Mahmoud H.K.1, Fathy G.M.2, Elhaddad A.1, Fahmy O.A.3, Abdel-Mooti M.1, Abdelfattah R.1 and Bokhary M.2.
1 National Cancer Institute, Hematology and Bone Marrow Transplantation unit.
2 Nasser Institute Hospital for research and treatment, Hematology and Bone Marrow Transplantation unit.
3 Kasr Alainy, faculty of medicine, Cairo University, Hematology and Bone Marrow Transplantation unit.
Published: May 1, 2020
Received: January 11, 2020
Accepted: April 2, 2020
Mediterr J Hematol Infect Dis 2020, 12(1): e2020023 DOI
10.4084/MJHID.2020.023
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Hematopoietic
stem cell transplantation (HSCT) is now an established treatment
modality with definitive indications for many hematological disorders.
However, HSCT requires tremendous resources, and it is increasingly
challenging for transplantation experts to practice in the developing
world and to reach a compromise between requirements and available
resources. Based on 30 years of experience and 4256 transplants (60%
allogeneic and 40% autologous), this article focuses on the challenges
our HSCT program encountered since it started in 1989 and what
opportunities we see to solve them. Since 1997, HSCT procedures
increased dramatically with the opening of 15 HSCT units distributed
all over Egypt.
|
Introduction
Hematopoietic stem cell transplantation (HSCT) is currently considered the standard of care for many hematological disorders.[1]
However, this treatment modality requires tremendous resources.
Performing HSCT procedures in developing countries (where many patients
have low socioeconomic standards) usually encounters financial,
ethical, technological, administrative, and medico-legal challenges.
There is a constant need to reach a compromise between requirements and
available resources.[2]
Thalassemia and sickle
cell disease/anemia constitute the most common inherited recessive
disorders associated with consanguinity, which is a common phenomenon
in Egypt.[3] Based on 30 years of experience and 4256 transplants, as shown in table 1,
this article is an update of our previously reported results published
in 2008 and focuses on the challenges and opportunities that
continuously face our HSCT program and how we try to solve them.[4]
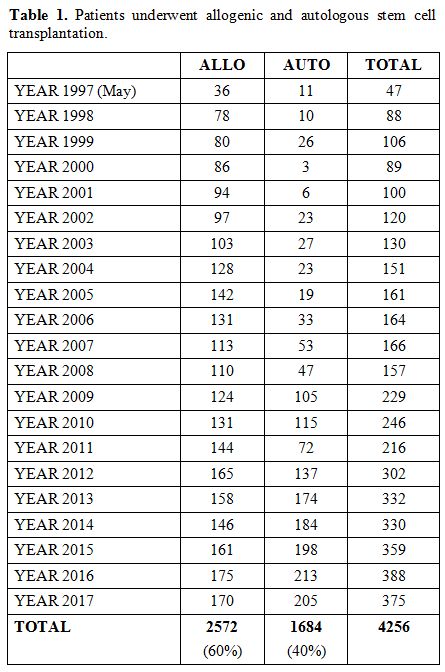 |
Table
1. Patients underwent allogenic and autologous stem cell transplantation. |
Challenges facing hematopoietic stem cell transplantation in Egypt
A. Offering transplant to every indicated patient
B. Sources of stem cells
C. Availability of donors
D. Socio-economic challenges
E. Hepatitis
F. Genetic diseases
G. GVHD management
H. Minimal residual disease (MRD)
I. New drugs
A. Offering transplant to every indicated patient.
The population of Egypt in 2020 exceeded 100 million.
There
are fifteen transplant centers, and the transplant rate/million is 8.4,
which is considerably higher than the number we reported previously in
2008, where the transplant rate/million was 2.8.[4] We are still far away from western standards, where transplant rates are between 36-40 /million.[5]
B. Stem Cell Sources
Stem
cells obtained by bone marrow harvesting were the only source until the
late-80s when peripheral blood stem cells (PBSCs) collection became
available at our centers. We were one of the first teams who almost
entirely changed the source of stem cells from BMSCs to PBSCs. In a
paper published by our group in 1999 comparing PBSCT to BMSCs, PBSCT
was found to be associated with faster hematopoietic recovery, and the
incidence of aGVHD did not exceed that seen with BMSCs.[6]
This change in stem cell source dramatically improved the motivation of
donors by avoiding hospital stays and painful collection procedures.
C. Availability of donors
Approximately
25–30% of patients who have siblings are expected to have an HLA
identical donor. This figure approximates 40% among the Egyptian
population owing to the larger size of the families. The probability of
finding a matched donor depends on several factors, among which are the
panel size, frequency of a specific HLA type in the population, and
ethnic groups of both the donor and the recipient. Less than 3% of
donors listed in the international registries are of oriental origin,
which further complicates the process of finding matched donors for our
patients.[7] Egypt does not possess a local donor
registry. This obstacle has been mitigated with the initiation of
haploidentical transplants at our centers.[8] Since June 2015, our team has performed 43 haploidentical transplants, as shown in table 2.
Stem cell sources were either PBSCs or BMSCs. PTCy prophylaxis,
together with cyclosporine, was administered to all patients in
addition to mycophenolate mofetil in selected cases.[9,10]
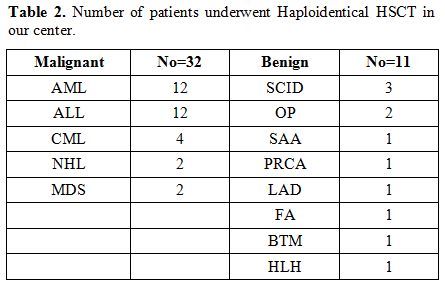 |
Table 2. Number of patients underwent Haploidentical HSCT in our center. |
D. Socio-economic challenges
Healthcare systems in Egypt:
The Healthcare system in Egypt consists of both public parastatal and
private sectors. Public health coverage is provided through the
Ministry of Health, which operates a series of free health care
facilities. There are two major parastatal organizations, the Health
Insurance Organization (HIO), and the Curative Care Organization (CCO).
Health Insurance Organization covers disabled individuals, graduates,
employees, and widows. Curative Care Organization works in different
governorates, and contracts for care delivery with other organizations.
Private insurance plans are also available, as well as a network of
private health care services and health facilities.
Financial Constraints:
The cost of sophisticated molecular techniques and newer drugs is
sometimes limiting. However, developing countries must have the
expertise to offer “state of the art” treatment strategies, including
HSCT. Such an approach will provide a potentially curative treatment
locally at a much lower cost than in western countries. A stem cell
transplantation (SCT) in Egypt cost ranges between 11,000 to 17,000 US
dollars depending on the type of transplant (autologous, allogeneic, or
haploidentical). The cost of SCT procedures in our centers is no more
than 10% of the cost in western countries.[11] The
post-transplant follow-up period further increases the socio-economic
burden on our patients. That is because strict hygienic conditions at
home are paramount, and a considerable proportion of patients find it
challenging to comply with hygiene recommendations, even if the
treatment is provided for free.[12] Moreover, the
follow-up dropout rate is relatively high among Egyptian patients
(10%), and this is because most transplant units are located in the
capital and major cities, while the vast majority of patients reside
far away.
E. Hepatitis
In Egypt, the prevalence of Hepatitis B virus (HBV) infection among adults aged 15-59 years is 1.4%.[13]
More seriously, 15% of the population is seropositive for Hepatitis C
virus (HCV). The incidence rate of HCV is 2.4 per 1000 person-year. Ten
percent of our HCV patients are chronically infected, and 90% of them
harbor genotype 4 of the virus.[14,15] HBV infection
or reactivation in patients undergoing chemotherapy or HSCT may
progress to hepatic failure, while this is much less in HCV infection.[16]
Antiviral prophylaxis is beneficial to HBsAg and anti-HBc positive
patients since the incidence of HBV reactivation in patients not
receiving antiviral prophylaxis has been reported to be 4.1%.[17,18]
Lamivudine
or third-generation antivirals (Entecavir or Tenofovir) are the most
commonly used for HBV suppression. Entecavir and tenofovir are
preferred over lamivudine due to the possibility of lamivudine
resistance. Prophylaxis is started at least one week prior to or in
concordance with the conditioning regimen of HSCT, and suppression
continues to 12-24 months after the transplantation. The inability to
detect HBV DNA and HBsAg negativity in addition to the appearance of
anti-HBs antibodies is an indicator of HBV resolution and allows for
discontinuation of antiviral therapy safely.[19]
Vaccination against HBV should be offered to patients undergoing auto- or allo-HSCT before starting the conditioning regimen.[17]
Currently,
it is recommended to treat hepatitis C virus infection prior to HSCT.
However, the treatment of HCV concurrently with HSCT may be a better
alternative for selected patients when it is not safe to delay
transplant.[20] The new direct-acting antivirals
(DAAs) for suppression and treatment of active HCV infection are
currently available in Egypt with acceptable prices and are covered by
third-party payers.
In 2004, our team demonstrated that the high
prevalence of HCV and HBV among our patients is strongly associated
with hepatic GVHD and SOS. Hence, early antiviral therapy was advised
in an attempt to delay and ameliorate liver disease progression.[21]
F. Genetic diseases
Thalassemia:
Thalassemia is the most common hereditary hemoglobinopathy in Egypt.
There are 10,000 registered cases in addition to more than 20,000
non-registered cases, 95% of whom are beta-thalassemia major (BTM). The
carrier state is between 9 and 11%.[22] Challenges
with thalassemia are the lack of prenatal diagnosis, inadequate
chelation therapy before transplant, and siblings are frequently
affected by the disease. Consequently, patients present with high
Pesaro risk scores, high prevalence of (HCV and HBV) (~75%), and the
referral to HSCT clinics is usually delayed. As a sequel to the factors
above, delayed engraftment frequently occurs.[21] Our team performed 201 cases of BTM, and after a median follow-up period of 12 years, the OS was 82.4% (Figure 1).
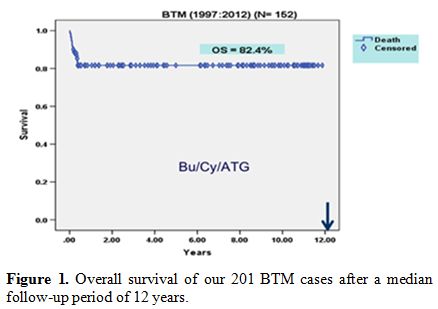 |
Figure 1. Overall survival of our 201 BTM cases after a median follow-up period of 12 years. |
Fanconi Anemia (FA):
There is a strong association of parental consanguinity with Fanconi
anemia. As a result, siblings are frequently affected by the disease.[23]
As in other types of hereditary anemia, diagnosis of FA and detecting
its associated mutations is usually late, and patients are referred to
HSCT at older ages. FA is the third indication of allogeneic-HSCT for
non-malignant hematological disorders in Egypt after hemoglobinopathies
and idiopathic aplastic anemia. Our team performed 63 transplants for
FA, and the overall survival of our patients was 64.5% (Figure 2) after six years of follow up.[24,25]
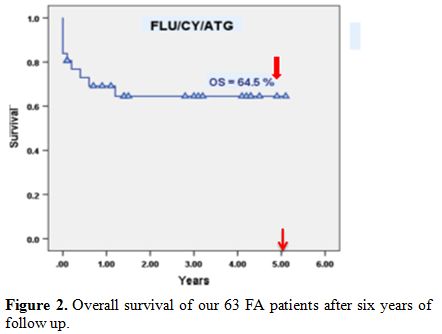 |
Figure 2. Overall survival of our 63 FA patients after six years of follow up. |
G. Challenges facing GVHD management in Egypt
Corticosteroids
with or without a calcineurin inhibitor (CI) is the first line of
treatment of acute and chronic GVHD. Less than half of patients respond
to corticosteroids depending on the severity of the disease.[26,27]
Different therapeutic options for steroid-refractory acute GVHD are
available, including rabbit/equine antithymocyte globulin (ATG),
alemtuzumab (Campath), interleukin-2 receptor antibodies as daclizumab
and basiliximab, anti-TNFα drugs (such as infliximab), and
extracorporeal photopheresis (ECP). At our centers, we started to use
novel drugs for the management of cGVHD, including bortezomib,
ruxolitinib, and ibrutinib.[28] However, many other
drugs with reported efficacy in this setting are not readily available
in Egypt, and the main challenge to the use of such novel approaches is
the price, which is usually beyond the capability of many patients.
Additionally, it is not yet covered by third-party payers. We started
implementing PTCy as a graft-versus-host disease prophylaxis in
HLA-matched HSCT in 52 cases. Pre-transplant conditioning regimens used
were either FLU/BU (160 mg/m2 of Fludarabine, and 16 mg/kg oral Busulfan both of which were divided over four days), or FLU/CY (120 mg/m2 FLU divided over four days, and 25 mg/kg/d CY for four days).[29]
Cyclophosphamide was administered at a dose of 50 mg/kg per day on days
3 and 4 post-transplantation, and cyclosporine was started on day 5.
The cumulative 1-year incidence of cGVHD was 13.4%. Incidence of aGVHD
grades I-II and III-IV were 3.8% and 11.5%, respectively. Overall
survival (OS) for the total number of cases at one year was 73.1%,
including both benign and malignant diseases. Disease-free survival
(DFS) was 69.5%, as depicted in Figure 3.
Considering our AML cases separately (29 cases), it is noteworthy that
the relapse rate was not passively affected, as the OS was 70%, and the
DFS was 66.7%, as shown in Figure 4. Our results support the use of PTCy for HLA-matched sibling donor PBSCT due to the significant reduction in the cGVHD rate.[30]
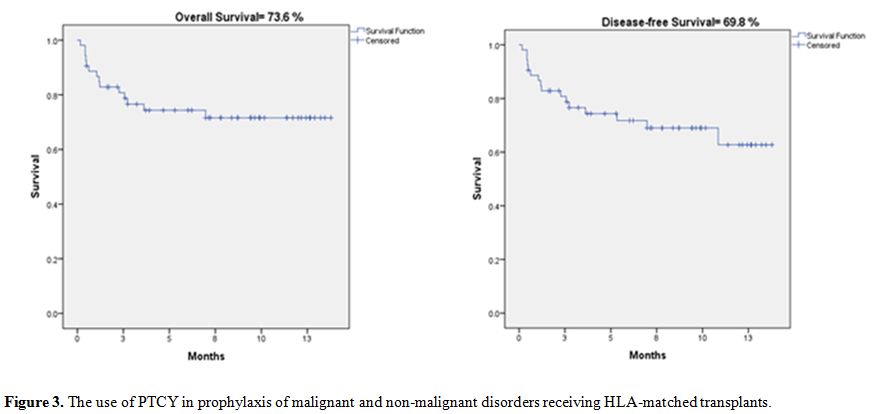 |
Figure 3.
The use of PTCY in prophylaxis of malignant and non-malignant disorders receiving HLA-matched transplants. |
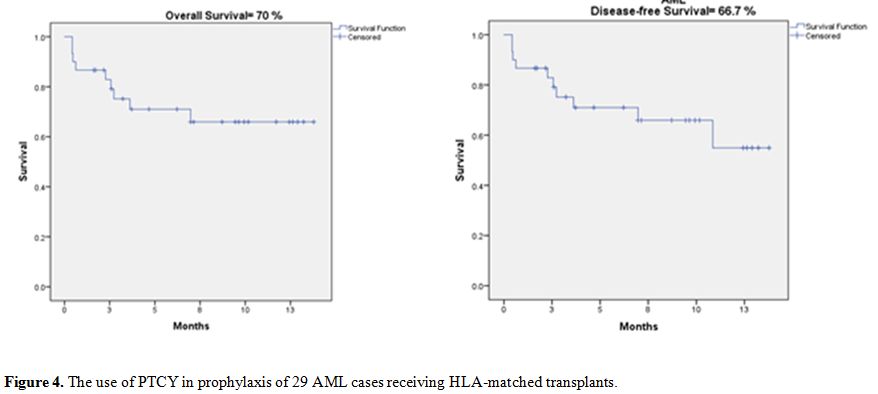 |
Figure 4. The use of PTCY in prophylaxis of 29 AML cases receiving HLA-matched transplants. |
H. Minimal residual disease (MRD)
Evaluation
of morphologic remission only is not sufficient for risk stratification
of a disease and cannot be relied upon for the determination of the
risk of relapse.[31] The detection of minimal
residual disease (MRD) after conventional chemotherapy is currently the
most important tool in predicting the outcome and prognosis of patients
with multiple hematological malignancies.[31,32] In
Egypt, MRD is evaluated almost exclusively for acute lymphoblastic
leukemia during and after induction therapy. The most common techniques
utilized to detect MRD are the multicolor flow cytometry and
quantitative polymerase chain reaction (PCR). Next-Generation
Sequencing (NGS) is expected to improve risk stratification further
using the MRD concept.[33] In our country, the MRD
mentioned above tools are available. However, their cost is the main
challenge facing their routine use. Multi-parametric flow and PCR are
the most utilized tools with an acceptable cost, while NGS is still
rarely ordered due to its high cost and non-availability in many of our
centers.
I. New drugs
Antifungals:
The evolution of various diagnostic and therapeutic alternatives for
invasive fungal infections led to better control of such a problem.
However, in our country, the implementation of such strategies (either
diagnostic or therapeutic) is limited due to financial and logistic
issues. Posaconazole is recommended for antifungal prophylaxis in HSCT
patients suffering GVHD,[34] but due to its high cost, we usually use voriconazole. Our primary antifungal prophylaxis is still fluconazole.[35]
Diagnostic
tools with considerable sensitivity and specificity for most of the
commonly known invasive fungal infections are now available (Antigen
detection, Beta-d-glucan, Galactomannan, and PCR). Such tools may
potentially help to improve the prognosis of invasive fungal infections
through earlier detection and commencing treatment early. However, the
implementation of these new rapid diagnostic tests may be hindered by
cost and infrastructure problems.
Eculizumab:
Transplant- related thrombotic thrombocytopenic purpura (TTP) and
hemolytic uremic syndrome (HUS) are associated with transplant-related
mortality in allogeneic HSCT patients. Previously, the majority of
patients developed end-stage renal disease with low chances of
survival. Eculizumab, a recombinant humanized monoclonal antibody
against the complement protein C5, was found to improve the outcome of
this condition, mainly if complement activation is the cause.[36]
Unfortunately, Eculizumab is not yet available in Egypt. Supportive
measures are the only interventions available for the treatment of TTP
and HUS in our centers.
Defibrotide: Sinusoidal Obstruction Syndrome (SOS) is a life-threatening complication of HSCT.[37]
Mortality in patients with SOS multi-organ dysfunction may exceed 80%
even with supportive measures. Defibrotide, a polydisperse
oligonucleotide with local antithrombotic, anti-ischemic, and
anti-inflammatory activity when administered to patients with clinical
SOS, is associated with improved survival rates of 41% in patients with
multi-organ failure and 70% in patients without multi-organ failure.[38]
The mean incidence of SOS in our patients with autologous and
allogeneic HSCTs is 7% and 10%, respectively. Unfortunately,
Defibrotide is not available in Egypt. Supportive measures (including
fluid restriction, plasma expanders, and diuretics) are the only
interventions available for the treatment of SOS in our centers.
Antivirals for CMV:
Cytomegalovirus (CMV) viremia or disease is one of the most common
complications of allogeneic HSCT. The first line of treatment for CMV
viremia is gancyclovir or valganciclovir,[39,40] and
both agents are available in Egypt. Their most commonly reported
adverse event is myelosuppression, and in some cases, resistance may
occur.[41] Second-line drugs such as foscarnet or
cidofovir, as well as the newer antivirals, including terminase
inhibitors (letermovir) and direct kinase inhibitors (maribavir), are
not yet available in our country.[42] The use of CMV-specific T-cell therapy is also not available.
Immunization post-HSCT
Assuring
that the patients’ vaccination status is up to date per vaccination
schedule is a significant challenge in our country, mainly due to cost
and difficulties in counseling patients regarding the schedules,
administration, adverse effects, and periodically monitoring titer
levels. Post-HSCT, patients’ humoral immunity is impaired, and a
considerable decline in titers of vaccine-preventable diseases takes
place.[43]
In our centers, it is standard
practice to revaccinate patients after transplantation, usually
starting after one year so that humoral immune reconstitution has fully
taken place..
Anti-infectious Prophylaxis
The
increasing incidence of multidrug-resistant gram-negative bacteria
(MDRGN bacteria) is one of the most significant challenges we currently
face in our centers. Antimicrobial options are becoming scarce, and
success rates of eradicating infections are decreasing steadily over
time.[44] In response to the heightened incidence of
MDRGN isolates, our group started adopting institution-specific
strategies based on local susceptibility data (biannual antibiogram).
Implementing institution-specific strategies has improved response
rates to MDRGN isolates and considerably decreased number of days on
broad-spectrum antimicrobials and consequently opportunistic fungal
infections, adverse events, drug interactions, and selection
pressure.
Pneumocystis Jirovecii Pneumonia (PJP)
Diagnosing
PJP in our centers is complicated and highly based on clinical
experience and imaging studies (chest CT scans). The definitive way to
diagnose PJP is with sputum PCR. Other methods include sputum direct
fluorescent antibody (DFA) and serum Beta-D-Glucan.[45]
The tests mentioned above are not available in our country.
Cotrimoxazole is used for PJP prophylaxis and treatment in our centers..
Conclusions
HSCT
centers in Egypt face many challenges not only financial but also
social and technical ones. There is an urgent need to improve
techniques of genetic testing, new anti-GvHD medications as well as the
availability of newer antimicrobial, antifungal, and antiviral
drugs.
References
- Juric, M.K., et al., Milestones of Hematopoietic
Stem Cell Transplantation - From First Human Studies to Current
Developments. Front Immunol, 2016. 7: p. 470. https://doi.org/10.3389/fimmu.2016.00470 PMCid:PMC5101209
- Aljurf,
M., et al., Worldwide Network for Blood and Marrow Transplantation
Recommendations for Establishing a Hematopoietic Stem Cell
Transplantation Program in Countries with Limited Resources, Part II:
Clinical, Technical, and Socioeconomic Considerations. Biol Blood
Marrow Transplant, 2019. https://doi.org/10.1016/j.bbmt.2019.04.012 PMid:31002990
- Anwar,
W.A., M. Khyatti, and K. Hemminki, Consanguinity and genetic diseases
in North Africa and immigrants to Europe. Eur J Public Health, 2014. 24
Suppl 1: p. 57-63. https://doi.org/10.1093/eurpub/cku104 PMid:25107999
- Mahmoud, H., et al., Hematopoietic stem cell transplantation in Egypt. Bone Marrow Transplant, 2008. 42 Suppl 1: p. S76-S80. https://doi.org/10.1038/bmt.2008.136 PMid:18724311
- Passweg,
J.R., et al., Hematopoietic stem cell transplantation in Europe 2014:
more than 40 000 transplants annually. Bone Marrow Transplant, 2016.
51(6): p. 786-92. https://doi.org/10.1038/bmt.2016.20 PMid:26901709 PMCid:PMC4895175
- Mahmoud,
H., et al., Peripheral blood vs bone marrow as a source for allogeneic
hematopoietic stem cell transplantation. Bone Marrow Transplant, 1999.
24(4): p. 355-8. https://doi.org/10.1038/sj.bmt.1701906 PMid:10467322
- Besse,
K., et al., On Modeling Human Leukocyte Antigen-Identical Sibling Match
Probability for Allogeneic Hematopoietic Cell Transplantation:
Estimating the Need for an Unrelated Donor Source. Biol Blood Marrow
Transplant, 2016. 22(3): p. 410-7. https://doi.org/10.1016/j.bbmt.2015.09.012 PMid:26403513
- Maher,
M., A. Elhaddad, M. Samra, R. Abdelfatah, M. Elgammal, and H. Kamel,
Egyptian Experience in Haploidentical Hematopoietic Stem Cell
Transplantation. Clin Lymph Myel Leuk, 2018. 18(Supplement 1): p. pp.
S304-S305: SCT-024, 201814. https://doi.org/10.1016/j.clml.2018.07.262
- Sugita,
J., HLA-haploidentical transplantation with post-transplant
cyclophosphamide. Rinsho Ketsueki, 2017. 58(10): p. 2124-2134.
- Moiseev,
I.S., et al., Risk-adapted GVHD prophylaxis with post-transplantation
cyclophosphamide in adults after related, unrelated, and haploidentical
transplantations. Eur J Haematol, 2018. 100(5): p. 395-402. https://doi.org/10.1111/ejh.13030 PMid:29360184
- Lin,
Y.F., et al., The costs and cost-effectiveness of allogeneic peripheral
blood stem cell transplantation versus bone marrow transplantation in
pediatric patients with acute leukemia. Biol Blood Marrow Transplant,
2010. 16(9): p. 1272-81. https://doi.org/10.1016/j.bbmt.2010.03.016 PMid:20348004 PMCid:PMC2919628
- Hashmi,
S.K., et al., Cost and quality issues in establishing hematopoietic
cell transplant program in developing countries. Hematol Oncol Stem
Cell Ther, 2017. 10(4): p. 167-172. https://doi.org/10.1016/j.hemonc.2017.05.017 PMid:28732192
- Ismail,
S.A., D.F. Cuadros, and L. Benova, Hepatitis B in Egypt: A
cross-sectional analysis of prevalence and risk factors for active
infection from a nationwide survey. Liver Int, 2017. 37(12): p.
1814-1822. https://doi.org/10.1111/liv.13469 PMid:28481452
- Miller,
F.D. and L.J. Abu-Raddad, Evidence of intense ongoing endemic
transmission of hepatitis C virus in Egypt. Proc Natl Acad Sci U S A,
2010. 107(33): p. 14757-62. https://doi.org/10.1073/pnas.1008877107 PMid:20696911 PMCid:PMC2930444
- El-Akel,
W., et al., National treatment programme of hepatitis C in Egypt:
Hepatitis C virus model of care. J Viral Hepat, 2017. 24(4): p.
262-267. https://doi.org/10.1111/jvh.12668 PMid:28145032
- Torres,
H.A. and M. Davila, Reactivation of hepatitis B virus and hepatitis C
virus in patients with cancer. Nat Rev Clin Oncol, 2012. 9(3): p.
156-66. https://doi.org/10.1038/nrclinonc.2012.1 PMid:22271089
- Gentile,
G., et al., Screening, monitoring, prevention, prophylaxis and therapy
for hepatitis B virus reactivation in patients with haematologic
malignancies and patients who underwent haematologic stem cell
transplantation: a systematic review. Clin Microbiol Infect, 2017.
23(12): p. 916-923. https://doi.org/10.1016/j.cmi.2017.06.024 PMid:28668465
- Hwang,
J.P., A.G. Barbo, and R.P. Perrillo, Hepatitis B reactivation during
cancer chemotherapy: an international survey of the membership of the
American Association for the Study of Liver Diseases. J Viral Hepat,
2015. 22(3): p. 346-52. https://doi.org/10.1111/jvh.12305 PMid:25220947 PMCid:PMC4833504
- Sarmati,
L., et al., Recommendations for screening, monitoring, prevention,
prophylaxis and therapy of hepatitis B virus reactivation in patients
with haematologic malignancies and patients who underwent haematologic
stem cell transplantation-a position paper. Clin Microbiol Infect,
2017. 23(12): p. 935-940. https://doi.org/10.1016/j.cmi.2017.06.023 PMid:28668466
- Cunningham,
H.E., et al., Successful treatment of hepatitis C virus infection with
direct-acting antivirals during hematopoietic cell transplant. Transpl
Infect Dis, 2019. 21(3): p. e13091. https://doi.org/10.1111/tid.13091 PMid:30972834
- Ramgopal,
A., et al., Safety of allogeneic hematopoietic stem cell
transplantation in beta-thalassemia patients with chronic hepatitis C
infections treated at a pediatric center. Pediatr Transplant, 2019.
23(6): p. e13520. https://doi.org/10.1111/petr.13520 PMid:31209983
- Adam, S., et al., Quality of Life Outcomes in a Pediatric Thalassemia Population in Egypt. Hemoglobin, 2017. 41(1): p. 16-20. https://doi.org/10.1080/03630269.2017.1312434 PMid:28440111
- Ben
Haj Ali, A., et al., Cytogenetic and molecular diagnosis of Fanconi
anemia revealed two hidden phenotypes: Disorder of sex development and
cerebro-oculo-facio-skeletal syndrome. Mol Genet Genomic Med, 2019.
7(7): p. e00694. https://doi.org/10.1002/mgg3.694 PMid:31124294 PMCid:PMC6625148
- Gamal
M Fathy, A.E.-H., Hossam K Mahmoud, Omar Fahmy, Raafat Abdelfattah,
Mohamed Abdel- Mooti, Mahmoud Bokhary and Shaimaa Ibrahim, ATG Based
Conditioning Regimen in Stem Cells Transplantation of Fanconi Anemia: A
Single Center Experience of 63 Patients. Annals of Bone Marrow
Research, 2017. 2(1): p. 8-12. https://doi.org/10.17352/abmr.000004
- Mahmoud,
H.K., et al., Allogeneic hematopoietic stem cell transplantation for
non-malignant hematological disorders. J Adv Res, 2015. 6(3): p.
449-58. https://doi.org/10.1016/j.jare.2014.11.001 PMid:26257943 PMCid:PMC4522586
- Zhang, L., J. Yu, and W. Wei, Advance in Targeted Immunotherapy for Graft-Versus-Host Disease. Front Immunol, 2018. 9: p. 1087. https://doi.org/10.3389/fimmu.2018.01087 PMid:29868032 PMCid:PMC5964137
- Saini,
N., R. Nath, and J. Cerny, Calcineurin inhibitor-free GVHD prophylaxis
with sirolimus and mycophenolate mofetil combination. Ann Hematol,
2017. 96(9): p. 1563-1568. https://doi.org/10.1007/s00277-017-3062-2 PMid:28710649
- Saleem,
M.S., et al., Challenges in managing graft-versus-host disease in
developing countries: a perspective. Bone Marrow Transplant, 2019.
54(5): p. 641-647. https://doi.org/10.1038/s41409-018-0333-z PMid:30237541
- George,
B., et al., Post-Transplant Cyclophosphamide as Sole Graft-versus-Host
Disease Prophylaxis Is Feasible in Patients Undergoing Peripheral Blood
Stem Cell Transplantation for Severe Aplastic Anemia Using Matched
Sibling Donors. Biol Blood Marrow Transplant, 2018. 24(3): p. 494-500. https://doi.org/10.1016/j.bbmt.2017.10.034 PMid:29100905
- Patel,
D.A., Haploidentical Stem Cell Transplantation With
Post-Transplantation Cyclophosphamide for Aggressive Lymphomas: How Far
Have We Come and Where Are We Going? World J Oncol, 2019. 10(1): p.
1-9. https://doi.org/10.14740/wjon1164 PMid:30834047 PMCid:PMC6396776
- Sung, P.J. and S.M. Luger, Minimal Residual Disease in Acute Myeloid Leukemia. Curr Treat Options Oncol, 2017. 18(1): p. 1. https://doi.org/10.1007/s11864-017-0447-3 PMid:28110381
- Patkar,
N., et al., Standardizing minimal residual disease by flow cytometry
for precursor B lineage acute lymphoblastic leukemia in a developing
country. Cytometry B Clin Cytom, 2012. 82(4): p. 252-8. https://doi.org/10.1002/cyto.b.21017 PMid:22467604
- Ladetto,
M., et al., Next-generation sequencing and real-time quantitative PCR
for minimal residual disease detection in B-cell disorders. Leukemia,
2014. 28(6): p. 1299-307. https://doi.org/10.1038/leu.2013.375 PMid:24342950
- Sipsas,
N.V., et al., Management of Invasive Fungal Infections in Adult
Patients with Hematological Malignancies in Greece during the Financial
Crisis: Challenges and Recommendations. J Fungi (Basel), 2018. 4(3). https://doi.org/10.3390/jof4030094 PMid:30096956 PMCid:PMC6162614
- Offner,
F., et al., Impact of previous aspergillosis on the outcome of bone
marrow transplantation. Clin Infect Dis, 1998. 26(5): p. 1098-103. https://doi.org/10.1086/520274 PMid:9597235
- Ardissino,
G., et al., Haploidentical Hematopoietic Stem Cell Transplant
Complicated by Atypical Hemolytic Uremic Syndrome and Kidney Transplant
From the Same Donor With No Immunosuppression but C5 Inhibition.
Transplantation, 2019. 103(2): p. e48-e51. https://doi.org/10.1097/TP.0000000000002505 PMid:30365467
- Richardson,
P., et al., Systematic review of defibrotide studies in the treatment
of veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS).
Bone Marrow Transplant, 2019. 54(12): p. 1951-1962. https://doi.org/10.1038/s41409-019-0474-8 PMid:30804485 PMCid:PMC6957462
- Corbacioglu,
S. and P.G. Richardson, Defibrotide for children and adults with
hepatic veno-occlusive disease post hematopoietic cell transplantation.
Expert Rev Gastroenterol Hepatol, 2017. 11(10): p. 885-898. https://doi.org/10.1080/17474124.2017.1370372 PMid:28825848
- Boeckh,
M., et al., Valganciclovir for the prevention of complications of late
cytomegalovirus infection after allogeneic hematopoietic cell
transplantation: a randomized trial. Ann Intern Med, 2015. 162(1): p.
1-10. https://doi.org/10.7326/M13-2729 PMid:25560711 PMCid:PMC4465336
- Uppuluri,
R., et al., Cytomegalovirus reactivation posthematopoietic stem cell
transplantation (HSCT) and type of graft: A step toward rationalizing
CMV testing and positively impacting the economics of HSCT in
developing countries. Pediatr Blood Cancer, 2017. 64(11). https://doi.org/10.1002/pbc.26639 PMid:28544502
- Leung,
P.Y.M., et al., Ganciclovir-resistant post-transplant cytomegalovirus
infection due to combined deletion mutation at codons 595-596 of the
UL97 gene. Transpl Infect Dis, 2019. https://doi.org/10.1111/tid.13168 PMid:31498954
- El
Helou, G. and R.R. Razonable, Safety considerations with current and
emerging antiviral therapies for cytomegalovirus infection in
transplantation. Expert Opin Drug Saf, 2019. https://doi.org/10.1080/14740338.2019.1662787 PMid:31478398
- Ogonek,
J., et al., Immune Reconstitution after Allogeneic Hematopoietic Stem
Cell Transplantation. Front Immunol, 2016. 7: p. 507. https://doi.org/10.3389/fimmu.2016.00507
- Ghose, C. and C.W. Euler, Gram-Negative Bacterial Lysins. Antibiotics (Basel), 2020. 9(2). https://doi.org/10.3390/antibiotics9020074 PMid:32054067
- Fishman, J.A., Pneumocystis jiroveci. Semin Respir Crit Care Med, 2020. 41(1): p. 141-157. https://doi.org/10.1055/s-0039-3399559 PMid:32000290
[TOP]





