Ravindra Kumar1, Mendi Prem Shyam Sundar Singh1, Soumendu Mahapatra1, Sonam Chaurasia1, Malay Kumar Tripathi2, John Oommen3, Praveen K Bharti4 and Rajasubramaniam Shanmugam1.
1 Division of Genetic Disorder, ICMR-National Institute of Research in Tribal Health, Jabalpur, Madhya Pradesh.
2 Community Health Center, Lanjigarh, Bishwanathpur, Kalahandi, Odisha.
3 Christian Medical Hospital, Rayagada, Odisha.
4 Division of Vector Borne Diseases, ICMR-National Institute of Research in Tribal Health, Jabalpur, Madhya Pradesh.
Corresponding
author: Dr. Rajasubramaniam Shanmugam. Division of Genetic Disorder,
ICMR-National Institute of Research in Tribal Health, Nagpur Road, P.O.
Garha, Jabalpur, Madhya Pradesh, India 482003. E-mail:
raja.rmrct@gmail.com
Published: March 1, 2020
Received: October 18, 2020
Accepted: February 10, 2020
Mediterr J Hematol Infect Dis 2020, 12(1): e2020015 DOI
10.4084/MJHID.2020.015
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Introduction:
The aim of the study was to enumerate the clinical, hematological, and
molecular spectrum of G6PD deficiency in malaria endemic regions of
south west Odisha.
Methods:
Diagnosis of G6PD deficiency was made by using the Di-chloroindophenol
Dye test in two south west districts (Kalahandi and Rayagada) of Odisha
State. Demographic and clinical history was taken from each individual
using a pre-structured questionnaire. Molecular characterization of
G6PD deficiency was done using PCR-RFLP and Sanger sequencing.
Results:
A total of 1981 individuals were screened; among them, 59 (2.97%)
individuals were G6PD deficient. The analysis revealed that G6PD
deficiency was more among males (4.0%) as compared to females (2.3%).
Prevalence of G6PD deficiency was significantly higher among tribal
populations (4.8%) as compared to non-tribal populations (2.4%)
(p=0.012, OR=2.014, 95%CI=1.206-3.365). Twenty four individuals with
G6PD deficiency had mild to moderate anemia, whereas 26 G6PD deficient
individuals had a history of malaria infection. Among them, 3 (11.5%)
required blood transfusion during treatment. Molecular analysis
revealed G6PD Orissa as the most common (88%) mutation in the studied
cohort. G6PD Kaiping (n=3), G6PD Coimbra (n=2) and G6PD Union (n=1)
were also noted in this cohort.
Conclusion:
The cumulative prevalence of G6PD deficiency in the present study is
below the estimated national prevalence. G6PD deficiency was higher
among tribes as compared to non-tribes. Clinical significance for G6PD
deficiency was noted only in malaria infected individuals. Rare G6PD
Kaiping and G6PD Union variants were also present.
|
Introduction
Glucose
6 Phosphate Dehydrogenase (G6PD) deficiency is the most common
inherited red cell enzymopathy in humans. It is estimated that 4.9% of
the global population has G6PD deficiency accounting for more than 400
million people around the world.[1] It is more
prevalent in the areas where malaria is endemic or has been endemic
especially in Africa, Asia, Europe and the Mediterranean region.[1]
The distribution of G6PD deficiency in India is very heterogeneous and
the prevalence of G6PD deficiency varies 0.3 to 30.7% per cent.[2]
According to an estimate around 390000 children are born with G6PD
deficiency each year in India.[3]
The clinical
spectrum of G6PD deficiency is quite heterogeneous, ranging from mostly
asymptomatic individuals to patients having neonatal jaundice, acute
hemolytic anemia when exposed to exogenous agents (acute infections,
drugs or fava beans) and chronic non-spherocytic hemolytic anemia.[4]
It is now well accepted that G6PD deficiency protects individuals against severe Plasmodium falciparum malaria infection. Malaria parasites require optimum RBC redox status for their survival, replication, and development,[5] which is diminished in G6PD deficient RBCs. Individuals with diminished G6PD enzyme activity, when infected with P. falciparum,
develop less severe symptoms than individuals having regular G6PD
enzyme activity. Ironically to this protective mechanism, G6PD
deficient individual’s RBCs are prone to destruction by hemolytic
agents, as antimalarial, causing significant impediments in malaria
treatment. Primaquine is a frequently used drug in combination therapy
for the treatment of P. falciparum and P. vivax. This drug induces serious hemolytic events in G6PD deficient individuals.[6,7]
As a result, G6PD deficiency imposes an obstacle to the success of both
malaria elimination and the National health program. This warrants the
need to undertake systematic studies on G6PD deficiency in the Indian
population, especially in malaria endemic regions. In India, Odisha
contributes to the highest number of cases and deaths due to malaria.[8] In Odisha, out of the 30 districts, 10 southern districts contributed around 64% of deaths due to malaria.[9]
Although a number of prevalence studies of G6PD deficiency in Odisha
have been carried out, however, in these studies and the methodologies
used were variable.[10-12] In addition, the clinical manifestations and molecular spectrum of these deficient cases were not well documented.
The
present study was thus planned to document the clinical and
hematological manifestations in G6PD deficient individuals in the
malaria endemic south west region of Odisha and to evaluate the
underlying mutation spectrum in G6PD deficient individuals.
Material and Methods
Study area.
This prospective cross-sectional study was conducted in one hospital
setting and 3 community settings from south west villages of Odisha.
Patients attending Biswanathpur (19.1607°N, 84.7727°E) community health
center, Kalahandi, (ii) Residents of Bissamcuttack town (19.5088°N,
83.5044°E), Rayagada (iii) residents of village Paiko-Dakuluguda
(19.5842°N, 83.5394°E), Rayagada and (iv) residents of village
Kachapaju (19.4984°N, 83.6295°E), Rayagada, Odisha (Figure 1) were included.
 |
Figure 1. Map showing Kalahandi and Rayagada districts (study area) in Odisha state, India. |
Sample collection.
After the prior approval, scientific advisory committee and
Institutional ethical committees (IEC registration number:
ECR/734/Inst/MP/2015, project approval ID: 201701), a detailed
demographic and clinical history of all recruited individuals were
taken in predesigned structured proforma. All individuals were briefed
on the purpose of the study and written informed consent was obtained
from adult individuals or from parent/guardian in case of minors prior
to obtaining samples. A total of 1981 individuals were recruited for
the study and 1-2 mL of peripheral blood was drawn in EDTA vial under
aseptic conditions from all the recruited individuals.
Laboratory analysis.
G6PD deficiency status was determined at field site using standard
Dichloroindophenol (DCIP) decolorizing method in all samples. All the
samples were stored at 4°C and transported to the laboratory under cold
chain. G6PD enzyme activity was measured in all deficient patients, as
described earlier.[13]
Complete blood count was
done using an automated cell counter (Sysmex KX-21, Transasia, Japan).
Individuals were grouped according to the severity of anemia on the
basis of age specific cut off values for hemoglobin levels.[14]
Genomic
DNA was extracted from all the G6PD deficient samples by standard
salting out method. Three common mutations (G6PD Orissa, G6PD
Mediterranean, and G6PD Kerala-Kalyan) in G6PD deficient individuals
were identified using the polymerase chain reaction-restriction
fragment length polymorphism (PCR-RFLP) method as described earlier.[10]
In remaining samples, all the 13 exons of the G6PD gene were amplified
using the PCR. PCR product was purified using exonuclease 1 and Shrimp
alkaline phosphatase restriction enzymes and sequenced using the Sanger
sequencing method on ABI PRISM® 3100 Genetic Analyzer (Applied
Biosystem, Foster City, CA, USA).
Statistical analysis.
All data were entered in Microsoft Excel for Windows and analyzed on
IBM SPSS (IBM Corp release 2017; IBM SPSS Statistics for Windows,
Version 25.0, Armonk, NY: IBM Corp). The Kolmogorov-Smirnov test was
used to check the normality of the continuous variables. Data for the
continuous variables were given as the mean ± standard deviation or
median (25-75 percentiles). Categorical or discrete variables were
presented as numbers (%). Fuzzy tool was used to select the age,
gender, and tribe matched controls for comparison from a large cohort
of G6PD healthy individuals (for Table 3).
Fisher’s exact test was used to calculate the significance difference
in the frequency of discrete variables in G6PD deficient and normal
individuals. Depending upon the normality, the Mann Whitney U test or
independent sample student “t” test was applied to see the significant
difference in continuous variables in G6PD deficient and normal
individuals. P <0.05 was considered significant.
Results
A
total of 773 males and 1208 females were enrolled in the study. The
median age of the studied subjects was 23 years (13-35 years). Out of
1981 subjects, 59 (2.97%) were G6PD deficient (Table 1). The prevalence of G6PD deficiency varies from 0.5% to 9.6% in different studied cohorts (Figure 2). Prevalence was significantly higher among males (31/773, 4.0%) as compared to females (28/1180, 2.3%) (p = 0.041).
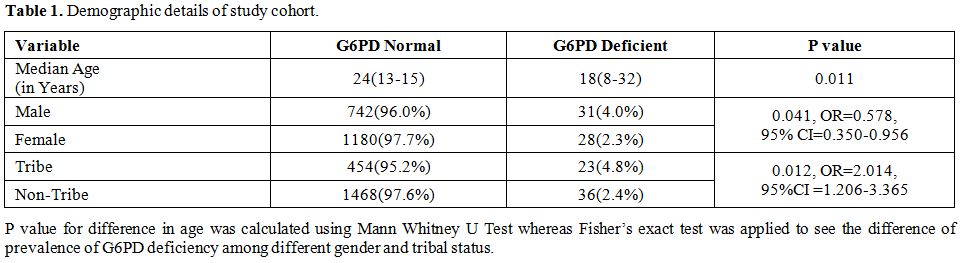 |
Table 1. Demographic details of study cohort. |
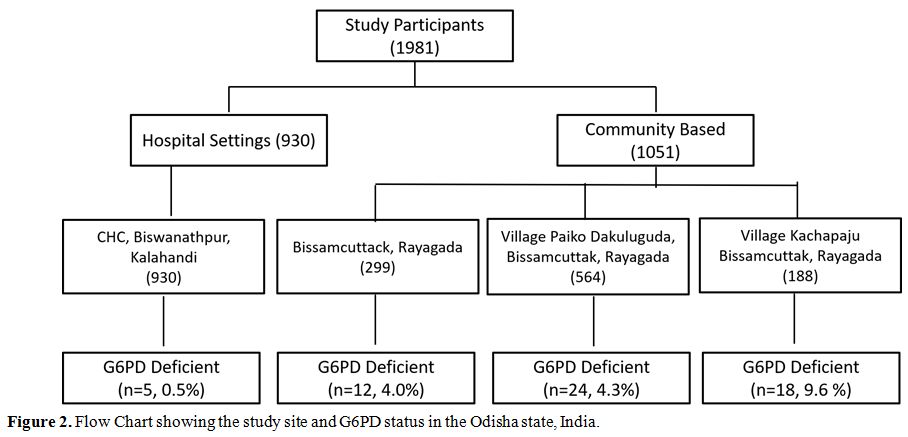 |
Figure 2. Flow Chart showing the study site and G6PD status in the Odisha state, India. |
The
median G6PD enzyme activity was 0.585 IU/g Hb (0.33-2.65 IU/g Hb)
(Normal range = 6.75-11.95 IU/g Hb for adults). Median G6PD enzyme
activity in deficient females was 2.17 IU/g Hb (0.72-3.78 IU/g Hb) and
it was significantly higher than those in deficient males (median 0.37,
0.29-0.55 IU/g Hb) (p<0.0001) (Figure 3).
Four hundred seventy-seven individuals in the study were from tribal
communities (24.1%), mainly Kondh and Dongaria Kondh. G6PD deficiency
was significantly higher in tribes (4.8%) as compared to non-tribes
(2.4%) (p = 0.007, OR=2.066, 95% CI=1.212-3.523).
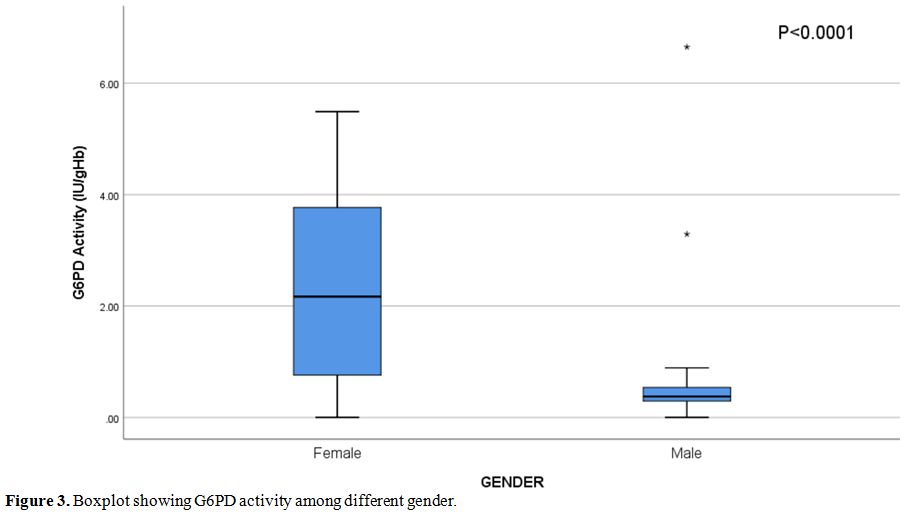 |
Figure 3. Boxplot showing G6PD activity among different gender. |
Hemoglobin
estimation could be performed only in 1538 samples, and it was observed
that 60.7% of the subjects were anemic. Among them, 406 (26.4%) were
mildly anemic, 477 (31.0%) had moderate anemia, whereas 50 (3.3%)
subjects had severe anemia (Table 2).
Severe anemia was higher in individuals selected from hospital settings
as compared to those from community settings (p <0.001). Twenty-four
individuals had both G6PD deficiency and anemia out of which only 3
individuals had a moderate degree of anemia (Hb range 7-9.9 gm/dL).
None of these individuals had severe anemia (Hb<7.0 gm/dL).
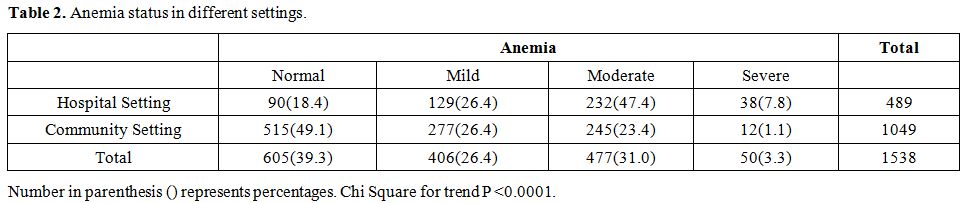 |
Table 2. Anemia status in different settings. |
Communities
in Odisha state are reported to suffer from various hemoglobinopathies.
Therefore, hemoglobin electrophoresis was performed to ascertain
whether the screened individuals also carried any hemoglobin disorder.
We observed the high prevalence of sickle cell disease (1.7% 32/1897)
and trait (18.5%, 350/1897). Beta thalassemia trait was observed in
only 0.9% (18/1897) individuals. No beta thalassemia major was
encountered. Electrophoresis could not be performed on 84 samples due
to low sample volume. Only one individual had both G6PD deficiency and
sickle cell disease and showed severe hemolytic disease. Eight sickle
cell trait individuals also had G6PD deficiency. All these 8
individuals had no history of blood transfusion.
The presence of
malaria was tested using the bivalent rapid diagnostic kit. Two hundred
fifty-four samples were positive for malaria; among them, 238 were
positive for P. falciparum (Pf), 15 for P. vivax
(Pv) infection. One patient had a mixed infection of both Pf and Pv.
Two Pf positive females were also found to be G6PD deficient. No male
individual with G6PD deficiency was found positive for Pf or Pv
infection.
A total of 843 (42.6%) individuals had a prior
history of malaria; however, due to lack of previous medical records,
the type of parasite infection could not be ascertained. Out of these
843 individuals, only 26 [12 male and 14 female] had G6PD deficiency.
No association of malaria with G6PD deficiency was observed (p =
0.792). Out of these 26 G6PD deficient individuals, three developed
severe anemia during malaria treatment and required blood transfusion.
There was a significant association with the need for blood transfusion
during malaria treatment and G6PD deficiency (p=0.026, OR=3.816,
95%CI=1.079-13.496).
None of the G6PD deficient subjects had
splenomegaly or hepatomegaly at the time of screening. Twenty-two per
cent of G6PD deficient individuals had a history of anemia, fever, and
joint pain. Four individuals also had a previous history of blood
transfusion. To compare the association of G6PD deficiency with
clinical symptoms, age, gender, and tribe, matched equal subjects were
randomly selected from people having regular G6PD enzyme activity. Table 3
shows the association of G6PD deficiency with a clinical history or
symptoms and it was observed that there was no significant difference
in the frequency of pallor, fatigue, history of anemia, fever, joint
pains or body pain in G6PD deficient as compared to normal individuals.
Although the G6PD deficient individuals more frequently reported a
prior history of blood transfusion than the not affected subjects, yet
it did not reach statistical significance (p=0.679). Red cell indices
like mean hemoglobin, mean corpuscular volume and mean corpuscular
hemoglobin levels were also similar in both the groups (Table 4).
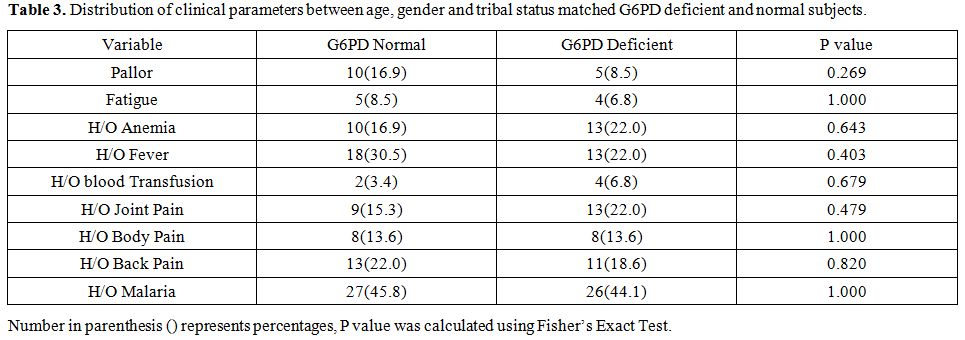 |
Table 3. Distribution of clinical
parameters between age, gender and tribal status matched G6PD deficient
and normal subjects. |
 |
Table 4. Hematological profile of age, gender and tribal status matched G6PD deficient and normal subjects. |
Molecular
characterization of the G6PD gene could not be done in 8 samples due to
low sample quantity. G6PD Orissa (C.131C>G, p.Ala44Gly) (Class III
variant) was found to be the most common mutation and was seen in 45
(88.2%) subjects. Other commonly known mutations like G6PD
Mediterranean (Class II variant), G6PD Kerala Kalyan (Class III) were
not encountered.
On the other hand, G6PD Coimbra (C.592C>T,
p.Arg198Cys)(Class II) was observed in 2 children of the Kondh tribe,
one of whom also had a history of malaria infection. Both children had
no history of blood transfusion or anemia.
Rare G6PD mutant variant G6PD Kaiping (c.1388G>A, p.Arg463His) (Figura 4a)
was observed in 3 individuals. One of them (20 year old male) belonged
to Porja Tribe. Clinical evaluation revealed pallor, prominent lymph
node, and purpuric spot. He also had a prior history of jaundice. G6PD
enzyme activity was 0.31 IU/g Hb. The second individual belonged to
Kondh tribal community (32 years old). He had no history of jaundice or
anemia, and his general health condition was good. The G6PD enzyme
activity was 0.48 IU/g Hb. The third individual was a 55 years old
female non-tribe with the G6PD enzyme activity of 2.34 IU/g Hb. She had
a history of malaria with severe anemia and blood transfusion.
Furthermore, rare variant, G6PD Union (C.1360C>T, p.Arg454Cys) (Figure 4b),
was identified in a 21 year old non-tribal woman in the heterozygous
state. In this case, the G6PD enzyme activity was 3.3 IU/g Hb. This
woman also reported suffering from malaria earlier.
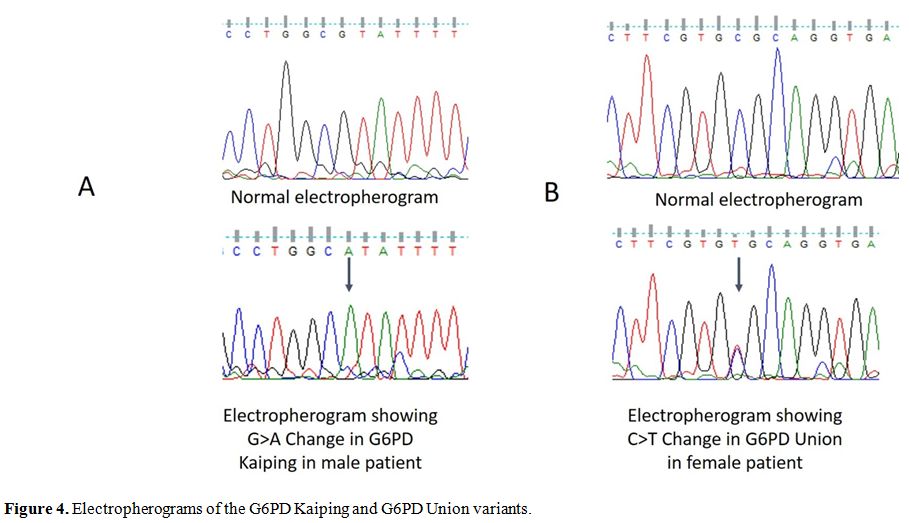 |
Figure 4. Electropherograms of the G6PD Kaiping and G6PD Union variants. |
Discussion
The
Indian population comprises numerous tribal communities, each with
common physical, cultural, and genetic traits. Due to the high degree
of endogamy and consanguineous marriages, hereditary diseases are
common among tribal communities leading to a high degree of morbidity
and mortality. The Odisha state in India is recognized as a malaria
hyper-endemic area and G6PD deficiency is a common inherited red cell
enzyme disorder in this region.[10] The various
tribal communities inhabiting Odisha state have been screened for G6PD
deficiency from time to time and prevalence of G6PD deficiency ranges
from 0.3 to 30.7% in different tribes.[2,10,11,15]
Recently Kumar et al. carried out a meta-analysis and reported an 8.5%
prevalence of G6PD deficiency in the Indian population.[2]
In the present studies, a 2.97% prevalence for G6PD deficiency was
observed. However, the prevalence of G6PD deficiency varies from 0.5%
to 9.6% in different studied cohorts. The prevalence of G6PD deficiency
was higher in the community-based cohort, whereas in the hospital
setting a very low prevalence of G6PD deficiency was observed. This
suggests that for the identification of accurate estimates of
prevalence, community-based screening is the best approach.
Furthermore, the distribution of G6PD deficiency in the community-based
cohort was also heterogeneous. The cline witnessed may be due to
migration and isolation rather than to the effect on selection. The
observed prevalence of G6PD deficiency in tribals was 4.82% and 2.39%
in non-tribal communities.
G6PD deficiency was higher among
males as compared to females, which is in concordance with previous
studies and inheritance patterns. Since G6PD deficiency is X linked
disorder, males can be G6PD deficient (hemizygous) or G6PD normal
genotype, and females can be homozygous or heterozygous for G6PD
mutation. Furthermore, due to random X chromosome inactivation, RBCs in
heterozygous females have a mosaic pattern based on the G6PD allele
expressed. The relative ratio of the two RBC populations governs the
G6PD activity in females. These ratios range from a high proportion of
RBCs with the normal G6PD enzyme to a high proportion of G6PD deficient
RBCs.[16] May et al. reported that only 14% of
heterozygous females have deficient G6PD activity, and 33.3% had
intermediate activity, whereas over 50% of heterozygous females had
normal G6PD enzyme activity.[17]
Individuals
having malaria infection with G6PD deficiency are prone to drug induced
hemolytic anemia. In the present studies, it was observed that the
individuals having G6PD deficiency required blood transfusion during
malaria treatment. However, due to the absence of medical records, we
were unable to ascertain the information about the type of malaria
infection or drug administered. Likely a recent study in Africa,[18]
no clinical association between G6PD deficiency and malaria was
observed, and that further confirms that G6PD deficient subjects remain
asymptomatic unless perturbated by exogenous factors such as
antimalarial drugs.
More than 400 G6PD variants have been
described so far on the basis of their biochemical and functional
characteristic. On the basis of molecular defect alone, 217 unique G6PD
variants have been described to date.[18,19,20]
However, of all recognized G6PD variants, only 10% variants have been
characterized at the structural and functional levels. The spectrum of
G6PD mutations found in India has not been studied in detail. Around 13
mutations have been reported so far from India.[20]
Based on previous reports, G6PD Mediterranean mutation (563C→T) has
been identified as the most prevalent mutation followed by G6PD
Kerala-Kalyan (949G→A) and G6PD Orissa (131C→G). Other mutations such
as G6PD Chatham, G6PD Insuli, G6PD Coimbra, G6PD Nilgiri, G6PD Gond,
G6PD Namoru, G6PD Dindori, G6PD Jammu, G6PD Porbandar, and G6PD Andhra
Pradesh have been reported sporadically.[21-24] It is
interesting to note that none of the individuals tested carried G6PD
Mediterranean or G6PD Kerala-Kalyan mutation suggesting an extensive
heterogeneity in the spectrum of mutation across different populations.
In the present studies, G6PD Kaiping (c.1388G>A, p.Arg463His)
and G6PD Union (C.1360C>T, p.Arg454Cys) mutations were encountered;
these mutations were hitherto not reported from any Indian population.
We noted G6PD Kaiping mutation in 3 unrelated individuals belonging to
3 different ethnicities suggesting a common origin or hotspot for G6PD
mutations. G6PD Kaiping has been classified as Class II variant (G6PD
residual activity less than 10%). G6PD Kaiping is a common G6PD
mutation in China[25] and also reported from other South East Asian countries like Vietnam,[26] Thailand,[27] and Indonesia.[28] Wang et al. in 2008[29]
reported G6PD Kaiping in 2 Malaysians of Indian Origin. It is
interesting to note that the mutations found by us in this endemic
malaria area of India are entirely different from those found in the
endemic malaria area of Burkina-Faso (Africa).[18]
On the other hand, G6PD Union (class II variant) has a worldwide distribution.[30] Rovira et al.[31]
reported that G6PD Union individuals found in Spain are of gypsy
origin. Gypsies belong to a tribal group that originated from the North
West of India that migrated into central and Western Europe. The
presence of this mutation supports the migratory link.
In the
present studies, the clinical history of the individuals was obtained
only through personal interviews, most of them were unable to recall
the name of the antimalarial drugs which they were administered for
malaria due to low literacy, as a consequence we were unable to analyze
the effect of antimalarial drug in G6PD deficient individuals. This
lack of information is a bias or limitation of the study.
We
carried out a systematic analysis involving clinical hematological and
molecular approach for the G6PD deficiency in both hospital and
community settings. The cumulative frequency of G6PD was found to be
low in hospital settings as compared to community settings. G6PD
deficient individuals were at high risk for hemolytic anemia and needed
blood transfusions during malaria treatment. The presence of various
G6PD mutants, in particular, G6PD Kaiping and Union in the study area,
indicates migratory link and genetic drift. However, further
community-based studies need to be carried out to determine the
prevalence, distribution and phenotypic correlation of these variants
in different ethnic groups and in different geographical areas.
Acknowledgment
Authors
would like to thank Health Officials of Kalahandi District, Odisha
State Health Department and Christian Medical Hospital, Bissamcuttack,
Rayagada for providing infrastructure facilities for collecting samples
from Kalahandi and Rayagada District respectively. Authors are also
thankful to Director, ICMR-NIRTH, Jabalpur for providing infrastructure
facility and administrative support to carry out this work. Authors are
also thankful to Ms. Sweta Mishra and Ms Nazia for helping in
sequencing. The manuscript has been approved by the Publication
Screening Committee of ICMR - NIRTH Jabalpur and assigned with the
number ICMR-NIRTH/PSC/16/2018.
References
- Nkhoma ET, Poole C, Vannappagari V, Hall SA,
Beutler E. The global prevalence of glucose-6-phosphate dehydrogenase
deficiency: a systematic review and meta-analysis. Blood Cells Mol
Dis.2009;42:267-78. https://doi.org/10.1016/j.bcmd.2008.12.005 PMid:19233695
- Kumar
P, Yadav U, Rai V. Prevalence of glucose-6-phosphate dehydrogenase
deficiency in India: An updated meta-analysis. Egyptian Journal of
Medical Human Genetics.2016;17:295-302. https://doi.org/10.1016/j.ejmhg.2016.01.004
- Verma
IC, Bijarnia S. The burden of genetic disorders in India and a
framework for community control. Community Genet.2002;5:192-6. https://doi.org/10.1159/000066335 PMid:14960891
- Luzzatto L, Nannelli C, Notaro R. Glucose-6-Phosphate Dehydrogenase Deficiency. Hematol Oncol Clin North Am.2016;30:373-93. https://doi.org/10.1016/j.hoc.2015.11.006 PMid:27040960
- Mbanefo
EC, Ahmed AM, Titouna A, Elmaraezy A, Trang NT, Phuoc Long N, Hoang Anh
N, Diem Nghi T, The Hung B, Van Hieu M, Ky Anh N, Huy NT, Hirayama K.
Association of glucose-6-phosphate dehydrogenase deficiency and
malaria: a systematic review and meta-analysis. Sci Rep.2017;7:45963. https://doi.org/10.1038/srep45963 PMid:28382932 PMCid:PMC5382680
- Watson J, Taylor WR, Menard D, Kheng S, White NJ. Modelling primaquine-induced haemolysis in G6PD deficiency. Elife.2017;6. https://doi.org/10.7554/eLife.23061 PMid:28155819 PMCid:PMC5330681
- Fanello
CI, Karema C, Avellino P, Bancone G, Uwimana A, Lee SJ, d'Alessandro U,
Modiano D. High risk of severe anaemia after
chlorproguanil-dapsone+artesunate antimalarial treatment in patients
with G6PD (A-) deficiency. PLoS One.2008;3:e4031. https://doi.org/10.1371/journal.pone.0004031 PMid:19112496 PMCid:PMC2603295
- National Vector Born Disease Control Program. Malaria Situation in India. 2017.
- Sahu
SS, Gunasekaran K, Raju HK, Vanamail P, Pradhan MM, Jambulingam P.
Response of malaria vectors to conventional insecticides in the
southern districts of Odisha State, India. Indian J Med
Res.2014;139:294-300.
- Kaeda JS, Chhotray
GP, Ranjit MR, Bautista JM, Reddy PH, Stevens D, Naidu JM, Britt RP,
Vulliamy TJ, Luzzatto L. A new glucose-6-phosphate dehydrogenase
variant, G6PD Orissa (44 Ala-->Gly), is the major polymorphic
variant in tribal populations in India. Am J Hum Genet.1995;57:1335-41.
- Balgir
RS. Genetic diversity of hemoglobinopathies, G6PD deficiency, and ABO
and Rhesus blood groups in two isolates of a primitive Kharia Tribe in
Sundargarh District of Northwestern Orissa, India. J Community
Genet.2010;1:117-23. https://doi.org/10.1007/s12687-010-0016-y PMid:22460244 PMCid:PMC3185996
- Balgir
RS, Dash BP, Murmu B. Blood Groups, Hemoglobinopathy and G-6-PD
Deficiency Investigations Among Fifteen Major Scheduled Tribes of
Orissa, India. The Anthropologist.2004;6:69-75. https://doi.org/10.1080/09720073.2004.11890830
- Beutler
E, Blume KG, Kaplan JC, Löhr GW, Ramot B, Valentine WN. International
Committee for Standardization in Haematology: recommended methods for
red-cell enzyme analysis. Br J Haematol.1977;35:331-40. https://doi.org/10.1111/j.1365-2141.1977.tb00589.x PMid:857853
- WHO. Last accessed date: 2019 12 May 2019. Available from: https://apps.who.int/iris/bitstream/handle/10665/85839/WHO_NMH_NHD_MNM_11.1_eng.pdf?ua=1.
- Mukherjee
MB, Colah RB, Martin S, Ghosh K. Glucose-6-phosphate dehydrogenase
(G6PD) deficiency among tribal populations of India - Country scenario.
Indian J Med Res.2015;141:516-20.
- Domingo
GJ, Advani N, Satyagraha AW, Sibley CH, Rowley E, Kalnoky M, Cohen J,
Parker M, Kelley M. Addressing the gender-knowledge gap in
glucose-6-phosphate dehydrogenase deficiency: challenges and
opportunities. Int Health.2019;11:7-14. https://doi.org/10.1093/inthealth/ihy060 PMid:30184203 PMCid:PMC6314154
- May
J, Meyer CG, Grossterlinden L, Ademowo OG, Mockenhaupt FP, Olumese PE,
Falusi AG, Luzzatto L, Bienzle U. Red cell glucose-6-phosphate
dehydrogenase status and pyruvate kinase activity in a Nigerian
population. Trop Med Int Health.2000;5:119-23. https://doi.org/10.1046/j.1365-3156.2000.00529.x PMid:10747271
- Ouattara,
A. K., Yameogo, P., Diarra, B., Obiri-Yeboah, D., Yonli, A. T.,
Compaore, T. R., Soubeiga, S. T., Djigma, F. W., & Simpore, J.
(2016). Molecular heterogeneity of Glucose-6-Phosphate Dehydrogenase
deficiency in Burkina Faso: G-6-Pd Betica Selma and Santamaria in
People with symptomatic malaria in Ouagadougou. Mediterr. J Hematol
Infect Dis, 8, e2016029. https://doi.org/10.4084/mjhid.2016.029 PMid:27413522 PMCid:PMC4928536
- Minucci
A, Moradkhani K, Hwang MJ, Zuppi C, Giardina B, Capoluongo E.
Glucose-6-phosphate dehydrogenase (G6PD) mutations database: review of
the "old" and update of the new mutations. Blood Cells Mol
Dis.2012;48:154-65. https://doi.org/10.1016/j.bcmd.2012.01.001 PMid:22293322
- Gómez-Manzo
S, Marcial-Quino J, Vanoye-Carlo A, Serrano-Posada H, Ortega-Cuellar D,
González-Valdez A, Castillo-Rodríguez RA, Hernández-Ochoa B,
Sierra-Palacios E, Rodríguez-Bustamante E, Arreguin-Espinosa R.
Glucose-6-Phosphate Dehydrogenase: Update and Analysis of New Mutations
around the World. Int J Mol Sci.2016;17. https://doi.org/10.3390/ijms17122069 PMid:27941691 PMCid:PMC5187869
- Devendra
R, Shanmugam R, Singh M, Vishwakarma CP, Godbhole S, Singh N, Gupta V,
Kedar P, Mukherjee MB. Identification of a novel S184F mutation causing
glucose-6-phosphate-dehydrogenase deficiency in a tribal family of
Madhya Pradesh, Central India. Meta Gene.2017;12:4. https://doi.org/10.1016/j.mgene.2017.03.001
- Chalvam
R, Colah RB, Mohanty D, Ghosh K, Mukherjee MB. Molecular heterogeneity
of glucose-6-phosphate dehydrogenase deficiency among the tribals in
Western India. Blood Cells Mol Dis.2009;43:156-7. https://doi.org/10.1016/j.bcmd.2009.05.002 PMid:19559632
- Nishank
SS, Chhotray GP, Kar SK, Ranjit MR. Molecular variants of G6PD
deficiency among certain tribal communities of Orissa, India. Ann Hum
Biol.2008;35:355-61. https://doi.org/10.1080/03014460801961289 PMid:18568599
- Sarkar
S, Biswas NK, Dey B, Mukhopadhyay D, Majumder PP. A large, systematic
molecular-genetic study of G6PD in Indian populations identifies a new
non-synonymous variant and supports recent positive selection. Infect
Genet Evol.2010;10:1228-36. https://doi.org/10.1016/j.meegid.2010.08.003 PMid:20713184
- Jiang
W, Yu G, Liu P, Geng Q, Chen L, Lin Q, Ren X, Ye W, He Y, Guo Y, Duan
S, Wen J, Li H, Qi Y, Jiang C, Zheng Y, Liu C, Si E, Zhang Q, Tian Q,
Du C. Structure and function of glucose-6-phosphate
dehydrogenase-deficient variants in Chinese population. Hum
Genet.2006;119:463-78. https://doi.org/10.1007/s00439-005-0126-5 PMid:16607506
- Matsuoka
H, Thuan DT, van Thien H, Kanbe T, Jalloh A, Hirai M, Arai M, Dung NT,
Kawamoto F. Seven different glucose-6-phosphate dehydrogenase variants
including a new variant distributed in Lam Dong Province in southern
Vietnam. Acta Med Okayama.2007;61:213-9.
- Nuchprayoon
I, Sanpavat S, Nuchprayoon S. Glucose-6-phosphate dehydrogenase (G6PD)
mutations in Thailand: G6PD Viangchan (871G>A) is the most common
deficiency variant in the Thai population. Hum Mutat.2002;19:185. https://doi.org/10.1002/humu.9010 PMid:11793482
- Kawamoto
F, Matsuoka H, Kanbe T, Tantular IS, Pusarawati S, Kerong HI, Damianus
W, Mere D, Dachlan YP. Further investigations of glucose-6-phosphate
dehydrogenase variants in Flores Island, eastern Indonesia. J Hum
Genet.2006;51:952-7. https://doi.org/10.1007/s10038-006-0044-y PMid:16927025
- Wang
J, Luo E, Hirai M, Arai M, Abdul-Manan E, Mohamed-Isa Z, Hidayah N,
Matsuoka H. Nine different glucose-6-phosphate dehydrogenase (G6PD)
variants in a Malaysian population with Malay, Chinese, Indian and
Orang Asli (aboriginal Malaysian) backgrounds. Acta Med
Okayama.2008;62:327-32.
- Luzzatto L, Notaro R. Malaria. Protecting against bad air. Science.2001;293:442-3. https://doi.org/10.1126/science.1063292 PMid:11463901
- Rovira
A, Vulliamy TJ, Pujades A, Luzzatto L, Corrons JL. The
glucose-6-phosphate dehydrogenase (G6PD) deficient variant G6PD Union
(454 Arg-->Cys) has a worldwide distribution possibly due to
recurrent mutation. Hum Mol Genet.1994;3:833-5. https://doi.org/10.1093/hmg/3.5.833 PMid:8081374
[TOP]







