Xiaoyuan Gong1, Mianzeng Yang1, Dong Lin1, Hui Wei1, Ying Wang1, Bingcheng Liu1, Chunlin Zhou1, Kaiqi Liu1, Shuning Wei1, Benfa Gong1, Guangji Zhang1, Yuntao Liu1, Yan Li1, Xingli Zhao1, Shaowei Qiu1, Runxia Gu1, Yingchang Mi1 and Jianxiang Wang1.
1 State Key
Laboratory of Experimental Hematology, National Clinical Research
Center for Blood Diseases, Institute of Hematology & Blood Diseases
Hospital, Chinese Academy of Medical Sciences & Peking Union
Medical College, Tianjin 300020, China.
Correspondence to: Dr. Jianxiang Wang. State Key Laboratory of
Experimental Hematology, National Clinical Research Center for Blood
Diseases, Institute of Hematology & Blood Diseases Hospital,
Chinese Academy of Medical Sciences & Peking Union Medical College,
Tianjin 300020, China. Tel: 86-22-23909120, Fax: 86-22-23909047.
E-mail:
wangjx@ihcams.ac.cn
Published: January 1, 2020
Received: September 7, 2019
Accepted: November 15, 2019
Mediterr J Hematol Infect Dis 2020, 12(1): e2020003 DOI
10.4084/MJHID.2020.003
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
The
study of candidemia in Chinese leukemia patients has been limited. This
retrospective study aims to investigate the characteristics and
prognostic factors of candidemia among leukemia patients.
From
2009 to 2015, 30 isolates of candidemia were detected in 19 patients
with acute leukemia after chemotherapy. The overall incidence of
candidemia was 2.12 episodes per 1000 admissions. Candida tropicalis was the most common Candida
species (n = 17; 89.5%). The vast majority of candidal infections are
endogeneous. The overall 30-day crude mortality rate was 31.6%.
Neutrophil recovery (P = 0.000) and initiation of empiric antifugal
treatment before first positive blood culture (P = 0.041) were
associated with a significant improvement in overall survival.
Early
diagnosis, followed by rapid antifungal treatment remains the
cornerstone of successful management. The widespread use of newer
antifungal agents as prophylaxis among patients with acute leukemia may
result in a decreased candidemia incidence.
|
Introduction
Candidemia is an infection that can threaten the life of patients with acute leukemia.[1] Most published data about candidemia in China summarized from non-neutropenic patients.[2-4]
There are absences of the study of the incidence, microbiologic
characteristics, and clinical outcome of candidemia among patients with
acute leukemia in China. To clear up these issues, we performed a
retrospective research of candidemia in patients with acute leukemia
who had been successively treated at our center between 2009 and 2015.
Case Report
This
retrospective study reviewed and analyzed 30 isolates of candidaemia
involving 19 patients with acute leukemia from January 2009 to December
2015.
Candidemia is defined as the positivity of no less than
one blood culture linking to clinical symptoms of bloodstream infection
such as hypotension or fever. All patients with acute leukemia and no
less than one blood culture positive for Candida spp.
were identified. We collected information regarding patient
characteristics, type of underlying leukemia, depth of neutropenia,
duration of, and recovery from neutropenia, presence of a central
venous catheter (CVC) and subsequent removal, prior or concurrent use
of antifungal agents and/or broad spectrum antibiotics, laboratory and
microbiological data, treatment options and clinical outcomes.
Catheter-associated candidemia was diagnosed when there was no other obvious source of infection, and the identical Candida spp. was separated from both the catheter-tip culture and the peripheral blood.[5,6] Patients with neutrophil counts less than 0.5×109/L
were diagnosed with neutropenia. Prolonged neutropenia was defined as
neutropenia lasting for over 12 days before the beginning of fungemia.
Neutrophil recovery was defined as the resolution of neutropenia beyond
0.5×109/L. Mortality resulting from
candidemia was defined as death within 30 days after the initial blood
culture under the condition of stable haematological disease and none
of the possible reasons for death.
Two patients developed
candidemia while being treated with intravenous voriconazole as a
prescription for presumed pulmonary fungal disease. Other patients
received oral fluconazole as routine antifungal prophylaxis during
chemotherapy.
The overall incidence of candidemia was 2.12
episodes per 1000 admissions. Candida tropicalis was the most common
Candida species (n=17; 89.5%), followed by Candida albicans (n=2;
10.5%) and one (5%) patient had concomitant bacteremia.
The median
age of the population was 38 years old (range: 17 to 64 years), and the
sex ratio was roughly equal. The most common underlying disease was
acute myeloid leukemia (94.7%). Of the 19 patients with candidemia in
this study, 15 (78.9%) had a newly diagnosed disease, 3 (15.8%) were in
remission, and one patient (5.31%) had relapsed disease. All patients
present with persistent or refractory fever lasted for ten days (range:
2-60 days), and the average peak body temperature was 40℃. The fever
may persistent for a long time even after recovery from neutropenia.
Erythematous papules appeared on ten (52.6%) patient’s skin, mainly on
trunk and upper extremities (Table 1).
No septic shock happened in these patients. Mucosal Candida
colonizations (mouth or stools) were observed in eight patients (42.1%)
before chemotherapy.
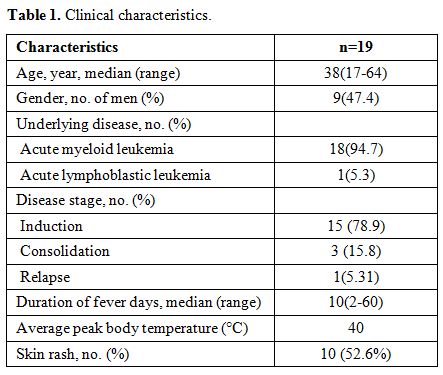 |
Table
1. Clinical characteristics. |
The overwhelming
majority of these patients had central venous catheterization (n=18;
94.7%). Before the infection, all patients were neutropenic for an
average of 14 days (range: 6 to 20 days). Median time from initiation
of chemotherapy to diagnosis of candidaemia was 14 days (range: 9 to 35
days). All patients had received broad-spectrum antibiotics within the
preceding eight days (range: 3-20 days) and cephalosporins, imipenem
and piperacillin were the most commonly prescribed drugs. Several
previously identified risk factors for candidemia were present in our
patients.[6] They are listed in Table 2.
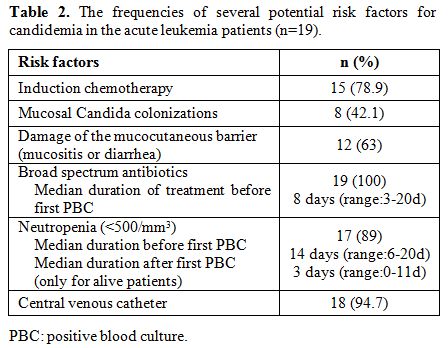 |
Table 2. The frequencies of several potential risk factors for candidemia in the acute leukemia patients (n=19). |
6 patients died
within 6.5 days (range: 5-20 days) of candidemia, leading to a crude
mortality of 31.6% and in cases due to Candida tropicalis the mortality
was 35.3%, which was similar with the report of Sipsas et al.[1] Almost all surviving patients (12/13; 92.3%) developed a chronic disseminated candidiasis after candidemia.
All isolates of Candida spp.
were sensitive in vitro to fluconazole, itraconazole, amphotericin B
and voriconazole. Susceptibility to echinocandin was not performed as a
result of the condition limit. There was no trend of increasing minimum
inhibitory concentration observed during the period of 2009-2015.
We
further analyzed whether there were any differences in demographic
characteristics or risk factors and disease characteristics between
those patients who died and those who survived (Table 3).
We found that the resolution of candidemia was associated with
neutrophil recovery (P=0.000) and initiation of empiric antifugal
treatment before the first positive blood culture (P=0.041). Age,
gender, and prolonged neutropenia before the onset were unrelated to
the clinical outcome.
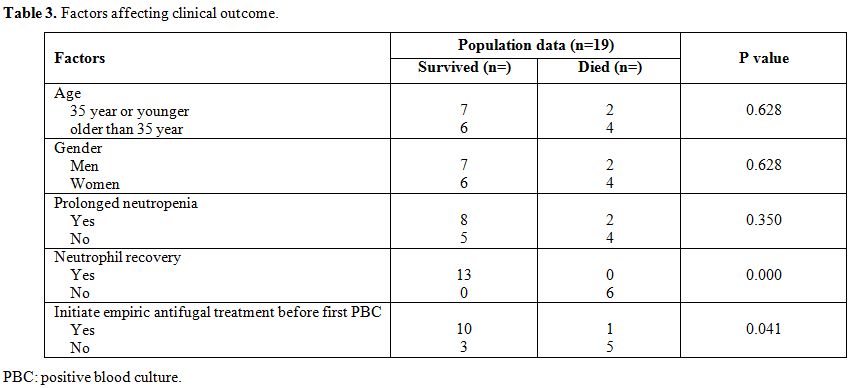 |
Table 3. Factors affecting clinical outcome. |
As candidemia
increases mortality rates by 20–49%, immediate and targeted treatment
initiation is necessary. In our study, 11 patients (57.9%) received
empirical treatment when a candidal infection was suspected (example,
patients present with persist fever had no respondence to
broad-spectrum antibacterial antibiotics neither had the manifestation
of skin rash), the others begun antifungal treatment after preliminary
positive results of blood cultures were reported by microbiology
departments. Twelve patients (63.2%) received monotherapy and the other
combination therapy. Antifungal treatments were caspofungin (n=5),
liposomal amphotericin B (n=5), voriconazole (n=1), fluconazole (n=1),
liposomal amphotericin B+voriconazole (n=4), caspofungin+liposomal
amphotericin B (n=2) and caspofungin+ voriconazole (n=1). The median
duration of intravenous antifungal treatment was 14 days (3–49 days)
and 15 days (3–49 days) in all patients and surviving patients
respectively.
Fifteen patients’ CVC was removed as soon as the
first positive blood culture was reported. Results of CVC culture were
accessible in these patients and indicated that no patient had an
infection of CVC.
Discussion
While
the incidence of candidemia seems to be reasonably low in patients with
leukemia submitted to intensive chemotherapy, crude and attributable
mortality rates have maintained persistently high, and similar to those
reported before,[1,7] despite the
introductions of new antifungal agents. This study has disclosed
several important issues about the epidemiology, manifestations, and
therapy of candidemia in Chinese patients, which offers a better
understanding and improves the management of this disease.
Candida albicans was the most common Candida spp. isolated from general patients or non-neutropenic patients with candidemia in Europe, the US and China.[2,8,9] C. parapsilosis and C. glabrata were predominant species in hematological patients with candidemia in Europe and US.[1,10] In China, there was an overall increase in isolation of C. parapsilosis for cancer patients.[11] However, in our study, C. tropicalis
(89.5%) was the most common pathogen. This unique epidemiology probably
accounts for the predominance of acute leukemia, the majority of
neutropenic patients, and all patients treated with cytotoxic agents
known to alter the gastrointestinal tract (GIT). In 1986, Walsh et al.
have demonstrated the increased invasion of C. tropicalis in the GIT of neutropenic patients with mucositis.[12]
The physical integrity of the mucous membrane barriers altered by
chemotherapy facilitated the spread of infection into the systemic
circulation. Therefore, the epidemiology of candidemia varies among
different regions and patients.
The mortality rate significantly
increases due to the delayed antifungal treatment. Even the delay of
12-24 hours can lead to the twofold increases in crude mortality rate
in candidemia.[13] Blood cultures remain the mainstay
for the diagnosis of candidemia, but a median incubation time of 2 days
(range:1-5 days) was required for species identification and
susceptibility in our study, early diagnosis of candidemia is still
difficult. In the study, we initiate empirical antifungal treatment due
to highly suspicion of candidemia judged by clinicians from symptoms
and manifestations in some patients, resulted in a better survival
(P=0.041). Among ten patients presented with skin rash, 6 of them
suffered from the skin lesions before the preliminary positive results
of blood cultures were reported, then early antifungal treatments were
initiated. The presences of skin rash seem to benefit an early
diagnosis of this infection. This fact alerts that we should be
cautious when we do physical examination screening of fungal infections.
Early CVC removal is strongly recommended by guidelines and considered to be critical to successful treatment in early studies.[14-16]
Nevertheless, in our research, the removal of CVCs had no connections
with the improvement of clinical outcome, which indicate that the
majority of candidal infections are endogenous rather than CVC. It was
different from another study in patients with hematologic malignancies.[1]
The latest edition of clinical practice guideline for the management of
Candidiasis by IDSA (Infectious Diseases Society of America) points out
that endogenous sources of candidiasis other than a CVC (example,
gastrointestinal tract) predominate in neutropenic patients, so
catheter removal should be considered on an individual basis for these
patients.[17] This newest suggestion is consistent
with our study. However, the fact is that the preservation of CVC has
been low. In one study covering children with C. parapsilosis complex infections, the preservation of catheter was 33.3% within caspofungin treatment.[18]
From
the year 2009 to 2014, three cases of candidemia occurred in our center
per year on average. Interestingly, there was no candidemia occurred in
the year of 2015, it seems that the widespread use of newer antifungal
agents (voriconazole or posaconazole) as prophylaxis among patients
with acute leukemia did result in a decreased candidemia incidence.
However, further prospective studies and continued surveillance are
needed to confirm this hypothesis.
There were several limitations
to our study. First, the study was performed only at a single
leukemia-chemotherapy center; consequently, it may not reflect local
practice patterns and be suitable for transplant submitted patients or
other hematologic malignancy. Second, this study was a retrospective
investigation. Third, the limited number of cases suffering from
candidemia may have compromised the statistical power of the study.
However, this study attempts to focus on candidemia in acute leukemia
patients receiving chemotherapy and still offers valuable information
concerning this issue.
Conclusions
In
summary, although the incidence of candidemia seems to be quite low in
patients with leukemia receiving intensive chemotherapy as well as the
availability of new effective antifungal drugs, its high mortality rate
continues to be a crucial problem. Early diagnosis, followed by rapid
antifungal treatment, remains the cornerstone of successful management.
Catheter removal should be considered on an individual basis. The
widespread use of newer antifungal agents as prophylaxis among patients
with acute leukemia may result in a decreased candidemia incidence.
References
- Sipsas NV, Lewis RE, Tarrand J, et al. Candidemia
in patients with hematologic malignancies in the era of new antifungal
agents (2001-2007): stable incidence but changing epidemiology of a
still frequently lethal infection. Cancer. 2009;115(20):4745-52. https://doi.org/10.1002/cncr.24507 PMid:19634156
- Wu
JQ, Zhu LP, Ou XT, et al. Epidemiology and risk factors for non-Candida
albicans candidemia in non-neutropenic patients at a Chinese teaching
hospital. Med Mycol. 2011;49(5):552-5.
- Li
D, Zhang W, Zheng S, et al. Surveillance study of candidemia in cancer
patients in North China. Med Mycol. 2013;51(4):378-84. https://doi.org/10.3109/13693786.2012.727481 PMid:23046201
- Wu
Z, Liu Y, Feng X, et al. Candidemia: incidence rates, type of species,
and risk factors at a tertiary care academic hospital in China. Int J
Infect Dis. 2014;22:4-8. https://doi.org/10.1016/j.ijid.2013.11.011 PMid:24583564
- De
Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive
fungal disease from the European Organization for Research and
Treatment of Cancer/Invasive Fungal Infections Cooperative Group and
the National Institute of Allergy and Infectious Diseases Mycoses Study
Group (EORTC/MSG) Consensus Group. Clin Infect Dis.
2008;46(12):1813-21. https://doi.org/10.1086/588660 PMid:18462102 PMCid:PMC2671227
- Vigouroux
S, Morin O, Moreau P, et al. Candidemia in patients with hematologic
malignancies: analysis of 7 years' experience in a single center.
Haematologica. 2006;91(5):717-8
- Koehler P, Tacke D, Cornely OA. Our 2014 approach to candidaemia. Mycoses. 2014;57(10):581-3. https://doi.org/10.1111/myc.12207 PMid:24863378
- McCarty TP, Pappas PG. Invasive Candidiasis. Infect Dis Clin North Am. 2016;30(1):103-24. https://doi.org/10.1016/j.idc.2015.10.013 PMid:26739610
- Bassetti
M, Merelli M, Righi E, et al. Epidemiology, species distribution,
antifungal susceptibility, and outcome of candidemia across five sites
in Italy and Spain. J Clin Microbiol. 2013;51(12):4167-72. https://doi.org/10.1128/JCM.01998-13 PMid:24108614 PMCid:PMC3838046
- Gamaletsou
MN, Walsh TJ, Zaoutis T, et al. A prospective, cohort, multicentre
study of candidaemia in hospitalized adult patients with haematological
malignancies. Clin Microbiol Infect. 2014;20(1):O50-7. https://doi.org/10.1111/1469-0691.12312 PMid:23889746
- Sun
M, Chen C, Xiao W, et al. Increase in Candida Parapsilosis Candidemia
in Cancer Patients. Mediterr J Hematol Infect Dis. 2019;11(1):e2019012.
https://doi.org/10.4084/mjhid.2019.012 PMid:30671218 PMCid:PMC6328045
- Walsh
TJ, Merz WG. Pathologic features in the human alimentary tract
associated with invasiveness of Candida tropicalis. Am J Clin Pathol.
1986;85(4):498-502 https://doi.org/10.1093/ajcp/85.4.498 PMid:3953503
- Morrell
M, Fraser VJ, Kollef MH. Delaying the empiric treatment of candida
bloodstream infection until positive blood culture results are
obtained: a potential risk factor for hospital mortality. Antimicrob
Agents Chemother. 2005;49(9):3640-5. https://doi.org/10.1128/AAC.49.9.3640-3645.2005 PMid:16127033 PMCid:PMC1195428
- Pappas
PG, Kauffman CA, Andes D, et al. Clinical practice guidelines for the
management of candidiasis: 2009 update by the Infectious Diseases
Society of America. Clin Infect Dis. 2009;48(5):503-35. https://doi.org/10.1086/596757 PMid:19191635
- Rodriguez
D, Park BJ, Almirante B, et al. Impact of early central venous catheter
removal on outcome in patients with candidaemia. Clin Microbiol Infect.
2007;13(8):788-93. https://doi.org/10.1111/j.1469-0691.2007.01758.x PMid:17610598
- Gokcebay
DG, Yarali N, Isik P, et al. Candida Associated Bloodstream Infections
in Pediatric Hematology Patients: A Single Center Experience. Mediterr
J Hematol Infect Dis. 2016;8(1):e2016018. https://doi.org/10.4084/mjhid.2016.018 PMid:26977277 PMCid:PMC4771141
- Pappas
PG, Kauffman CA, Andes DR, et al. Clinical Practice Guideline for the
Management of Candidiasis: 2016 Update by the Infectious Diseases
Society of America. Clin Infect Dis. 2016;62(4):e1-50. https://doi.org/10.1093/cid/civ1194 PMid:26810419
- Devrim
I, Isguder R, Agin H, et al. Outcome of Candida Parapsilosis Complex
Infections Treated with Caspofungin in Children. Mediterr J Hematol
Infect Dis. 2016;8(1):e2016042. https://doi.org/10.4084/mjhid.2016.042 PMid:27648205 PMCid:PMC5016015


