Sanaa Kamal1, Sara Abdelhakam1, Dalia Ghoraba1, Mohamed Amer Mohsen2, Ahmed Abdel Salam3, Hoda Hassan4 and Leila Nabeigh5.
1 Department of Tropical Medicine, Ain Shams Faculty of Medicine, Cairo, Egypt.
2 Department of Radiodiagnosis, Misr University of Science and Technology, Cairo, Egypt.
3 Department of Pediatrics, Misr University of Science and Technology, Cairo, Egypt.
4 Department of Hematology and Clinical Pathology, Faculty of Medicine, Cairo University, Cairo, Egypt.
5 Department of Pathology, Ain Shams Faculty of Medicine, Cairo, Egypt.
Correspondence to: Sanaa M. Kamal, MD, PhD. Professor of Medicine,
Department of Gastroenterology, infectious diseases and tropical
medicine, Ain Shams Faculty of Medicine, Abbassia, Cairo, Egypt.
Tel.: +201158106588, Fax: +2020294311. E-mail:
sanaakamal@ainshamsmedicine.net
Published: November 1, 2019
Received: August 21, 2019
Accepted: September 19, 2019
Mediterr J Hematol Infect Dis 2019, 11(1): e2019060 DOI
10.4084/MJHID.2019.060
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
The course of hepatitis C infection (HCV) in patients with thalassemia
has not been adequately studied, and management has not been optimized.
The current prospective longitudinal study assessed the clinical
course, outcome, progression, and management of recently acquired HCV
in patients with transfusion-dependent thalassemia major versus acute
HCV without thalassemia.
Methods:
A well-characterized cohort of patients with thalassemia and recent HCV
infection or recent HCV without thalassemia were enrolled and
prospectively followed. The blood transfusion needs and chelating
agents were determined. Liver functions tests, HCV-RNA, iron, and
ferritin levels were measured. Patients with chronic HCV evolution
received treatment for HCV. The fibrosis progression rate was
determined in chronic HCV patients with or without thalassemia by
paired liver biopsies or serial transient elastography (TE), or serum
markers of liver fibrosis. Liver iron content (LIC) was assessed by R2
MRI.
Results:
Self-limited acute HCV was observed in 17% of patients with acute HCV
and thalassemia versus 35% of patients without thalassemia (P=0.031).
The fibrosis progression rates were significantly higher in patients
with chronic HCV and thalassemia compared to those with chronic HCV
alone (1.14±0.48) and (0.35±0.14) (P<0.0001), respectively. A direct
linear correlation was observed between the fibrosis progression rate
and each of LIC (R=+0.67; P=0.01) and ferritin (R=0.77; P<0.01). In
patients with chronic HCV and thalassemia, the sustained virologic
response (SVR) to pegylated interferon-based therapy and direct
antiviral agents (DAAS) were 33% and 82% respectively (P<0.0001),
while in chronic HCV patients without thalassemia, the SVR rates to
PEG-IFN/RBV and DAAs were 51% and 92% respectively. Five patients with
concomitant HCV and thalassemia died during the study due to cardiac
causes (n=3) and liver cancer (n=2).
Conclusions:
Patients with acute HCV and thalassemia have low rates of spontaneous
resolution of HCV infection, and the majority develop chronic
HCV. Direct-acting antiviral combinations are associated with high
SVR rates and low adverse event in treatment naïve and experienced
patients with chronic HCV and thalassemia. Liver fibrosis is
accelerated in thalassemia patients with chronic HCV; therefore, early
diagnosis, treatment with DAAs, adequate iron chelation, and
non-invasive monitoring liver status are recommended to prevent
cirrhosis and hepatocellular carcinoma.
|
Introduction
Hepatitis
C infection (HCV) is a major cause of liver-related morbidity,
cirrhosis, hepatocellular carcinoma, and liver transplantation.[1,2] In Western countries, HCV prevalence ranges between 2% in the United States of America and 1.6% % in Europe.[3] Significantly higher incidence and prevalence rates are reported from Southeast Asia, Africa and Western Pacific.[1]
In Egypt, HCV has been a huge public health and economic burden with
prevalence rates exceeding 15%, however, the incidence of HCV in Egypt
is gradually declining after adoption of a nationwide program for
prevention and treatment of HCV infection.[4-6] HCV infection results in acute hepatitis which may be either subclinical or associated with symptoms.[5]
The rates of self-limited acute HCV vary; however, more than half of
HCV infections progress to chronic hepatitis that may progress to
cirrhosis and liver cancer in some patients.[7]
Progression of HCV related liver fibrosis is highly variable and may be
accelerated in the presence of coinfections such as HIV, HBV, or
schistosomiasis or comorbidities.[7-12]
Beta-thalassemia
comprises a group of hereditary disorders characterized by a genetic
deficiency in the synthesis of beta-globin chains resulting in chronic
hemolytic anemia that requires long-term transfusion therapy and iron
chelation.[13] Despite the advances in the management
of thalassemia patients, long-term transfusion therapy remains a risk
for increased iron compartmentalization in different organs such as the
liver, heart, endocrine glands such as pituitary gland, pancreas,
ovaries, testes, thyroid, parathyroid and adrenals leading to various
complications.[13-16]
In Egypt, as in several
Mediterranean countries, β-thalassemia represents a major health
problem since it constitutes 85% of hereditary hemoglobinopathies. The
injurious impact of iron overload on the liver is further accentuated
by the high prevalence of HCV infection among patients with
thalassemia.[17,18,19] Before the adoption of
obligatory screening for HCV at blood banks in Egypt, the prevalence of
HCV among multitransfused thalassemic patients reached almost 85%.[20,21]
Despite the current strict control on HCV screening in blood banks, a
recent study showed that 37% of Egyptians with thalassemia have HCV
infection.[19] Given the high prevalence of HCV in
Egypt, interfamilial and iatrogenic HCV transmission are important
modes of HCV transmission among thalassemics in Egypt.[21]
Concomitant HCV infection and iron overload are major causes of
advanced liver fibrosis and cirrhosis among Egyptian multi-transfused
thalassemia patients.[22,23]
Accurate
assessment of the liver fibrosis progression rates requires performing
at least one baseline liver biopsy for the initial diagnosis and
staging of liver fibrosis, and successive (one or more) liver biopsies)
performed several years after the baseline biopsy. However, liver
biopsy is an invasive procedure that may be associated with
complications and may not be ethically justified if patients are not
offered therapy. Histopathologic scoring systems are limited by biopsies’
size, variability of inter- and intra-observer reproducibility,
sampling errors, and potential fibrosis heterogeneity throughout the
liver.[24] Thus, non-invasive methods for assessment
of HCV related hepatic fibrosis including direct or indirect serum
biomarkers of fibrosis or assessment of liver stiffness by transient
elastography or magnetic resonance elastography have been used to
determine the degree of liver disease and predict the rate of hepatic
fibrosis progression in patients with chronic HCV to prioritize therapy
to those with accelerated rates of hepatic fibrosis progression.[25-28]
To
date, neither the course of HCV infection in patients with thalassemia
nor their response to antiviral therapy particularly direct-acting
antiviral agents (DAAs) has been adequately assessed in longitudinal
studies. Therefore, we conducted this prospective, longitudinal study
to investigate the clinicopathologic features, course, progression of
disease in patients with thalassemia and recent HCV infection. We also
evaluated the diagnostic and prognostic performance of transient
elastography and various panels of non-invasive fibrosis biomarkers
individually or in combination to assess their potential role as
non-invasive diagnostic tools for monitoring liver fibrosis in patients
with thalassemia who developed chronic hepatitis C.
Patients and Methods
Study design and study population.
We conducted the current longitudinal, prospective study at six
hospitals and liver centers in Cairo, Upper Egypt and Delta Egypt,
between February 2004 and December 2018. Consecutive Egyptian
thalassemia patients with proven acute HCV genotype 4 (HCV-G4)
infection were enrolled in the current study in addition to patients
with proven acute HCV mono-infection. The study was approved by the
Office for Human Protections Research Board of the participating
institutions. The protocol and all procedures of the study were
conducted in accordance with Good Clinical Practice guidelines and in
conformity with the ethical guidelines of the Declaration of Helsinki.
All patients presented written informed consent before enrollment and
before any study-related procedure.
Patients with thalassemia
and recent HCV infection were invited to join the study if they
fulfilled the criteria of acute HCV which include elevated serum
alanine aminotransferase (ALT) at least five times the upper limit of
normal (40 U/L), seroconversion from a previous documented negative HCV
antibody test prior potential HCV exposure into a positive antibody
test after a suspected risky exposure (anti-HCV tested using a Cobas
e411 analyzer with Elecsys Anti-HCV assay; Roche Diagnostics, Mannheim,
Germany) and recent detection of HCV (PCR; COBAS Amplicor HCV test
version 2.0; Roche Molecular Systems, Pleasanton, CA, USA) in the
presence or absence of symptoms such as jaundice, dark urine, malaise,
abdominal pain.
Patients were excluded from the study if he/she
had previous HCV infection, hepatitis A, hepatitis B, autoimmune
hepatitis, alcoholic liver disease, drug-induced hepatitis, and
decompensated liver disease with a history of variceal hemorrhage,
ascites, or hepatic encephalopathy, clinical symptoms of cardiac
dysfunction; coinfection with schistosomiasis or human immunodeficiency
virus (HIV), a leucocyte count less than 3000/mm3, neutropenia (<1500 cells/mm3),
serum creatinine above the upper limit of normal (ULN); significant
proteinuria (urinary protein/creatinine ratio [UPCR] ≥1.0 mg/mg),
thrombocytopenia (<90 000 cells/mm3), or organ transplantation.
Enrolled
patients were followed for spontaneous resolution of acute HCV or
development of chronic HCV. Spontaneous HCV clearance was defined as
undetectable HCV RNA tested at least in two instances six months after
the estimated seroconversion date or the first positive HCV PCR or date
of potential exposure. Detectable viremia beyond six months implies
chronic hepatitis C. Patients with proven chronic HCV were screened for
eligibility to treatment and followed during therapy and after
treatment completion (Figure 1).
Patients who were ineligible to therapy or those who declined therapy
were enrolled and followed for assessment of hepatic fibrosis
progression or consideration for DAAs. As control groups, patients with
chronic HCV without thalassemia were also enrolled and followed for at
least five years.
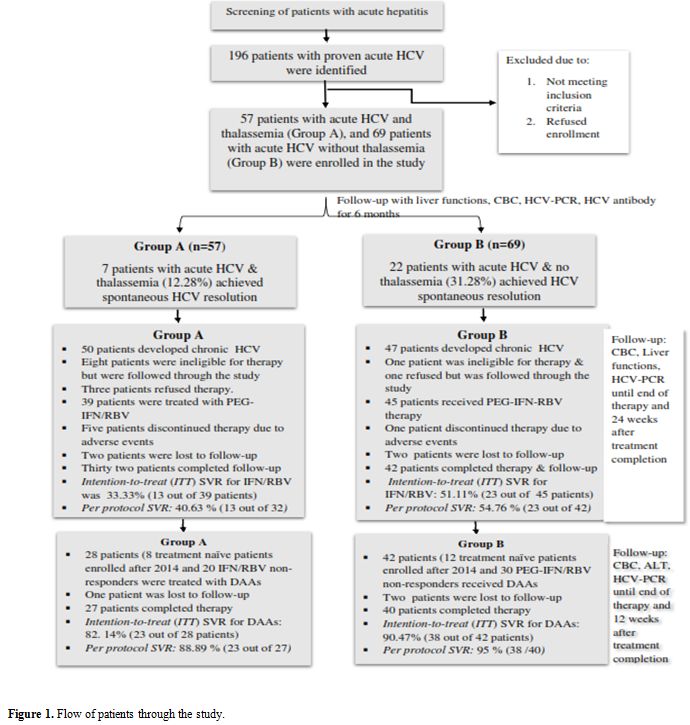 |
Figure 1. Flow of patients through the study. |
Laboratory assays.
Complete blood picture, liver functions tests (aspartate
aminotransferase (AST); alanine aminotransferase (ALT); alkaline
phosphatase (ALP) and gamma-glutamyltransferase (GGT), conjugated and
unconjugated bilirubin levels) were performed monthly for all patients
at enrollment and during the acute phase and every three months in the
chronic phase of HCV infection. Serum iron, ferritin
(electrochemiluminescent immunoassay, ELECSYS 2010; Hitachi High
Technologies Corp, Tokyo, Japan; Ferritin values exceeding 500 ng/mL)
indicated iron overload, serum iron (mcg/dl).
Qualitative
HCV-PCR (COBAS® AmpliPrep/COBAS® TaqMan® HCV Qualitative Test, v2;
Roche Diagnostics Branchburg, NJ, USA) with low limit of detection
(LoD) = 15 IU/mL was performed to at all patients after potential risky
exposure then repeated after 4 weeks to monitor the first positive HCV
positivity. Quantitative HCV-PCR (Lower limit of quantitation (LLoQ):
15 IU/mL (OBAS® AmpliPrep/COBAS® TaqMan® HCV Test), was performed at
weeks 12, 24 and 48 weeks to all patients. In patients with chronic HCV
evolution eligible for HCV therapy, quantitative HCV-PCR was conducted
before initiation of treatment, at treatment weeks 4, 12, 24, end of
therapy and 12 or 24 weeks after therapy completion according to the
treatment regimen.
Interventions.
Antiviral therapy for chronic HCV: Until 2014, patients with chronic HCV were treated with 48 weeks of PEG-IFN α-2a (180 µg,
Pegasys; Hoffman La Roche, Basel, Switzerland) and ribavirin (RBV)
(10.6 mg/kg/day; Copegus, Hoffman La Roche, Basel, Switzerland) for
patients without thalassemia or RBV 600 mg/day in patients with
thalassemia. Beyond 2014, patients received with sofosbuvir 400 mg and
daclatasvir 60 mg daily for 12 weeks. The primary endpoint was
sustained virological response (SVR) defined as undetectable serum HCV
RNA 12/24 weeks after the discontinuation of treatment (according to
the therapeutic regimen) using COBAS® AmpliPrep/COBAS® TaqMan® HCV
Quantitative Test, v2.0 (Roche Diagnostics, Branchburg, NJ, USA) with a
lower limit of detection (LoD) of 15 IU/mL
Iron chelation:
The history of iron chelation, age of start of chelation therapy,
frequency of chelation and type of chelators were reported in all
enrolled thalassemia patients. Patients received deferoxamine (DFO) or
deferasirox (DFX) or a combination of both.
Histological Assessment.
After obtaining each patient’s consent, patients who developed chronic
HCV in both groups were subjected to a liver biopsy as a requirement
for assessing their eligibility to therapy. A subset of patients who
developed chronic HCV and either failed or did not receive PEG-IFN/RBV
therapy had second liver biopsies during screening for DAA
treatment. Liver biopsies were performed under ultrasound
guidance with local analgesia. Biopsies were divided into two sections:
one for histology examination and another for liver iron concentration
(LIC). Biopsies were stained by hematoxylin-eosin, Masson’s trichrome,
and Perls’ stains and evaluated histologically by an experienced
pathologist (L.N.). Perl’s stained slides were assessed for liver iron
according to Scheuer and Rowe[29,30] in which
stainable iron is graded on a 0-4 scale where 0 implies absence of
granules at magnification x 400, grade 1 indicates granules are barely
discernable at x 250 magnification and easily confirmed at x 100, grade
2 is considered when discrete granules were resolved at x 100
magnification, grade 3 depicts discrete granules resolved at x 25
magnification while grade 4 is given when masses are visible at
magnification x 10 or naked eye. LIC was determined by atomic
absorption spectrophotometry with normal LIC levels ranging between 0.4
and 2.2 mg/g of dry liver weight. (dw). LIC values of between 3 and 7
mg/g dw are considered mildly elevated while values between 7-14 are
moderate and LIC >14 mg/g dw represent severe iron overload. [30,31]
Liver biopsies were graded according to the Metavir scoring system.[32,33]
Briefly, the METAVIR scoring system assesses histologic lesions in
chronic hepatitis C using two separate scores, one for the
necroinflammatory grade (A for activity) and another for the stage of
fibrosis (F). These scores are defined as follows; stages of fibrosis
(F): F0, no fibrosis; F1, portal fibrosis without septa; F2, portal
fibrosis with rare septa, F3, numerous septa without cirrhosis; F4,
cirrhosis. Grade for activity (A): A0, no histologic necroinflammatory
activity; A1, minimal activity, A2, moderate activity, A3, severe
activity.
Fibrosis Progression Rate Assessment.
In chronic HCV patient with paired liver biopsies, the direct
progression rate of fibrosis per year was estimated as the difference
between fibrosis scores of the baseline and follow-up biopsies divided
by the interval between the two biopsies. In patients with a single
biopsy, the indirect fibrosis progression rate per year was estimated
as the ratio between the fibrosis stage (in METAVIR score) and the
estimated duration of infection in years.[33,34]
Transient Elastography. Transient
elastography (TE) was annually performed in patients with chronic HCV
with or without thalassemia to monitor liver stiffness (LSM) and
fibrosis progression by using Fibroscan® (Echosens, Paris, France)
device according to the manufacturer’s instructions and as previously
described. The liver stiffness measurement (LSM) results were reported
in kilopascals (kPa) where higher kPa reflected a stiffer liver and
more severe liver fibrosis. According to the TE values, patients are
grouped into three categories: those with elastography values of
≤7.0 kPa corresponding to METAVIR stages F0 or F1; those with
elastography values > 7kPa-≤ 15kPa who have moderate to severe
fibrosis (stages F2 and F3) and are at risk for fibrosis progression
and the third group includes patients with high elastography values
> 15.0 kPa (METAVIR stage of F4 or some cases of F3) who have a high
likelihood of cirrhosis.[35,36]
Serum fibrosis biomarkers.
In patients with chronic HCV with or without thalassemia, serial
measurements of the fibrosis markers: human N-terminal procollagen III
propeptide (PIIINP) (BioSource -International Inc. Nivelles, Belgium),
YKL-40 (YKL-40 ELISA Kit, LifeSpan Biosciences Inc. Seattle WA, USA)
and serum hyaluronic acid (HA, Hyaluronic Acid Test Kit (Corgenix,
Westminster, Colorado, USA) was performed according to the
manufacturers’ instructions.
Liver Iron Content assessment by R2 MRI method. Liver iron content was assessed non-invasively in a subset of patients by FerriScan® R2-MRI using a 1.5T scanner (MAGNETOM Avanto Fit, Siemens Healthcare, Erlangen, Germany) as previously described.[29,30]
LIC values were expressed in mg Fe/g dry weight (dw). According to the
FerriScan values, LIC levels were graded as: Grade 1=normal LIC < 3
mg Fe/g dw, Grade 2=mild overload LIC 3–7 mg Fe/g dw, Grade 3=moderate
LIC overload 7–15 mg Fe/g dw, and Grade 4=severe LIC overload ≥15 mg
Fe/g dw.[37,38]
Statistical analysis.
Continuous variables were expressed as mean ± SD or median (range).
Continuous variables that follow a Gaussian distribution were analyzed
using unpaired t-test (two
unpaired groups) or one-way ANOVA (three or more unmatched groups) or
repeated measures ANOVA (three or more matched groups). Nonparametric
tests, Mann-Whitney test, and Kruskal-Wallis tests were applied for the
analysis of variables that followed non-Gaussian distribution.
Categorical variables were compared by chi-square test or Fisher’s
exact test. Association between two variables was performed by Pearson
or Spearman correlation according to the type of data. Survival
time/time to event was measured by Kaplan Meier survival curve or Cox
proportional hazard regression. Formal hypotheses were two-sided with a
type I nominal error rate of 0.05. Results were expressed as mean
values ± standard deviation (SD). For all statistical purposes, P
< 0.05 was considered statistically significant. All statistical
analyses were performed using SPSS (Statistical Package for Social
Sciences) software version 22 (IBM, Armonk, New York, USA).
Results
From 2004 through 2018, 57 patients with β-thalassemia
and recent HCV infection (Group A), and 69 patients with acute HCV
without thalassemia (Group B) fulfilled the inclusion criteria,
provided informed and were enrolled in the study (Figure 1). Baseline demographic and clinical characteristics of enrolled patients are shown in Table 1.
No significant differences in age, gender, or BMI. The risk factors for
HCV transmission were comparable between the two groups except for
blood transfusion. Patients with concomitant HCV and thalassemia showed
significantly reduced hemoglobin levels and total iron-binding
capacity, as well as elevated serum iron, transferrin, and ferritin
levels in comparison to those with acute HCV infection without
thalassemia (Table 1). During
the acute phase of HCV infection, the mean total ALT and AST levels and
HCV-RNA levels were slightly higher in patients with HCV and
thalassemia compared to those without thalassemia although the
difference was not statistically significant. (Figure 2).
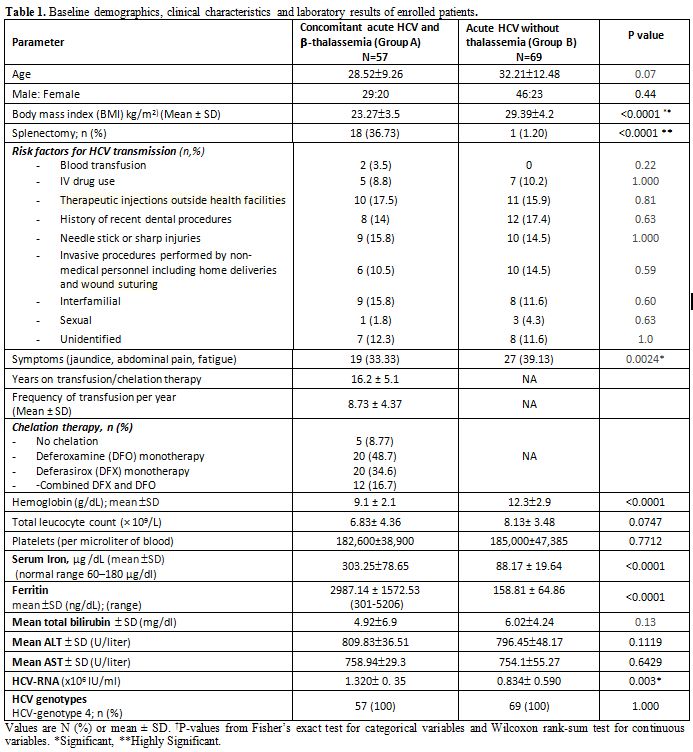 |
Table 1. Baseline demographics, clinical characteristics and laboratory results of enrolled patients. |
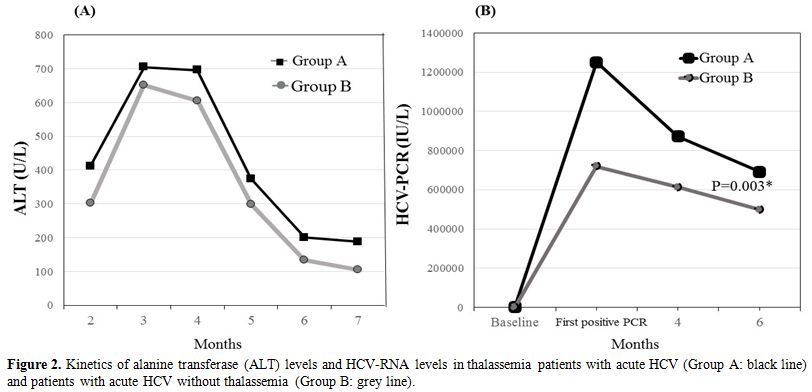 |
Figure
2. Kinetics of alanine transferase (ALT) levels and HCV-RNA levels in
thalassemia patients with acute HCV (Group A: black line) and patients
with acute HCV without thalassemia (Group B: grey line). |
Outcome of acute HCV in patients with thalassemia and without thalassemia.
Spontaneous resolution of acute HCV occurred in 10 out of 57 patients
(17.54%) with concomitant acute HCV and thalassemia compared to 24 out
of 69 patients without thalassemia (34.78%) (P= 0.043) (Figure 1).
Other than the abnormal baseline iron profile, no significant
differences in demographics or clinical features were observed between
patients who achieved spontaneous HCV clearance compared to those who
did not (data not shown).
Response rates to the antiviral treatment regimen in patients who developed chronic HCV with and without thalassemia.
Through the study, 47 and 45 patients in groups A and B respectively
developed chronic HCV and were assessed for eligibility to either
PEG-IFN based therapy (before 2014) or DAAs (after 2014) according to
the standard of care available. In Group A, 39 patients were eligible
to pegylated interferon and ribavirin therapy, and 8 treatment naïve
patients enrolled after 2014 were treated with DAAs. In group B, 47
patients developed chronic HCV; of them, 42 patients received treatment
(23 IFN-based therapy and 19 received DAAs) (Figure 1).
In both groups, the sustained response rates (SVR) were significantly
higher in patients who were treated with DAAs compared with PEG-IFN
based therapy. Patients with thalassemia and chronic HCV experienced
significantly less 80/80/80 adherence to PEG-IFN with more ribavirin
reduction and more adverse events compared to Group B patients. In
patients with chronic HCV and thalassemia, the SVR rates were 34.62%
and 90% with PEG-IFN based treatment and DAAs therapy respectively (P
<0.0001). In patients with chronic HCV without thalassemia, the SVR
rates were 60.87% and 94.74% with PEG-IFN/RBV treatment and DAAs
therapy respectively (P <0.0001). No significant differences in SVR
rates to DAAs were observed between patients with chronic HCV with and
without thalassemia (Figure 1).
Blood
transfusion demands of patients with HCV and thalassemia during acute
HCV infection and during chronic HCV antiviral therapy. During
the acute phase of HCV infection, blood transfusion demands were not
significantly increased in patients with concomitant thalassemia.
However, blood transfusion demands were increased by 15% in patients
with thalassemia and chronic HCV during PEG-IFN and RBV therapy,
compared with the pre-treatment transfusion amounts. In patients with
SVR, the blood transfusion amounts decreased significantly after
treatment completion to almost approaching the pre-treatment
transfusion demands (6.23 units/month during therapy vs. 5.03
units/month after therapy, p = 0.03) (data not shown).
Liver histology and fibrosis progression rates in the study groups.
Liver biopsies were assessed for necroinflammation and fibrosis in
patients who developed chronic HCV. Baseline biopsies were performed as
a prerequisite for PEG-IFN/RBV eligibility screening (before 2014). The
liver biopsies were repeated in treatment-experienced patients who
failed PEG-IFN based regimen and were considered for DAAs therapy. The
mean interval between the two biopsies was 83.19±18.92 months and
85.53±19.22 months in Groups A and B respectively (P= 0.609). Baseline
and follow-up liver biopsies showed that patients with thalassemia and
chronic HCV had significantly higher grading and staging scores (Table 2)
The direct liver fibrosis progression rates were assessed in patients
with paired liver biopsies (39 and 45 chronic HCV patients with
thalassemia and without thalassemia respectively). The fibrosis
progression rates were significantly higher in patients with chronic
HCV and thalassemia (1.14.84±0.48) compared to those with chronic HCV
alone (0.24±0.14) (P < 0.0001) (Table 2).
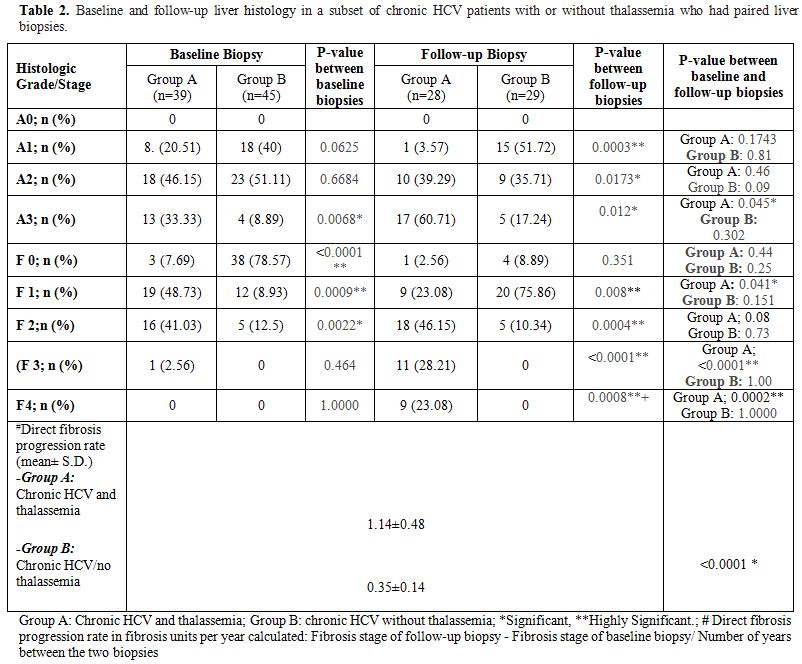 |
Table 2. Baseline and
follow-up liver histology in a subset of chronic HCV patients with or
without thalassemia who had paired liver biopsies. |
Non-invasive assessment of liver fibrosis and fibrosis progression.
The liver fibrosis and hepatic fibrosis progression were also monitored
non-invasively by serial transient elastography and serum fibrosis
markers measurements. At all study time points, TE scores were
significantly higher in patients with concomitant chronic HCV and
thalassemia compared to Group B patients. The serum markers PIIINP,
YKL-40, and HA, were significantly higher in Group A patients compared
to Group B patients (Table 3).
A significant correlation was observed between histologic liver
fibrosis and LSM in Group A patients (r = 0.82 (P< 0.0001)) and
group B patients (r=0.69; P<0.001) (Table 4). A correlation was also detected between LSM results and PIIINP, YKL-40 (Table 4).
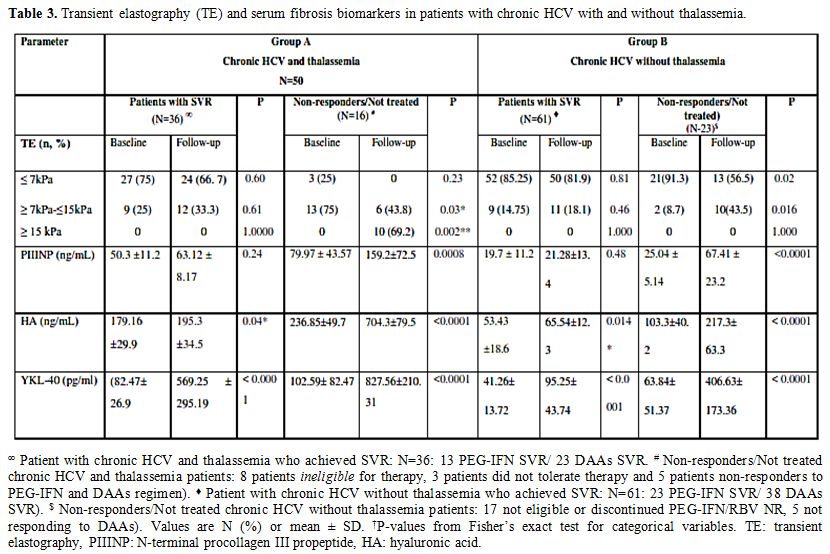 |
Table 3. Transient elastography (TE) and serum fibrosis biomarkers in patients with chronic HCV with and without thalassemia. |
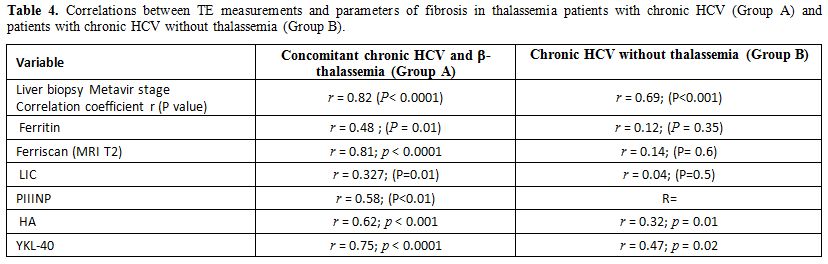 |
Table 4. Correlations between
TE measurements and parameters of fibrosis in thalassemia patients with
chronic HCV (Group A) and patients with chronic HCV without thalassemia
(Group B). |
Liver iron concentration (LIC).
At all study points, the liver iron content measured by either
histopathology or MRI was significantly higher in thalassemia patients
with chronic HCV and thalassemia compared to chronic HCV patients
without thalassemia. Heavy hepatic iron overload was observed patients
with concomitant HCV and thalassemia during PEG-IFN based therapy (Figure 3a). A significant correlation occurred between LIC and hepatic fibrosis rates (Figure 3b). Similar results were observed with ferritin (Figures 3c, 3d).
In thalassemia patients with chronic HCV, a linear correlation was
detected between LSM values and LIC levels (r= 0.327; P<0.01) while
in chronic HCV patients without thalassemia no correlation was found (Table 4).
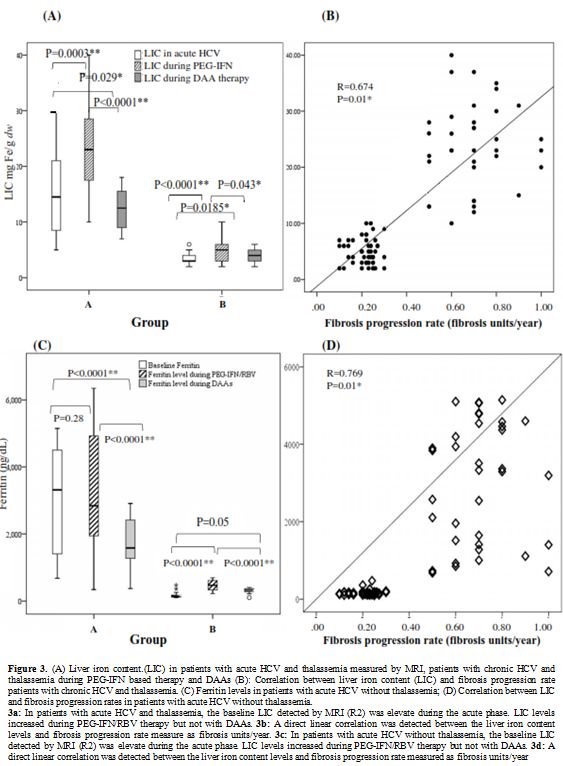 |
Figure 3. A) Liver iron
content.(LIC) in patients with acute HCV and thalassemia measured by
MRI, patients with chronic HCV and thalassemia during PEG-IFN based
therapy and DAAs (B): Correlation between liver iron content (LIC) and
fibrosis progression rate patients with chronic HCV and thalassemia.
(C) Ferritin levels in patients with acute HCV without thalassemia; (D)
Correlation between LIC and fibrosis progression rates in patients with
acute HCV without thalassemia.
3a: In patients with acute HCV and
thalassemia, the baseline LIC detected by MRI (R2) was elevate during
the acute phase. LIC levels increased during PEG-IFN/RBV therapy but
not with DAAs. 3b: A direct linear correlation was detected between the
liver iron content levels and fibrosis progression rate measure as
fibrosis units/year. 3c: In patients with acute HCV without
thalassemia, the baseline LIC detected by MRI (R2) was elevate during
the acute phase. LIC levels increased during PEG-IFN/RBV therapy but
not with DAAs. 3d: A direct linear correlation was detected between the
liver iron content levels and fibrosis progression rate measured as
fibrosis units/year
|
Follow-up and survival analysis.
Fibrosis progression was more accelerated in patients with chronic HCV
and thalassemia (Group A) compared to patients with chronic HCV without
thalassemia (Group B) (P=0.002; Figure 4a).
Survival
analysis revealed a significant difference between the study groups
(P=0.04). Five patients with concomitant HCV and thalassemia passed
away during the study. The causes of death were cardiac-related in 3
patients and due to hepatocellular carcinoma in two patients. Among
patients with chronic HCV without thalassemia, one patient passed away
after a traffic accident.
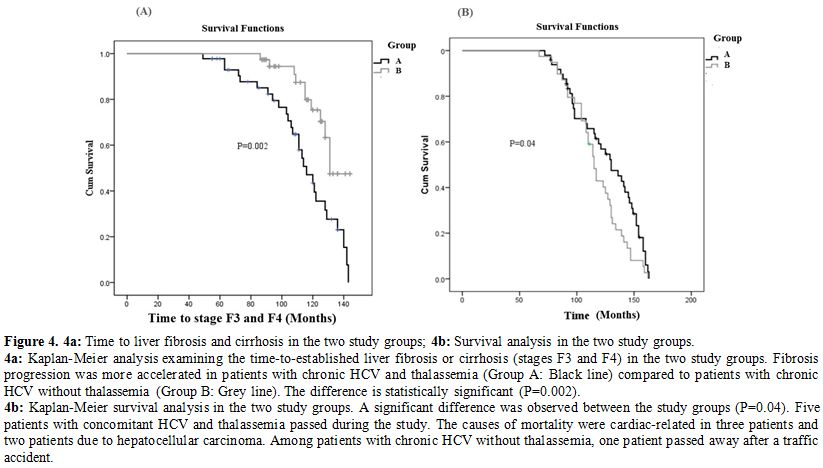 |
Figure 4. 4a: Time to liver fibrosis and cirrhosis in the two study groups; 4b: Survival analysis in the two study groups.
4a:
Kaplan-Meier analysis examining the time-to-established liver fibrosis
or cirrhosis (stages F3 and F4) in the two study groups. Fibrosis
progression was more accelerated in patients with chronic HCV and
thalassemia (Group A: Black line) compared to patients with chronic HCV
without thalassemia (Group B: Grey line). The difference is
statistically significant (P=0.002).
4b: Kaplan-Meier survival
analysis in the two study groups. A significant difference was observed
between the study groups (P=0.04). Five patients with concomitant HCV
and thalassemia passed during the study. The causes of mortality were
cardiac-related in three patients and two patients due to
hepatocellular carcinoma. Among patients with chronic HCV without
thalassemia, one patient passed away after a traffic accident.
|
Discussion
The
current prospective, longitudinal study compared the outcome and
progression of HCV infection in a well-characterized cohort of
thalassemia patients who acquired recent HCV infection and a cohort
with recent HCV infection without thalassemia. The study also
investigated the response to antiviral therapies. The risk factors for
HCV infection in this study reflect the previously reported exposures
related to acute viral of HCV in Egypt[4,5,11,12,21] and highlight the role of interfamilial HCV transmission as previously reported.[21,39]
In the current study, the rate of spontaneous resolution of acute HCV
in patients with concomitant acute HCV and thalassemia was
significantly lower compared to the rate of spontaneous eradication of
acute HCV mono-infection.[11,12,39-43] Very few studies investigated the outcome of acute HCV in a limited number of thalassemia patients. A study[44]
reported spontaneous HCV resolution rate of 44%. It is not clear why
patients with thalassemia have lower rates of spontaneous HCV
eradication, however, given the limited number of studies, the
non-homogeneity of cohorts, the differences in enrollment criteria it
is difficult to estimate the outcome of acute HCV in patients with
thalassemia and further large studies are required.
Our findings
showed that patients with concomitant thalassemia and HCV showed higher
tendency to develop chronic HCV and were considered for either PEG-IFN
based therapy or DAAs according to the standard of care available at
the study time points. The SVR of patients with concomitant chronic HCV
and thalassemia to PEG-IFN based therapy was significantly lower than
SVR in patients without thalassemia in agreement with previous reports.[45-47]
In the current study, the poor response of patients with chronic HCV
and thalassemia to PEG-IFN based therapy may be explained by frequent
occurrence of adverse events such as anemia due to ribavirin induced
hemolysis, interferon-induced bone marrow suppression and the higher
grading and staging scores observed in the baseline liver biopsies of
patients with concomitant chronic HCV and thalassemia due to the dual
liver injury caused by chronic hepatitis and hepatic iron overload.
Although a lower dose of ribavirin was used in thalassemia patients,
ribavirin increased hemolysis that necessitated elevating the blood
transfusion demands and increased risk of iron overload. Thus,
PEG-IFN/RBV regimen was not an efficient safe therapeutic strategy to
manage this patient population. In contrast, our study showed
significantly enhanced efficacy and safety of DAAs in treating patients
with concomitant chronic HCV genotype 4 and thalassemia in whom
significantly high SVR rates have been achieved even in
treatment-experienced patients in accordance with previous studies.[48-51]
The
fibrosis progression rates detected in our chronic HCV patients without
thalassemia were comparable to those in previous reports.[33-35]
However, patients with concomitant HCV and thalassemia showed a
significantly high progression of fibrosis rates which were similar to
those reported in patients with HIV or HBV or S. mansoni coinfections[9-12,33]
and almost one third of this cohort had established cirrhosis by the
end of the study. The accelerated hepatic fibrosis is probably
attributed to the cumulative effect of HCV induced liver injury and the
increased iron overload particularly in inadequately chelated patients,
and that was shown in this study and other studies[51,52]
by the direct correlation between LIC and fibrosis progression rates.
The accelerated rates of liver fibrosis and high incidence of liver
cirrhosis in this patient population highlight the importance of early
detection of HCV infection and prompt treatment with DAAs to prevent
advanced liver disease with its complications.
Our results
showed not only comparability of TE and serum fibrosis markers with the
liver biopsy results but also the capability of TE and fibrosis markers
in non-invasive monitoring of liver fibrosis progression in patients
with thalassemia and HCV in accordance with previous studies.[51,52]
Our study also showed that hepatic iron levels did not interfere with
the TE results suggesting that TE alone or in combination with serum
fibrosis biomarkers particularly markers measures a quantitative liver
fibrosis parameters such as which may be used in follow-up of this
patient population.
In the current study, three patients with
concomitant HCV and thalassemia died due to cardiac causes and other
two due to hepatocellular carcinoma (HCC). Besides early treatment of
thalassemia patients with chronic HCV, close monitoring of patients
(including those who achieved SVR) for HCC, iron overload and cardiac
status, is mandatory.
The current study has several strengths
that include the longitudinal prospective study design; the long follow
up which stretched for 14 years, the reasonable sample size, the
inclusion of patients with acute HCV with and without thalassemia, the
comprehensive investigations performed and inclusion of the antiviral
regimen available through the study. The limitation of the present
study is enrolling patients infected with HCV genotype 4 only because
this is the prevalent genotype in Egypt.
Conclusions
Thalassemia
patients have low rates of spontaneous eradication of HCV infection and
the majority develop chronic HCV. Liver fibrosis is accelerated in
thalassemia patients with chronic HCV due to the impact of HCV induced
liver injury and iron overload state in thalassemia patients.
Direct-acting antiviral agents are highly effective and safe in
treating naïve and experienced thalassemia patients with chronic HCV.
Therefore, early diagnosis and treatment of this with DAAs is
recommended to prevent cirrhosis and hepatocellular carcinoma which are
important causes of mortality in this patients population.
References
- Lavanchy D. The global burden of hepatitis C. Liver Int 2009; 29:74 81. https://doi.org/10.1111/j.1478-3231.2008.01934.x PMid:19207969
- Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis 2015; 5:558-567. https://doi.org/10.1016/S1473-3099(05)70216-4
- Gower
E, et al. Global epidemiology and genotype distribution of the
hepatitis C virus infection. J Hepatol. 2014; 61(1):S45-57. https://doi.org/10.1016/j.jhep.2014.07.027 PMid:25086286
- Kamal SM, Nasser IA. Hepatitis C genotype 4: what we know and what we don't yet know. Hepatology 2008; 47:1371-1383 https://doi.org/10.1002/hep.22127 PMid:18240152
- Angelico
M, Renganathan E, Gandin C, Fathy M, Profili MC, Refai W, et al.
Chronic liver disease in Alexandria governorate, Egypt: contribution of
schistosomiasis and hepatitis virus infections. J Hepatol 1997; 26:
236-243. https://doi.org/10.1016/S0168-8278(97)80036-0
- Talaat
M, Afifi S, Reaves E, Abu Elsood H, El-Gohary A, Refaey S, Hammad R,
Abdel Fadeel M, Kandeel A. Evidence of sustained reductions in the
relative risk of acute hepatitis B and C virus infections, and the
increasing burden of hepatitis a virus infection in Egypt: comparison
of sentinel acute viral hepatitis surveillance results, 2001-17. BMC
Infect Dis. 2019; 19: 159. https://doi.org/10.1186/s12879-019-3806-9 PMid:30764780 PMCid:PMC6376689
- Westbrook R.H. Dusheiko. G. Natural history of hepatitis C J Hepatol 2014; 61 (1 Suppl): S58-S68 https://doi.org/10.1016/j.jhep.2014.07.012 PMid:25443346
- Poynard
T, Bedossa P, Opolon P. Natural history of liver fibrosis progression
in patients with hepatitis C. Lancet 1997;349:825-832. https://doi.org/10.1016/S0140-6736(96)07642-8
- Newsum
AM, Kooij KW, Boyd A, Smit C, Wit FWNM, van der Meer JTM, Prins M,
Reiss P, van der Valk M; MOSAIC study group, ATHENA observational HIV
cohort and NVHB-SHM hepatitis working group. Progression of liver
fibrosis following acute hepatitis C virus infection in HIV-positive
MSM. AIDS. 2019; 1;33(5):833-84. https://doi.org/10.1097/QAD.0000000000002138 PMid:30649050
- Pol
S, Haour G, Fontaine H. Dorival C, Petrov-Sanchez V, Bourliere M, J.
Capeau J, Carrieri P, Larrey D, Larsen C, Marcellin, Pawlostky JM,
Nahon F, Zoulim P, Cacoub P, de Ledinghen V, Mathurin P, Negro F,
Pageaux GP, Yazdanpanah Y. Wittkop L, Zarski JP, Carrat F, For The
French Anrs Co22 Hepather Cohor. The negative impact of HBV/HCV
coinfection on cirrhosis and its consequences. Aliment Pharmacol Ther.
2017 Dec;46(11-12):1054-1060. https://doi.org/10.1111/apt.14352 PMid:28994127
- Kamal
SM, Rasenack JW, Bianchi L, Al Tawil A, El Sayed Khalifa K, Peter T,
Mansour H, et al. Acute hepatitis C without and with schistosomiasis:
correlation with hepatitis C-specific CD4 (+) T-cell and cytokine
response. Gastroenterology 2001; 12: 646-656. https://doi.org/10.1053/gast.2001.27024 PMid:11522749
- Kamal
SM, Graham CS, He Q, Bianchi L, Tawil AA, Rasenack JW, et al. Kinetics
of intrahepatic hepatitis C virus (HCV)-specific CD4+ T cell responses
in HCV and Schistosoma mansoni coinfection: relation to progression of
liver fibrosis. J Infect Dis 2004; 189:1140-1150. https://doi.org/10.1086/382278 PMid:15031780
- Origa R. β-Thalassemia. Genet Med. 2017 Jun;19(6):609-619. https://doi.org/10.1038/gim.2016.173 PMid:27811859
- Pennell
DJ, Udelson JE, Arai AE, Bozkurt B, Cohen AR, Galanello R.
Cardiovascular function and treatment in ß-thalassemia major: a
consensus statement from the American Heart Association. Circulation.
2013. 128(3):281-308 https://doi.org/10.1161/CIR.0b013e31829b2be6 PMid:23775258
- De
Sanctis V, Soliman AT, Yassin MA, Di Maio S, Daar S, Elsedfy H, Soliman
N, Kattamis C. Hypogonadism in male thalassemia major patients:
pathophysiology, diagnosis and treatment. Acta Biomed. 2018 Feb
16;89(2-S):6-15
- De
Sanctis V, Elsedfy H, Soliman AT, Elhakim IZ, Soliman NA, Elalaily R,
Kattamis C Endocrine profile of β-thalassemia major patients followed
from childhood to advanced adulthood in a tertiary care center.Indian J
Endocrinol Metab. 2016 Jul-Aug;20(4):451-9. https://doi.org/10.4103/2230-8210.183456 PMid:27366710 PMCid:PMC4911833
- De
Sanctis V, Kattamis C, Canatan D, Soliman AT, Elsedfy H, Karimi M, Daar
S, Wali Y, Yassin M, Soliman N, Sobti P, Al Jaouni S, El Kholy M,
Fiscina B, Angastiniotis M. β-Thalassemia Distribution in the Old
World: an Ancient Disease Seen from a Historical Standpoint. Mediterr J
Hematol Infect Dis. 2017 Feb 20;9(1):e2017. https://doi.org/10.4084/mjhid.2017.018 PMid:28293406 PMCid:PMC5333734
- Elalfy
MS, Adly A, Awad H, Tarif Salam M, Berdoukas V, Tricta F. Safety and
efficacy of early start of iron chelation therapy with deferiprone in
young children newly diagnosed with transfusion-dependent thalassemia:
A randomized controlled trial.Am J Hematol. 2018 Feb;93(2):262-268. https://doi.org/10.1002/ajh.24966 PMid:29119631
- Ragab
L, Helal S, Zaghloul N, El-Raziky M, Afifi R, Musallam KM, Taher AT
Clinicovirologic analysis of hepatitis C infection in
transfusion-dependent β-thalassemia major children. 2010; Int J Lab
Hematol 32:184-190 https://doi.org/10.1111/j.1751-553X.2009.01155.x PMid:19389113
- El-Beshlawy A, Youssry I. Prevention of hemoglobinopathies in Egypt. Hemoglobin. 2009;33 Suppl 1:S14-20 https://doi.org/10.3109/03630260903346395 PMid:20001619
- Said
F, El Beshlawy A, Hamdy M, El Raziky M, Sherif M, Abdel kader A, Ragab
Intrafamilial transmission of hepatitis C infection in Egyptian
multitransfused thalassemia patients. J Trop Pediatr.
2013;59(4):309-13. https://doi.org/10.1093/tropej/fmt017 PMid:23542535
- Angelucci
E, Brittenham GM, McLaren CE, Ripalti M, Baronciani D, Giardini C,
Galimberti M, Polchi P, Lucarelli G.. Hepatic iron concentration and
total body iron stores in thalassemia major. N Engl J Med.
2000;343(5):327-331 https://doi.org/10.1056/NEJM200008033430503 PMid:10922422
- Borgna-Pignatti
C, Rugolotto S, De Stefano P, Zhao H, Cappellini MD, Del Vecchio GC,
Romeo MA, Forni GL, Gamberini MR, Ghilardi R, Piga A, Cnaan A. Survival
and complications in patients with thalassemia major treated with
transfusion and deferoxamine. 2004; Haematologica 89:1187-1193
- Bedossa
P, Dargère D and Paradis V. Sampling variability of liver fibrosis in
chronic hepatitis C. Hepatology 2003; 38:1449-1457 https://doi.org/10.1053/jhep.2003.09022 PMid:14647056
- Castera L. Non-invasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology 2012; 142:1293-302. https://doi.org/10.1053/j.gastro.2012.02.017 PMid:22537436
- Rossi
E, Adams L, Prins A, et al. Validation of the FibroTest biochemical
markers scores in assessing liver fibrosis in hepatitis C patients.
Clin Chem 2003; 49(3):450-4. https://doi.org/10.1373/49.3.450 PMid:12600957
- Johansen
JS, Moller S, Price PA, et al. Plasma YKL-40 a new potential marker of
fibrosis in patients with alcoholic cirrhosis? Scand J gastroenterol
1997; 32: 582-90. https://doi.org/10.3109/00365529709025104 PMid:9200292
- Karsdal
MA, Hjuler ST, Luo Y, Rasmussen DG, Nielsen MJ, Leeming DJ, Goodman Z,
Arch RH, Patel K, Schuppan D. Assessment of liver fibrosis progression
and regression by a serological collagen turnover profile. Am J Physiol
Gastrointestinal Liver Physiol. 2019 Jan 1;316(1):G25-G31 https://doi.org/10.1152/ajpgi.00158.2018 PMid:30160980
- Scheuer
P.J., Williams R., Muir A.R. Hepatic pathology in relatives of patients
with haemochromatosis. J. Pathol. Bacteriol. 1962;84:53-64 https://doi.org/10.1002/path.1700840107 PMid:14498313
- Rowe
JW, Wands JR, Mezey E, Waterbury LA, Wright JR, Tobin J, Andres R.
Familial hemochromatosis: characteristics of the precirrhotic stage in
a large kindred. Medicine (Baltimore). 1977 May;56(3):197-211. https://doi.org/10.1097/00005792-197705000-00002 PMid:870791
- Alustiza
JM, Castiella A, De Juan MD, Emparanza JI, Artetxe J, Uranga M. Iron
overload in the liver diagnostic and quantification. Eur J Radiol.
2007; 61:499-506. https://doi.og/10.1016/j.ejrad.2006.11.012 PMid:17166681
- Bedossa,
P. & Poynard, T. An algorithm for the grading of activity in
chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology,
1996; 24, 289- 293. https://doi.org/10.1002/hep.510240201 PMid:8690394
- Poynard
T, Bedossa P, Opolon P. Natural history of liver fibrosis progression
in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR,
and DOSVIRC groups. Lancet. 1997 Mar 22;349(9055):825-32. https://doi.org/10.1016/S0140-6736(96)07642-8
- Deuffic-Burban
S, Poynard T, Valleron AJ. Quantification of fibrosis progression in
patients with chronic hepatitis C using a Markov model. J Viral Hepat.
2002;9(2):114-22. https://doi.org/10.1046/j.1365-2893.2002.00340.x PMid:11876793
- Poynard
T, Vergniol J, Ngo Y, Foucher J, Munteanu M, Merrouche W, Colombo M,
Thibault V, Schiff E, Brass CA, Albrecht JK, Rudler M, Deckmyn O,
Lebray P, Thabut D, Ratziu V, de Ledinghen V; FibroFrance Study Group;
Epic3 Study Group; Bordeaux HCV Study Group. Staging chronic hepatitis
C in seven categories using fibrosis biomarker (FibroTest™) and
transient elastography (FibroScan®). J Hepatol. 2014;60(4):706-14 https://doi.org/10.1016/j.jhep.2013.11.016 PMid:24291240
- Kennedy
P, Wagner M, Castéra L, Hong CW, Johnson CL, Sirlin CB, Taouli B.
Quantitative Elastography Methods in Liver Disease: Current Evidence
and Future Directions. Radiology: 2018; 286 ( 3): 738-763 https://doi.org/10.1148/radiol.2018170601 PMid:29461949 PMCid:PMC5831316
- Rose
C, Vandevenne P, Bourgeois E, Cambier N, Ernst O. Liver iron content
assessment by routine and simple magnetic resonance imaging procedure
in highly transfused patients. Eur J Haematol. 2006 Aug;77(2):145-9 https://doi.org/10.1111/j.0902-4441.2006.t01-1-EJH2571.x PMid:16608501
- Tipirneni-Sajja
A, Song R, McCarville MB, Loeffler RB, Hankins JS, Hillenbrand
CM.Automated vessel exclusion technique for quantitative assessment of
hepatic iron overload by R2*-MRI. J Magn Reson Imaging. 2018
Jun;47(6):1542-1551 https://doi.org/10.1002/jmri.25880 PMid:29083524 PMCid:PMC5927847
- Kamal
SM, Kassim SK, Ahmed AI, Mahmoud S, Bahnasy KA, Hafez TA, Aziz IA,
Fathelbab IF, Mansour HM. Host and viral determinants of the outcome of
exposure to HCV infection genotype 4: a large longitudinal study. Am J
Gastroenterol. 2014 Feb;109(2):199-211. https://doi.org/10.1038/ajg.2013.427 PMid:24445571
- Sharaf
Eldin N, Ismail S, Mansour H, et al. Symptomatic acute hepatitis C in
Egypt: diagnosis, spontaneous viral clearance, and delayed treatment
with 12 weeks of pegylated interferon alfa‐2a. PLoS One. 2008; 3( 12):
e4085. https://doi.org/10.1371/journal.pone.0004085 PMid:19115010 PMCid:PMC2605267
- Bakr
I, Rekacewicz C, El Hosseiny M, Ismail S, El Daly M, El-Kafrawy S,
Esmat G, Hamid MA, Mohamed MK, Fontanet A. Higher clearance of
hepatitis C virus infection in females compared with males. Gut. 2006
Aug;55(8):1183-7. https://doi.org/10.1136/gut.2005.078147 PMid:16434426 PMCid:PMC1856273
- Santantonio
T, Medda E, Ferrari C, Fabris P, Cariti G, Massari M, Babudieri S, Toti
M, Francavilla R, Ancarani F, Antonucci G, Scotto G, Di Marco V,
Pastore G, Stroffolini T. Risk factors and outcome among a large
patient cohort with community-acquired acute hepatitis C in Italy. Clin
Infect Dis. 2006;43:1154-9. https://doi.org/10.1086/507640 PMid:17029134
- Jauncey
M, Micallef JM, Gilmour S, et al. Clearance of hepatitis C virus after
newly acquired infection in injection drug users. Jjj Infect Dis. 2004;
190 (7): 1270‐ 1274. https://doi.org/10.1086/423943 PMid:15346337
- Lai
ME, Origa R, Danjou F, Leoni GB, Vacquer S, Anni F, Corrias C, Farci P,
Congiu G, Galanello R. Natural history of hepatitis C in thalassemia
major: a long-term prospective study. Eur J Haematol. 2013;
90(6):501-7. https://doi.org/10.1111/ejh.12086 PMid:23414443
- Kamal
SM, Fouly A, Mohamed MK, Lamonti S, Gohary M, Koziel MJ, Afdhal NA.
Peginterferon alpha-2b therapy with and without ribavirin in patients
with thalassemia: A randomized study. Journal of Hepatology. 2006;44
(2): S217 https://doi.org/10.1016/S0168-8278(06)80585-4
- Harmatz
P, Jonas MM, Kwiatkowski JL, Wright EC, Fischer R, Vichinsky E, et al.
Safety and efficacy of pegylated interferon alpha-2a and ribavirin for
the treatment of hepatitis C in patients with thalassemia.
Haematologica. 2008;93(8):1247-51 https://doi.org/10.3324/haematol.12352 PMid:18556414
- Sandoughdaran
S, Alavian SM, Sharafi H, Behnava B, Salimi S, Mehrnoush L, Karimi
Elizee P, Keshvari M. Efficacy of prolonged treatment with pegylated
interferon (Peg-IFN) and ribavirin in thalassemic patients with
hepatitis C who relapsed after previous Peg-IFN-Based Therapy. Hepat
Mon. 2015. 13;15(1):e23564. https://doi.org/10.5812/hepatmon.23564 PMid:25741371 PMCid:PMC4344648
- Origa
R, Ponti ML, Filosa A, Galeota Lanza A, Piga A, Saracco GM, Pinto V,
Picciotto A, Rigano P, Madonia S, Rosso R, D'Ascola D, Cappellini MD,
D'Ambrosio R, Tartaglione I, De Franceschi L, Gianesin B, Di Marco V,
Forni GL; Italy for THAlassemia and hepatitis C Advance - Società
Italiana Talassemie ed Emoglobinopatie (ITHACA-SITE). Treatment of
hepatitis C virus infection with direct-acting antiviral drugs is safe
and effective in patients with hemoglobinopathies. Am J Hematol. 2017
Dec;92(12):1349-1355. https://doi.org/10.1002/ajh.24911 PMid:28929515
- Mehta
R, Kabrawala M, Nandwani S, Desai P, Bhayani V, Patel S, Parekh V.
Safety and Efficacy of Sofosbuvir and Daclatasvir for hepatitis C virus
infection in patients with β-thalassemia major. J Clin Exp Hepatol.
2018;8(1):3-6. https://doi.org/10.1016/j.jceh.2017.06.002 PMid:29743790 PMCid:PMC5938522
- Mangia
A, Sarli R, Gamberini R, Piga A, Cenderello G, Piazzolla V, Santoro R,
Caruso V, Quarta A, Ganga R, Copetti M, Forni G. Randomised clinical
trial: sofosbuvir and ledipasvir in patients with transfusion-dependent
thalassaemia and HCV genotype 1 or 4 infection. Aliment Pharmacol Ther.
2017; 46(4):424-431 https://doi.org/10.1111/apt.14197 PMid:28660640
- Zachou
K, Arvaniti P, Gatselis NK, Azariadis K, Papadamou G, Rigopoulou E,
Dalekos GN.. Patients with Haemoglobinopathies and Chronic Hepatitis C:
A Real Difficult to Treat Population in 2016? Mediterr J Hematol Infect
Dis. 2017; 1;9(1):e2017003. https://doi.org/10.4084/mjhid.2017.003 PMid:28101309 PMCid:PMC5224816
- Maira
D, Cassinerio E, Marcon A, Mancarella M, Fraquelli M, Pedrotti P,
Cappellini MD. Progression of liver fibrosis can be controlled by
adequate chelation in transfusion-dependent thalassemia (TDT). Ann
Hematol. 2017; 96(11):1931-1936. https://doi.org/10.1007/s00277-017-3120-9 PMid:28875336
- Ferraioli
G, Lissandrin R, Tinelli C, Scudeller L, Bonetti F, Zicchetti M, Longo
F, Murgia M, Bernuzzi S, Zecca M, Casula P, Piga A, Filice C. Liver
stiffness assessed by transient elastography in patients with β
thalassaemia major. Ann Hepatol. 2016; 15(3):410-417. https://doi.org/10.5604/16652681.1198817







