCD
patients with regular, intermittent, occasional and those with no
transfusion was 16.7%,19.6%,14.5% and 0.5% respectively. A total of
thirty-six patients 63.23%) had genotype 1; two patients (3.86%) had
genotype 2, seven patients (13.46) each had genotype 3, and 4 (Figure 1). Furthermore, none of the SCD patients in this cohort had positive serology for HIV.
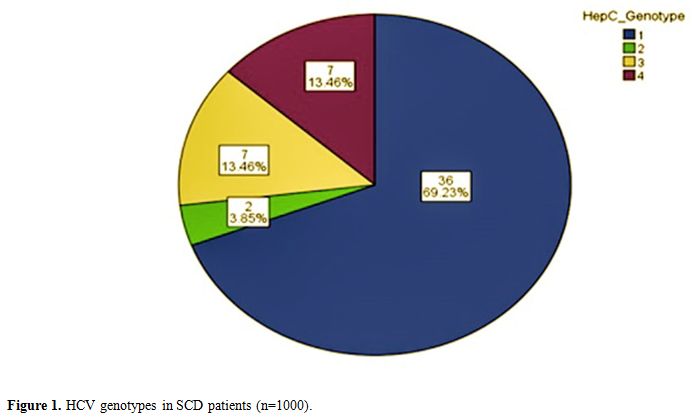 |
Figure
1. HCV genotypes in SCD patients (n=1000). |
Abdominal
liver ultrasound showed a normal liver in 78.8% (788) patients, whereas
20.8% (208) had hepatomegaly and only four patients had liver cirrhosis
(0.4%). A total of thirty-six patients (3.6%) in this study cohort had
died; however, in only two patients, death was due to cirrhosis of
liver.
. Discussion
The
survival rate of SCD patients has recently increased with improved
patient care, including blood transfusions, vaccinations and use of
antibiotics. However, repeated blood transfusions are still necessary
for many patients with SCD. Thus, multiply transfused patients with SCD
are at an increased risk of transfusion-associated infections such as
HBV, HCV, and HIV.
The screening for HBsAg became available in the late 1960s and had been implemented widely since 1970s.[18]
Despite regular HBV screening and national neonatal vaccination
programs since early nineties, HBV infection is still present. In Oman,
the prevalence of HBV infection was 5.8% in healthy adult population.[15] In another study, Al Awaidy, S. et al.[19]
reported that the prevalence of HBV among pregnant Omani women in 2006
was found to be 7.1%, with 0.5% being HBeAg positive. However, there is
no data related to HBV in SCD population from Oman.
In our
study, we found that the prevalence of HBV infection among SCD was 2.3%
with 16 (1.6%) HBV DNA positive, and 0.1% HBeAg positive.
Significantly, the prevalence is considerably lower than the earlier
studies in the general Omani population[15,19].
A possible explanation for this lower prevalence is the higher
prevalence of anti-Hbs (74.4%) in our SCD patient cohort. This high
prevalence of anti-HBs positivity reflects the success of prophylactic
hepatitis B vaccinations that are judiciously pursued by our
physicians, as well as the introduction of hepatitis B vaccination in
this relatively young patient population. The isolated total anti-HBc
pattern (anti-HBs negative/anti-HBc positive) found in 8.7% in our
study may reflect previous hepatitis B exposure and ability of human
body to eliminate the virus in some cases after infection.
Although
the burden of chronic HBV viral infection was low (1.6%) in this study,
it reflects the potential for chronic liver disease and hepatocellular
carcinoma. Treatment of chronic HBV infection can control viral
replication in most patients and reduces the risks for progression and
may even reverse liver fibrosis. Nevertheless, in this study cohort,
liver cirrhosis was only documented in 0.4% of patients with 0.2%
deaths related to liver disease.
There has been a significant drop
in transfusion-associated viral hepatitis since the implementation of
testing for HCV in the early 1990s.[20] In 1993, Al Dhahry, S.H et al[21]
reported the prevalence of antibodies to HCV among Omani patients with
renal disease. They observed anti-HCV antibodies in 26.5% patients
undergoing hemodialysis, 13.4% kidney transplant patients and 1%
non-dialyzed non-transplanted patients with various renal diseases.[21] In another study, Al Dhahry, S.H et al.[22]
reported the prevalence of HCV among Omani blood donors between 1991 to
2001 using a third-generation enzyme-linked immunosorbent assay (ELISA)
and recombinant immunoblot assay (RIBA) to confirm positive ELISA
tests.[22] Out of 30,012 samples from blood donors
that were screened for anti-HCV, 272 (0.91%) were positive. However,
among those, almost half (127) were confirmed positive by RIBA.[22] Al-Naamani, K et al,[23]
in a retrospective study of 200 thalassemic patients from Oman,
reported that the prevalence of anti-HCV antibodies in thalassemia
patients was 41%.[23] However, no previous data related to HCV in SCD population in Oman is available.
The
prevalence of anti-HCV positivity (12.6%) in this study was higher than
that observed in SCD patients from the United States (5.4%),[24] France (7.5%),[25] but lower than that reported from Egyptian SCD children (23%).[26]
Further, there is a 12-fold increase in the prevalence of HCV in SCD
patients in this study, as compared to that reported earlier by Al
Dahry et al.[22] from Oman, which was essentially a
population-based study. HCV is a major problem in SCD patients,
and this might be because we still do not have a specific HCV vaccine
as opposed to Hepatitis B vaccine. SCD patients are at great risk since
they are often multi-transfused, especially in those patients with a
severe disease background. The frequency of transfusion was identified
as a major risk factor for HCV infection, according to study conducted
in 1995 in Bahrain[27] and another in Siria reporting a cohort of frequently transfused hemoglobinopathy patients.[28]
However, although the risk of infection increased with increasing
frequency of transfusions, the prevalence of HCV in the current study
is much lower than reported in thalassemic patients from Oman (41%).[23]
Though there are several factors responsible for this high prevalence,
frequency of transfusions is indeed the most significant factor for the
high HCV prevalence in the thalassemia population from Oman.[23]
Further, although we did not study any close family contacts, lack of
transmission of HCV in very close family contacts of patients
undergoing multi-transfusions for thalassemia has been reported,
emphasizing that the nature of HCV transmission is predominantly by the
parenteral route.[29]
Further, the current study
found active HCV infection, confirmed by positive HCV RNA using RT-PCR,
in 5.2% of the anti-HCV positive patients. This datum reflects a
significant burden for chronic liver disease as these patients are at
risk of developing liver cirrhosis and progressing to end-stage liver
disease and liver cancer.[30] In 2016 the World
Health Organization (WHO) released their first global health sector
strategy with an ambitious plan to reduce the incidence of global
chronic hepatitis infections from the current 6–10 million cases of
chronic infection to 0.9 million cases worldwide, and to reduce the
annual deaths from chronic hepatitis from 1.4 million to less than 0.5
million by 2030.[31] It is now feasible to aim for
these objectives as the treatment for HCV has improved dramatically
with the addition of direct-acting antivirals, which are easy to take,
being an oral regimen that is highly effective, has minimal side
effects, and achieves cure rates of over 90%.[32,33]
This
study showed that HCV-genotype 1 was the most common genotype seen
(69.2%), which is similar to the most common HCV genotype reported in
thalassemic patients from Oman.[23] Although, the
genotype is not related to the mode of virus transmitted or with
histologic findings at presentation, patients with HCV genotype 1 tend
to develop more severe liver disease with lower rates of response to
interferon-based therapy than did patients with other HCV genotypes.[34]
HIV continues to be a major global public health issue. In 2016, an estimated 36.7 million people were living with HIV.[35] Some studies reported a low prevalence of HIV among SCD patients, with the prevalence in USA being 0.87%.[24]
In the current study, we did not identify any case with seropositivity
for HIV in this cohort, and no other cases have been identified with
SCD and HIV in our institution. Exposure to blood and blood products is
also common to two other diseases, i.e., hemophilia and thalassemia.
However, comparatively speaking, in our observation, although we had
seen respectively 3 and 4 HIV patients before 1990, currently,
among our 112 hemophilia and 200 thalassemia patients,[23]
we do not have any patient with positive serology for HIV-1 & 2.
The reason for the lower risk of HIV comorbidity with SCD is unclear.
Although the general population prevalence of HIV in Oman is low,[17]
SCD may have a unique effect in altering the risk of HIV infection or
progression, raising the possibility of SCD protective effect against
HIV infection.[36] Investigation of how the hemolytic
and immunological changes of SCD influence HIV might lead to a new
therapeutic or preventive approaches.[37]
Liver
involvement in patients with SCD includes a wide range of alterations,
from mild liver function test abnormalities to cirrhosis and acute
liver failure. Liver cirrhosis is a major public health problem and a
significant source of morbidity and mortality that is preventable and
underestimated. In HBV infection, it is estimated that 10% to 33% of
those who develop persistent infection end up with chronic hepatitis of
which 20% to 50% may develop liver cirrhosis,[38]
Studies in patients who had acquired hepatitis C by blood transfusion
for 15–25 years, revealed that 20% to 30% developed cirrhosis.[39]
In our current study we found that the proportion of liver cirrhosis
among HCV, HBV infected SCD patients was significantly low (0.4%), and
only two patients (0.2%) died due to liver cirrhosis.
SCD and homozygous β-thalassemia are common hemoglobinopathies in Oman,[5]
with financial consequences for national healthcare services. It is
thus prudent to follow the recommendations for blood banks and
transfusion services in Oman. The transfusions of such patients take
place in many hospitals throughout the country. Indications for blood
transfusions require local recommendations and guidelines to ensure
standardized levels of care.[40] Furthermore, not all
patients with positive results received blood transfusions. This
apparent discrepancy is mainly due to the fact that these patients also
receive blood transfusions at other health care facilities in the
Sultanate.
Conclusions
The
study documents the prevalence of HBV, HCV, and HIV infections among
SCD patients from Oman for the first time. Compared to the not affected
Omani population, the prevalence of HBV was significantly low
(anti-HBs), but that of HCV infection was significantly high
(anti-HCV). Persistence of viral infections was found to be 5.2% and
1.6% based on HCV PCR and HBV DNA respectively, indicating an ongoing
risk for chronic liver disease, cirrhosis, and liver cancer.
Importantly, no case with HIV was observed. Although the overall
mortality was 3.6%, liver cirrhosis was seen in only 0.2% of cases.
Acknowledgments
We wish to thank the hospital administration for the use of hospital material in this study.
References
- Rees, D.C., Williams, T.N. & Gladwin, M.T., Sickle-cell disease. The Lancet, 2010 376(9757), pp.2018–2031. http://dx.doi.org/10.1016/S0140-6736(10)61029-X.
- Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med. 1997 Sep 11;337(11):762-9 http://dx.doi.org/10.1056/NEJM199709113371107
- Steinberg MH. Genetic etiologies for phenotypic diversity in sickle cell anemia. Scientific World Journal. 2009; 9:46-67. http://dx.doi.org/10.1100/tsw.2009.10
- Al-Riyami,
A.A. Suleiman AJ, Afifi M, Al-Lamki ZM, Daar S., A community-based
study of common hereditary blood disorders in Oman. East Mediterr
Health J. 2001;7:1004-11. PMID: 15332742
- Al-Riyami,
A. & Ebrahim, G.J., Genetic Blood Disorders Survey in the Sultanate
of Oman. J Trop Pediatr. 2003 ;49 Suppl 1:i1-20. PMID: 12934793
- Alkindi
S, Al Zadjali S, Al Madhani A, et al Forecasting Hemoglobinopathy
Burden Through Neonatal Screening in Omani Neonates, Hemoglobin. 2010;
34, 135-144. http://dx.doi.org/10.3109/03630261003677213
- Shilpa
Jain, Nitya Bakshi, Lakshmanan Krishnamurti, Acute Chest Syndrome in
Children with Sickle Cell Disease Pediatr Allergy Immunol Pulmonol.
2017; 30: 191–201. http://dx.doi.org/10.1089/ped.2017.0814
- Vichinsky
EP, Neumayr LD, Earles AN, et al. Causes and outcomes of the acute
chest syndrome in sickle cell disease. National Acute Chest Syndrome
Study Group. N Eng. J Med ,2000; 342: 1855-1865. http://dx.doi.org/10.1056/NEJM200006223422502
- Josephson
CD, Su LL, Hillyer KL, Hillyer CD. Transfusion in the patient with
sickle cell disease: a critical review of the literature and
transfusion guidelines. Transfus Med Rev. 2007 Apr;21(2):118-33. http://dx.doi.org/10.1016/j.tmrv.2006.11.003
- Lavanchy D. Chronic viral hepatitis as a public health issue in the world. Best Pract Res Clin Gastroenterol. 2008;22:991-1008. http://dx.doi.org/10.1016/j.bpg.2008.11.002
- Margaret
E. Hellard, Roger Chou, and Philippa Easterbrook. WHO guidelines on
testing for hepatitis B and C – meeting targets for testing. BMC Infect
Dis. 2017; 17(Suppl 1): 703. http://dx.doi.org/10.1186/s12879-017-2765-2
- Poonam
Khetrapal Singh. Towards ending viral hepatitis as a public health
threat: translating new momentum into concrete results in South-East
Asia, Gut Pathog. 2018; 10: 9. http://dx.doi.org/10.1186/s13099-018-0237-x
- Calderon,
G.M. González-Velázquez F, González-Bonilla CR, et al. Prevalence and
risk factors of hepatitis C virus, hepatitis B virus, and human
immunodeficiency virus in multiply transfused recipients in Mexico.
Transfusion. 2009;49:2200-7. http://dx.doi.org/10.1111/j.1537-2995.2009.02248.x
- Ocak,
S. Kaya H, Cetin M, Gali E, Ozturk M. Seroprevalence of Hepatitis B and
Hepatitis C in Patients with Thalassemia and Sickle Cell Anemia in a
Long-term Follow-up, Arch Med Res. 2006 ;37:895-8. http://dx.doi.org/10.1016/j.arcmed.2006.04.007
- Al
Baqlani, S.A. Sy BT, Ratsch BA, et al. Molecular Epidemiology and
Genotyping of Hepatitis B Virus of HBsAg-Positive Patients in Oman,
PLoS One. 2014;9: e97759. http://dx.doi.org/10.1371/journal.pone.0097759
- Mohamoud,
Y.A., Riome, S. & Abu-raddad, L.J., Epidemiology of hepatitis C
virus in the Arabian Gulf countries: Systematic review and
meta-analysis of prevalence. Int J Infect Dis. 2016; 46:116-25. http://dx.doi.org/10.1016/j.ijid.2016.03.012
- Ali
Elgalib, Samir Shah, Zeyana Al-Habsi, et al, HIV viral suppression in
Oman: Encouraging progress toward achieving the United Nations ‘third
90’, Int. J. Inf. Dis. 2018:71,94–99. http://dx.doi.org/10.1016/j.ijid.2018.04.795
- Tawk,
H.M. Vickery K, Bisset L, Lo SK, Cossart YE; Infection in Endoscopy
Study Group. The significance of transfusion in the past as a risk for
current hepatitis B and hepatitis C infection: a study in endoscopy
patients. Transfusion. 2005 ;45:807-13. http://dx.doi.org/10.1111/j.1537-2995.2005.04317.x
- Al
Awaidy, S. Abu-Elyazeed R, Al Hosani H, et al. Sero-epidemiology of
hepatitis B infection in pregnant women in Oman, Qatar and the United
Arab Emirates. J Infect. 2006 ;52:202-6. http://dx.doi.org/10.1111/j.1537-2995.2005.04317.x
- Donahue,
J.G. Muñoz A, Ness PM, et al. The declining risk of post-transfusion
hepatitis C virus infection. N Engl J Med. 1992 ;327:369-73. http://dx.doi.org/10.1056/NEJM199208063270601
- Al-Dhahry
SH, Aghanashinikar PN, al-Hasani MK, Buhl MR, Daar AS. Prevalence of
Antibodies to Hepatitis C Virus among Omani Patients with Renal
Disease., Infection. 1993 ;21:164-7. PMID: 7690010
- Al
Dhahry, S.H., Nograles JC, Rajapakse SM, Al Toqi FS, Kaminski GZ.
Laboratory diagnosis of viral hepatitis C: The Sultan Qaboos University
Hospital experience. J Sci Res Med Sci. 2003 ;5:15-20. PMID: 24019730
PMCID: PMC3174725
- Al-Naamani,
K. Al-Zakwani I, Al-Sinani S, Wasim F, Daar S. Prevalence of Hepatitis
C among Multi-transfused Thalassaemic Patients in Oman: Single centre
experience., Sultan Qaboos Univ Med J. 2015 ;15:e46-51. PMID: 25685385
PMCID: PMC4318606
- Master,
S. Patan S, Cingam S & Mansour RP. Prevalence of Chronic Hepatitis
B, Hepatitis C and HIV in Adult Patients with Sickle Cell Disease.
Blood, 2016,128 (22), p.4863 (ASH abstracts)
- Arlet
JB, Comarmond C, Habibi A, et al. Prevalence and Characteristics of
Hepatitis C Virus Infection in Adult Sickle Cell Disease Patients
Living in France, J Infect Dis Epidemiol 2016, 2:020. (Open Access)
- Mousa,
S.M. El-Ghamrawy MK, Gouda H, Khorshied M, El-Salam Ahmed DA, Shiba H.
Prevalence of Hepatitis C among Egyptian Children with Sickle Cell
Disease and the Role of IL28b Gene Polymorphisms in Spontaneous Viral
Clearance. Mediterr J Hematol Infect Dis. 2016 ;8:e2016007. http://dx.doi.org/10.4084/MJHID.2016.007
- al-Mahroos,
F.T. & Ebrahim, A., Prevalence of hepatitis B, hepatitis C and
human immune deficiency virus markers among patients with hereditary
haemolytic anaemias. Ann Trop Paediatr. 1995;15:121-8. PMID: 7677412
- Yazaji,
W., Habbal, W., & Monem, F. (2016). Seropositivity of hepatitis b
and c among syrian multitransfused patients with hemoglobinopathy.
Mediterr J Hematol and Infect Dis, 8, e2016046. https://doi.org/10.4084/mjhid.2016.046
- Rosenthal
E, Hazani A, Segal D, Koren A, Kamal S, Rimon N, Atias D, Ben-Porath E.
Lack of transmission of hepatitis C virus in very close family contacts
of patients undergoing multitransfusions for thalassemia. J Pediatr
Gastroenterol Nutr. 1999 Jul;29(1):101-3. http://dx.doi.org/10.1097/00005176-199907000-00025
- Alter
HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus
infection: a perspective on long-term outcome. Semin Liver Dis.
2000;20:17–35. PMID: 10895429
- Organization WH. Global Health Sector Strategy on Viral Hepatitis, 2016–2021. http://www.who.int/health-accounts/platform_approach/en/. 2016.
- Ara
AK, Paul JP. New Direct-Acting Antiviral Therapies for Treatment of
Chronic Hepatitis C Virus Infection. Gastroenterol Hepatol (N Y).
2015;11:458–66. PMID: 27118941 PMCID: PMC4843024
- Schietroma
I, Scheri GC, Pinacchio C, Statzu M, Petruzziello A, Vullo V. Hepatitis
C Virus and Hepatocellular Carcinoma: Pathogenetic Mechanisms and
Impact of Direct-Acting Antivirals. Open Virol J. 2018; 12:16–25. http://dx.doi.org/10.2174/1874357901812010016
- Zein,
N.N. Rakela J, Krawitt EL, Reddy KR, Tominaga T, Persing DH. Hepatitis
C virus genotypes in the United States: epidemiology, pathogenicity,
and response to interferon therapy. Collaborative Study Group. Ann
Intern Med. 1996 ;125:634-9. http://dx.doi.org/10.7326/0003-4819-125-8-199610150-00002
- UNAIDS. Global AIDS update, 2016. http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf
- Obaro S. Does sickle cell disease protect against HIV/AIDS? Sex Transm Infect. 2012 Nov;88:533. http://dx.doi.org/10.1136/sextrans-2012-050613
- Nouraie,
M., Nekhai, S. & Gordeuk, V.R., Sickle cell disease is associated
with decreased HIV but higher HBV and HCV comorbidities in U.S.
hospital discharge records: a cross-sectional study. Sex Transm Infect.
2012 ;88:528-33. http://dx.doi.org/10.1136/sextrans-2011-050459
- Claus
Niederau, Chronic hepatitis B in 2014: Great therapeutic progress,
large diagnostic deficit, World J Gastroenterol. 2014 Sep 7; 20:
11595–11617. http://dx.doi.org/10.3748/wjg.v20.i33.11595.
- Alberti, A., Chemello, L. & Benvegnu, L., Natural history of hepatitis C. J Hepatol. 1999;31 Suppl 1:17-24. PMID: 10622555
- Arwa
Z. Al-Riyami and Shahina Daar, Transfusion in Haemoglobinopathies,
Review and recommendations for local blood banks and transfusion
services in Oman, Sultan Qaboos Univ Med J. 2018 Feb; 18(1): e3–e12. http://dx.doi.org/10.18295/squmj.2018.18.01.002

