Salvatore Perrone1, Chiara Lisi1, Elettra Ortu La Barbera1, Cristina Luise1, Miriam Lichtner2, Corrado Girmenia3 and Giuseppe Cimino1,4.
1 Hematology, Polo Universitario Pontino, S.M. Goretti Hospital, Latina, Italy.
2 Department of Public Health and Infectious Diseases, Sapienza University, S.M. Goretti Hospital, Latina, Italy.
3 Hematology, Dipartimento Medicina Traslazionale e di Precisione, AOU Policlinico Umberto I, Sapienza University of Rome, Italy.
4 Department of Medical Oncology, Sapienza University of Rome, Medical and Surgical Sciences and Biotechnology, Rome, Italy.
Corresponding
author: Salvatore Perrone, M.D. Hematology, Polo Universitario Pontino,
“Sapienza”. Via A. Canova S.M. Goretti Hospital, Latina, Italy. Tel:
+3907736553126. E-mail:
sperrone@hotmail.it
Published: May 1, 2020
Received: February 8, 2020
Accepted: April 5, 2020
Mediterr J Hematol Infect Dis 2020, 12(1): e2020026 DOI
10.4084/MJHID.2020.026
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background: Saprochaete capitata
is a rare and emerging opportunistic fungus, involving
immunocompromised hosts, in particular, neutropenic patients after
chemotherapy. Case Report: We report a case of disseminated and cerebral infection by Saprochaete capitata,
in a 68-year-old woman affected by acute myeloid leukemia that was
successfully managed with liposomal amphotericin B and isavuconazole.
Conclusions:
This case illustrates the feasibility of isavuconazole therapy in the
treatment of a S. capitata infection when co-administered with
midostaurin.
|
Introduction
Saprochaete capitata (formerly known as Blastoschizomyces capitatus and Geotrichum capitatum)
is a rare and emerging opportunistic fungus, involving
immunocompromised hosts, in particular, neutropenic patients after
chemotherapy, with crude mortality estimates as high as 60%.[1,2] Herein we describe the first case, to our knowledge, of disseminated S. capitata infection successfully managed with isavuconazole.
Case Report
In 2019, a 68-year-old woman presented with chest pain, dyspnoea, and gum pain lasting one week. Hyperleukocitosis (WBC 241x109/L)
was sustained by monoblastic cells. Therefore, bone marrow aspiration
was performed and confirmed an extensive infiltration (93% monocytic
blasts) by acute myeloid leukemia (AML) with translocation
t(9;11)(p21.3;q23.3) and Fms-related-tyrosine kinase 3 with a point
mutation in the tyrosine kinase domain (FLT3-TKD) positivity.
Therefore, she was treated with standard intensive chemotherapy ‘7+3’
(ARA-C 100mg/m2 ci. x 7 days and daunorubicin 60mg/m2
x 3) and midostaurin (50 mg bid, day 8 to 28), a recently
approved FLT3 inhibitor metabolized to its active metabolites GGP6221
and CGP52421 via CYP3A4 in the liver.[3,4] Due to the
known interference of midostaurin with potent CYP3A4 inhibitors with
the risk of side effects including QTc prolongation,[5]
as per protocol, we decided not to give the standard posaconazole
antifungal prophylaxis, and we planned a preemptive antifungal approach
with weekly serum galactomannan monitoring and precocious chest
Computed Tomography (CT) in case of persisting neutropenic fever. On
day +8, she developed neutropenic fever, and empirical anti-bacterial
treatment with piperacillin/tazobactam and teicoplanin was started.
After 48 hours, she was still febrile; therefore, she underwent a CT
scan that revealed a 20 mm lung nodule, with halo-sign. Considering a
possible invasive fungal infection (IFI) (serum galactomannan assay was
negative), we started liposomal amphotericin B (3 mg/kg/day). Two days
later, she was still febrile, and blood cultures showed S. capitata
fungemia, galactomannan raised to 0.6 in two blood samples; therefore,
liposomal amphotericin B dosage was increased to 5 mg/kg/day for 9
days, with defervescence within few days. Later, when neutropenia
recovered, the CT-scan showed the evolution of the lung nodule into a
cavitary lesion (Figure 1A), multiple liver abscesses (Figure 1B), and a single brain abscess in the left head of the caudate nucleus (Figure 1C-D).
We then decided to discharge the patient and to shift antifungal
treatment from intravenous liposomal amphotericin B to oral
isavuconazole. We chose oral isavuconazole instead of oral
voriconazole, which is a standard therapy of S. capitata infection[6,7] because isavuconazole is a mild/moderate inhibitor while voriconazole is a potent inhibitor of CYP3A4.[5] Isavuconazole was also chosen because of its in vitro activity against S. capitata[8,9] and the favorable pharmacokinetic profile in the central nervous system (CNS) infections.[10,11]
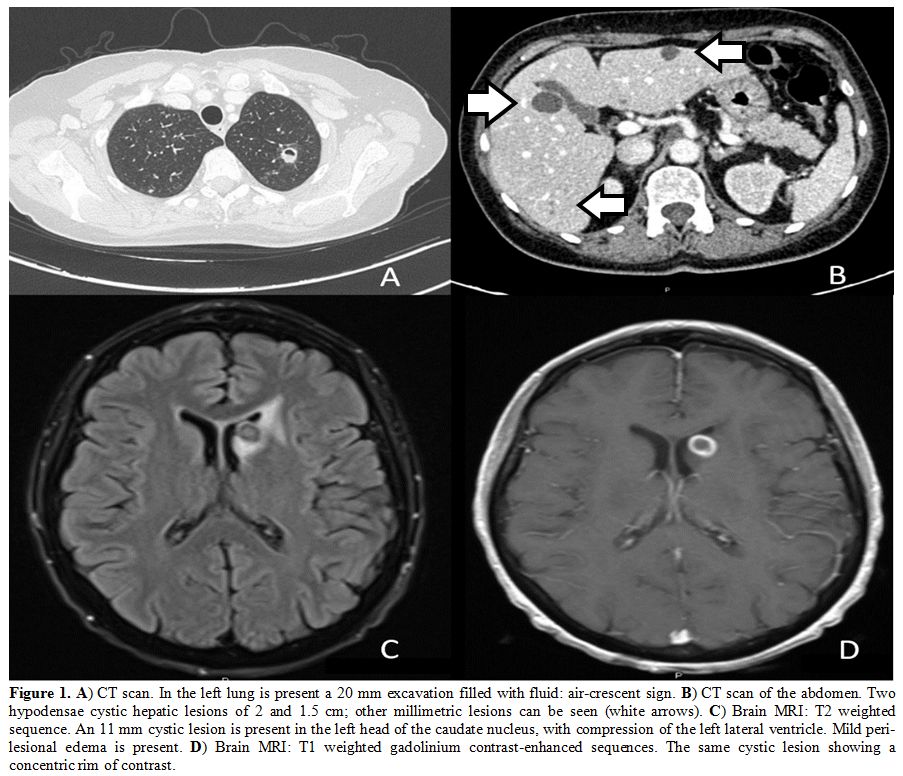 |
Figure
1. A) CT scan. In the left lung is present a 20 mm excavation filled with fluid: air-crescent sign. B)
CT scan of the abdomen. Two hypodensae cystic hepatic lesions of 2 and
1.5 cm; other millimetric lesions can be seen (white arrows). C)
Brain MRI: T2 weighted sequence. An 11 mm cystic lesion is present in
the left head of the caudate nucleus, with compression of the left
lateral ventricle. Mild peri-lesional edema is present. D) Brain MRI: T1 weighted gadolinium contrast-enhanced sequences. The same cystic lesion showing a concentric rim of contrast. |
The
patient successfully underwent two courses of consolidation with high
doses of cytarabine and midostaurin while under isavuconazole therapy
without any midostaurin related toxicity (the patient was monitored
biweekly for QTc prolongation on each ambulatory visit). During
maintenance with midostaurin, we performed a therapeutic drug
monitoring (TDM) of isavuconazole 5.2 µg/ml (normal range 2-5 µg/ml),
no adjustment was undertaken. Eight months later, AML is in complete
remission, and fungal infection is improving on isavuconazole (Figure 2), despite the prolonged neutropenia induced by the consolidation cycles.
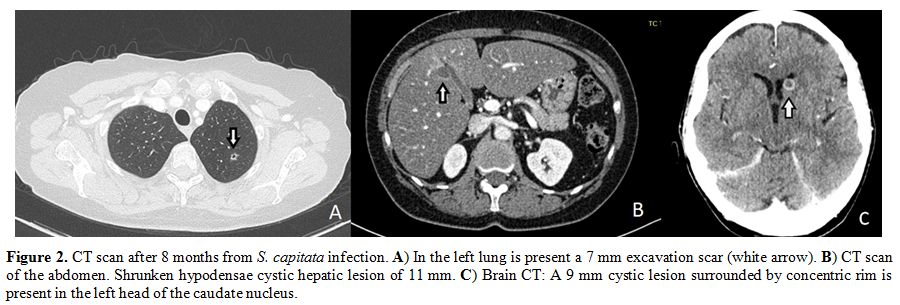 |
Figure 2. CT scan
after 8 months from S. capitata infection. A) In the left lung is
present a 7 mm excavation scar (white arrow). B) CT scan of the
abdomen. Shrunken hypodensae cystic hepatic lesion of 11 mm. C) Brain
CT: A 9 mm cystic lesion surrounded by concentric rim is present in the
left head of the caudate nucleus. |
Discussion
This case illustrates that isavuconazole may be an option in the treatment of S. capitata
infections, and that may be a safe choice if a co-administration with
midostaurin is required. A further case of safe isavuconazole and
midostaurin therapy in an AML patient with a possible pulmonary fungal
infection has been recently reported.[12]
Indeed,
our case raises the challenging question of the appropriateness of
administering midostaurin concomitantly with a CYP3A4 inhibitor.
Posaconazole
and voriconazole are drugs of the first choice in the primary
antifungal prophylaxis and therapy of invasive aspergillosis and other
IFIs, respectively.[13,14] However, in patients
affected by FLT3 positive AML caution is requested when triazoles are
administered concomitantly with midostaurin, given the possible
toxicity related to the increased exposure to the FLT3 inhibitor, being
posaconazole and voriconazole potent inhibitors of CYP3A4. Furthermore,
an increased risk of QTc prolongation should be considered when
patients receive midostaurin in association with other drugs that can
prolong QTc, as the above triazoles.[4,5]
This
limitation in the prevention and treatment of IFIs in FLT3 positive AML
patients represents a challenging issue in the clinical practice,
considering that IFIs significantly affect complete remission
achievement and long-term survival of AML patients.[15]
Again, the protective effects of mold active antifungal prophylaxis
during induction and salvage chemotherapy for AML may have long-lasting
benefits that extend even after the allogeneic stem cell transplant
procedure, which is indicated in FLT3 positive AML patients after the
achievement of complete remission because of the high risk of leukemia
relapse.[16]
On the other hand, the
contraindication of the concomitant use of midostaurin and triazoles is
controversial. Ouatas et al. analyzed data from the Ratify study
focusing on the subset of patients with concomitant use of midostaurin
and fluconazole, posaconazole, or voriconazole in prophylaxis.[3,17]
In that study, concomitant use of various CYP3A4 inhibitors and
antifungals agents was permitted with caution but without any specific
recommendation on how to perform dose adjustment. More than half of
patients received posaconazole or voriconazole during induction,
consolidation, or maintenance therapy. In those patients in which
midostaurin plasma levels were measured, a 1.44-fold increase in
midostaurin through levels was observed when the strong CYP3A4
inhibitors posaconazole or voriconazole were co-administered, and no
increase of adverse events nor impact on efficacy outcomes were
observed, therefore dose modification does not seem required.[17] Isavuconazole is not in-label for IFI prophylaxis, but retrospective studies[18,19] suggest it could be an interesting option to be investigated in settings similar to our case.
Interestingly,
few data exist about isavuconazole penetration in cerebral tissue, and
experiences are mediated from mice models.[20] In a
patient with AML and cerebral aspergillosis, isavuconazole
concentrations measured in the inflammatory brain tissue surrounding
the abscess were similar to plasma, while the concentration in the
liquid of the abscess was quasi-null.[10] Two other
patients with cerebral aspergillosis were treated with isavuconazole
and required surgery for the progression of the infection. Bioptic
samples showed increased drug concentration in the abscess and inflamed
meninges compared to unaffected brain tissue.[11]
Differently from these cases, the outcome of our patient was favorable.
Similarly, isavuconazole was also utilized with success to treat
Rhino-Orbital-Cerebral Mucormycosis.[23]
Conclusions
In conclusion, our case illustrates the feasibility of isavuconazole therapy in the treatment of a S. capitata
infection when co-administered with midostaurin. Considering that the
warning about the risk of severe side effects of midostaurin treatment
when administered concomitantly with potent CYP3A4 inhibitors, even if
not confirmed, it can be speculated that the use of a mild/moderate
CYP3A4 inhibitor, as isavuconazole, could be a safer choice
particularly in an outpatient setting, when the strict monitoring of
adverse events is less feasible. We think it could have clinical
implications when treating patients with a rare yeast infection.
However, prospective data in this setting would be helpful in the next
future, also considering the emergence of S. capitata in central Europe.[21,22]
References
- Girmenia, C., L. Pagano, B. Martino, D. D'Antonio,
R. Fanci, G. Specchia, et al., Invasive infections caused by
Trichosporon species and Geotrichum capitatum in patients with
hematological malignancies: a retrospective multicenter study from
Italy and review of the literature. J Clin Microbiol, 2005. 43(4): p.
1818-28. https://doi.org/10.1128/JCM.43.4.1818-1828.2005 PMid:15815003 PMCid:PMC1081342
- Mazzocato,
S., E. Marchionni, A.W. Fothergill, D.A. Sutton, S. Staffolani, R.
Gesuita, et al., Epidemiology and outcome of systemic infections due to
saprochaete capitata: case report and review of the literature.
Infection, 2015. 43(2): p. 211-5. https://doi.org/10.1007/s15010-014-0668-3 PMid:25078793
- Stone,
R.M., S.J. Mandrekar, B.L. Sanford, K. Laumann, S. Geyer, C.D.
Bloomfield, et al., Midostaurin plus Chemotherapy for Acute Myeloid
Leukemia with a FLT3 Mutation. N Engl J Med, 2017. 377(5): p. 454-464. https://doi.org/10.1056/NEJMoa1614359 PMid:28644114 PMCid:PMC5754190
- Abbas,
H.A., M. Alfayez, T. Kadia, F. Ravandi-Kashani and N. Daver,
Midostaurin In Acute Myeloid Leukemia: An Evidence-Based Review And
Patient Selection. Cancer Manag Res, 2019. 11: p. 8817-8828. https://doi.org/10.2147/CMAR.S177894 PMid:31632141 PMCid:PMC6782026
- Lindsay,
J., B.W. Teh, K. Micklethwaite and M. Slavin, Azole antifungals and new
targeted therapies for hematological malignancy. Curr Opin Infect Dis,
2019. 32(6): p. 538-545. https://doi.org/10.1097/QCO.0000000000000611 PMid:31688198
- Girmenia,
C., G. Pizzarelli, D. D'Antonio, F. Cristini and P. Martino, In vitro
susceptibility testing of Geotrichum capitatum: comparison of the
E-test, disk diffusion, and Sensititre colorimetric methods with the
NCCLS M27-A2 broth microdilution reference method. Antimicrob Agents
Chemother, 2003. 47(12): p. 3985-8. https://doi.org/10.1128/AAC.47.12.3985-3988.2003 PMid:14638517 PMCid:PMC296229
- Cornely,
O.A., M. Cuenca-Estrella, J.F. Meis and A.J. Ullmann, European Society
of Clinical Microbiology and Infectious Diseases (ESCMID) Fungal
Infection Study Group (EFISG) and European Confederation of Medical
Mycology (ECMM) 2013 joint guidelines on diagnosis and management of
rare and em erging fungal diseases. Clin Microbiol Infect, 2014. 20
Suppl 3: p. 1-4. https://doi.org/10.1111/1469-0691.12569 PMid:24606200
- Guinea,
J., S. Recio, P. Escribano, T. Pelaez, B. Gama and E. Bouza, In vitro
antifungal activities of isavuconazole and comparators against rare
yeast pathogens. Antimicrob Agents Chemother, 2010. 54(9): p. 4012-5. https://doi.org/10.1128/AAC.00685-10 PMid:20566770 PMCid:PMC2935032
- Thompson,
G.R., 3rd, N.P. Wiederhold, D.A. Sutton, A. Fothergill and T.F.
Patterson, In vitro activity of isavuconazole against Trichosporon,
Rhodotorula, Geotrichum, Saccharomyces and Pichia species. J Antimicrob
Chemother, 2009. 64(1): p. 79-83. https://doi.org/10.1093/jac/dkp138 PMid:19406849
- Lamoth,
F., T. Mercier, P. Andre, J.L. Pagani, O. Pantet, R. Maduri, et al.,
Isavuconazole brain penetration in cerebral aspergillosis. J Antimicrob
Chemother, 2019. 74(6): p. 1751-1753. https://doi.org/10.1093/jac/dkz050 PMid:30753519
- Rouzaud,
C., V. Jullien, A. Herbrecht, B. Palmier, S. Lapusan, M. Morgand, et
al., Isavuconazole Diffusion in Infected Human Brain. Antimicrob Agents
Chemother, 2019. 63(10). https://doi.org/10.1128/AAC.02474-18 PMid:31405852 PMCid:PMC6761565
- Tollkuci,
E., Isavuconazole therapy in an FLT3 mutated acute myeloid leukemia
patient receiving midostaurin: A case report. J Oncol Pharm Pract,
2019. 25(4): p. 987-989. https://doi.org/10.1177/1078155218764257 PMid:29558838
- Maertens,
J.A., C. Girmenia, R.J. Bruggemann, R.F. Duarte, C.C. Kibbler, P.
Ljungman, et al., European guidelines for primary antifungal
prophylaxis in adult haematology patients: summary of the updated
recommendations from the European Conference on Infections in
Leukaemia. J Antimicrob Chemother, 2018. 73(12): p. 3221-3230. https://doi.org/10.1093/jac/dky286
- Tissot,
F., S. Agrawal, L. Pagano, G. Petrikkos, A.H. Groll, A. Skiada, et al.,
ECIL-6 guidelines for the treatment of invasive candidiasis,
aspergillosis and mucormycosis in leukemia and hematopoietic stem cell
transplant patients. Haematologica, 2017. 102(3): p. 433-444. https://doi.org/10.3324/haematol.2016.152900 PMid:28011902 PMCid:PMC5394968
- Girmenia,
C., A. Micozzi, A. Piciocchi, G. Gentile, L. Di Caprio, D. Nasso, et
al., Invasive fungal diseases during first induction chemotherapy
affect complete remission achievement and long-term survival of
patients with acute myeloid leukemia. Leuk Res, 2014. 38(4): p. 469-74.
https://doi.org/10.1016/j.leukres.2014.01.007 PMid:24534569
- Busca,
A., A. Candoni, E. Audisio, R. Passera, B. Bruno, F. Monaco, et al.,
Long-Lasting Protective Effect of Posaconazole Prophylaxis in Patients
with Acute Myeloid Leukemia Receiving Allogeneic Hematopoietic Stem
Cell Transplantation. Biol Blood Marrow Transplant, 2016. 22(12): p.
2214-2219. https://doi.org/10.1016/j.bbmt.2016.09.019 PMid:27667012
- Ouatas,
T., V. Duval, K. Sinclair and N. Berkowitz, Concomitant use of
midostaurin with strong CYP3A4 inhibitors: an analysis from the ratify
trial. Blood, 2017. 130 (Supplement 1): p. 3814-3814.
- Fontana,
L., D.S. Perlin, Y. Zhao, B.N. Noble, J.S. Lewis, L. Strasfeld, et al.,
Isavuconazole Prophylaxis in Patients With Hematologic Malignancies and
Hematopoietic Cell Transplant Recipients. Clin Infect Dis, 2020. 70(5):
p. 723-730. https://doi.org/10.1093/cid/ciz282 PMid:30958538
- Bowen,
C.D., G.B. Tallman, M. Hakki and J.S. Lewis, Isavuconazole to prevent
invasive fungal infection in immunocompromised adults: Initial
experience at an academic medical centre. Mycoses, 2019. 62(8): p.
665-672. https://doi.org/10.1111/myc.12924 PMid:31050373
- Wiederhold,
N.P., L. Kovanda, L.K. Najvar, R. Bocanegra, M. Olivo, W.R.
Kirkpatrick, et al., Isavuconazole Is Effective for the Treatment of
Experimental Cryptococcal Meningitis. Antimicrob Agents Chemother,
2016. 60(9): p. 5600-3. https://doi.org/10.1128/AAC.00229-16 PMid:27324761 PMCid:PMC4997854
- Tanuskova,
D., J. Horakova, P. Svec, I. Bodova, M. Lengerova, M. Bezdicek, et al.,
First case of invasive Magnusiomyces capitatus infection in Slovakia.
Med Mycol Case Rep, 2017. 16: p. 12-15. https://doi.org/10.1016/j.mmcr.2017.03.004 PMid:28409093 PMCid:PMC5379865
- Birrenbach,
T., S. Bertschy, F. Aebersold, N.J. Mueller, Y. Achermann, K.
Muehlethaler, et al., Emergence of Blastoschizomyces capitatus yeast
infections, Central Europe. Emerg Infect Dis, 2012. 18(1): p.
98-101. https://doi.org/10.3201/eid1801.111192 PMid:22261201 PMCid:PMC3310123
- Andreani
G., Fadda G.L., Gned D., Dragani M., Cavallo G., Monticone V., Morotti
A., De Gobbi M., Guerrasio A., Barbui A.M., D'Avolio A., Cilloni D.
Rhino-orbital-cerebral mucormycosis after allogeneic hematopoietic stem
cell transplantation and isavuconazole therapeutic drug monitoring
during intestinal graft versus host disease. Mediterr J Hematol Infect
Dis 2019, 11(1): e2019061. https://doi.org/10.4084/mjhid.2019.061 PMid:31700586 PMCid:PMC6827600
[TOP]