Jialian Li1, Peng Wang1, Xue Li1, Qiaoyu Wang1, Jiayou Zhang2 and Yong Lin1.
1 Department
of Pharmacy, The Second Affiliated Hospital of Chengdu Medical College,
China National Nuclear Corporation 416 Hospital, Chengdu, Sichuan,
China.
2 Department of Hematology, The Second
Affiliated Hospital of Chengdu Medical College, China National Nuclear
Corporation 416 Hospital, Chengdu, Sichuan, China.
Corresponding
author: Dr Yong Lin. No. 4, North Section 4, Second Ring Road, Chengdu, China. E-mail:
Ly7171@163.com
Published: May 1, 2020
Received: January 22, 2020
Accepted: April 14, 2020
Mediterr J Hematol Infect Dis 2020, 12(1): e2020029 DOI
10.4084/MJHID.2020.029
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Objective:
The Iron chelation is essential to prevent iron overload damage of
vital organs, like heart, liver, and endocrine glands, in patients with
transfusion-dependent thalassemia. The most common chelation regimens
for β-thalassemia major (β-TM) patients used in China are a combination
therapy of deferoxamine and deferiprone (DFO+DFP), deferoxamine
(DFO) monotherapy, deferiprone (DFP) monotherapy and deferasirox (DFX)
monotherapy. Such patients use iron chelators their whole lives,
resulting in enormous treatment costs. This study analyses the
cost-utility of these four regimens from the Chinese healthcare system
perspective.
Methods: A
Markov decision model was used over a 5-year time horizon and was
populated using clinical data from a systematic literature review. We
obtained utility data from local and previous research. Costs were
estimated using Chinese national sources.
Results:
From the base-case analysis results, DFP was the most cost-effective
chelation regimen, followed by DFO, DFX, and DFO+DFP. DFP had 97.32%,
99.43%, and 58.04% likelihood of being cost-effective versus DFX,
DFO+DFP, and DFO, respectively, at a payment threshold of 193,932.00
CNY/QALY (QALY, quality-adjusted life-year).
Conclusions:
DFP was the most cost-effective chelation regimen for β-TM patients,
followed by DFO, DFX, and DFO+DFP. Using DFP as the primary treatment
regimen may potentially result in cost-savings and QALY gains for the
Chinese healthcare system. To increase these benefits, the Chinese
government should take measures to lower DFX and DFO drug costs, and
Chinese clinicians should choose the cheaper DFX and DFO, increase the
utility of DFO+DFP and reduce mortality and morbidity of DFP. Changes
in influential parameters easily affect the results of DFX versus
DFO+DFP and of DFP versus DFO; clinicians should focus on such
parameters and adjust the regimens accordingly.
|
Introduction
β-thalassemia is an autosomal recessive hereditary anemia characterized by reduced or absent β-globin chain synthesis.[1]
Patients with βthalassemia have been typically categorized as minor,
intermedia, or major based on their α-globin or β-globin chain
imbalance, the severity of the anaemia, and clinical picture at
presentation.[2] β-thalassemia is known to be highly
prevalent in Southeast and South Asia, the Middle East, the
Mediterranean countries, and North and Central Africa.[1] Additionally,
because of continued migration, β-thalassemia is now becoming
increasingly common in Europe and North America, making it a global
health concern.[2] Approximately 1.5% of the world's population carries the β-thalassemia gene,[3] and every year, the number of new-born children diagnosed with βthalassemia major(β-TM) exceeds 23,000.[4,5] In China, the average prevalence rate of β-thalassemia was 0.67%-2%.[6]
β-thalassemia may be classified clinically as transfusion-dependent or non–transfusion-dependent.[7,8]
β-TM patients require since early childhood regular red blood cell
(RBC) transfusions to maintain adequate hemoglobin levels improving
quality of life while reducing mortality.[7,8]
Unfortunately, the human body does not have an iron excretory pathway,
which leads to the accumulation of iron from the transfused blood,
known as iron overload.[6] The cumulative iron
overload subsequently leads to organ toxic effects and dysfunction, for
example, in the heart, liver, or endocrine glands, eventually leading
to death.[8] Three iron chelators are currently
available for the treatment of iron overload in β-TM patients:
deferoxamine (DFO), deferiprone (DFP), and deferasirox (DFX). The four
most commonly used chelation regimens in China are combination therapy
of DFO and DFP (DFO+DFP) (38.9%), DFO monotherapy (19.1%), DFP
monotherapy (19.0%) and DFX monotherapy (16.0%).[6]
DFO was the first iron chelator to be marketed, and it is the first-line drug for β-TM patients 2 years of age and older.[2]
Because DFO is administered as a subcutaneous infusion, the quality of
life and compliance of patients are low. Low compliance with DFO poses
a higher risk of iron overload-related complications and death.[9,10]
To obtain a higher quality of life, and better compliance, oral iron
chelators, DFP and DFX, were introduced. DFP is the second-line drug
for β-TM patients six years of age and older.[2,11] DFX is the first-line drug for patients 6 years of age and older in China and Europe.[2,12] Because cardiac complications related to iron overload is the leading cause of death in 52.3% of these patients,[13]
the guidelines of the US, Italy, Australia, and China all recommend
that β-TM patients with iron overload-related cardiac complications
should receive DFO+DFP.[11,14-16]
Because
β-TM patients need to use iron chelators throughout their whole lives,
the treatment cost is enormous. Paramore et al. reported that the
annual average chelation treatment cost of transfusion-dependent
β-thalassemia patients in the US was approximately USD 53,000.[17]
Esmaeilzadeh et al. found that the treatment of approximately 18,000
β-TM patients led to an annual loss of nearly USD 150 million for
Iran's healthcare system.[18] In China, the annual
average treatment cost of blood transfusion and iron excretion for β-TM
patients was over CNY 100,000, but the annual income of over 90% of
families with β-TM patients was less than CNY 60,000, which means that
most families with β-TM patients will fall into poverty due to the
illness.[6] As a result, it is crucial to analyse the cost-effectiveness of iron chelation regimens from a Chinese perspective.
According
to a previous systematic review, there is no published analysis of the
relative cost-effectiveness of iron chelator therapies from a Chinese
perspective.[19] Cost-effectiveness is not an entire
issue when in different countries (regions), the results are the
opposite for other countries (regions). The specific legislation of
regions where clinicians operate has a substantial influence on the
economics of drugs.[19] Thus, this study aims to
compare the cost-utility of the four iron chelation regimens (DFO, DFP,
DFX, DFO+DFP) from the Chinese healthcare system perspective.
Methods
A
Markov model was developed to determine the cost-utility of the four
chelation regimens (DFO, DFP, DFX, and DFO+DFP) for β-TM patients with
iron overload from the perspective of the Chinese healthcare system.
The data used in the Markov model included cost, utility, and clinical
transition probabilities. To obtain these data, we conducted a
systematic literature review. If the data collected by the systematic
review were insufficient, we conducted local research to supplement the
data.Outline of the economic model.
The model considered adults and children, regardless of treatment
history or disease status. A 5-year time horizon was specified in the
model, and the cycle length was one year. The People's Bank of China
regulates that for financial institutions, the national guiding
interest rate for one-year deposits is 1.5%.[20] Hence, we used a 1.5% discount rate to discount future costs and QALYs (quality-adjusted life year).[21] The National Bureau of Statistics of China announced that in 2018, per capita GDP was CNY 64,644.[22]Additionally,
a study by the World Health Organization (WHO) proposed that when the
incremental cost-effectiveness ratio (ICER) was less than three times
per capita GDP, the increased costs were acceptable, and the
intervention was cost-effective.[21] As a result, the
payment threshold used in the model was CNY 193,932. We constructed our
Markov model with three health statuses: β-thalassemia without cardiac
complications, β-thalassemia with cardiac complications, and death (Figure 1).
In addition to the cardiac complication, the major complications of
iron overload include chronic liver disease, diabetes mellitus,
hypogonadism, hypoparathyroidism, and hypothyroidism.[19,23,24] The cost and morbidity of these complications were also calculated in a model.
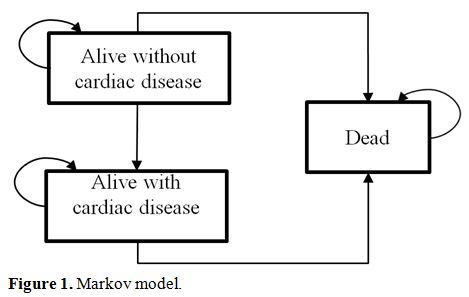 |
Figure 1. Markov model. |
Systematic literature review.
A systematic literature search in MEDLINE (PubMed), EMBASE (Ovid), the
Cochrane Central Register of Controlled Trials (CENTRAL, Cochrane
Library), the Cochrane Database of Systematic Reviews (CDSR, Cochrane
Library), China Biology Medicine (CBM, SinoMed), the China National
Knowledge Infrastructure (CNKI), VIP Data, and Wanfang Data was
conducted on March 2019, with no restrictions on the date. Besides, a
manual search was performed to identify conferences. Both Chinese and
English search terms were used. The search terms included
“thalassemia”, “beta-thalassemia”, “iron overload”, “iron-chelating
agents”, “deferoxamine”, “deferiprone”, and “deferasirox” and their
variations. The study selection and data extraction were conducted
independently by two researchers to confirm that they met the
pre-defined inclusion/exclusion criteria and data extraction form. Any
inconsistencies were resolved through discussion. The inclusion
criteria for the systematic review were as follows: population: β-TM patients or transfusion-dependent thalassemia major patients; intervention/comparison: DFO, DFP, DFX, or DFO+DFP; outcomes:
(1) clinical data: cardiac complication morbidity, cardiac complication
mortality, and non-cardiac complication mortality; (2) utility data:
the utility associated with cardiac complication or without cardiac
complication; and (3) cost data: the chelator cost, DFO administration
cost, cardiac complication therapy cost, and monitoring cost; study
design: randomized controlled trials (RCTs), non-RCTs, observational
studies, and pharmacoeconomic reviews. Because local/national context
has a substantial influence on the results of pharmacoeconomic
evaluations,[7] we used valuable localized in this
study. For the clinical data, we preferred to use data from China
(including Mainland China, Hong Kong, Macao, and Taiwan), followed by
data from Asia or all around the world. For the utility data, we used
data from China (including Mainland China, Hong Kong, Macao, and
Taiwan). For the cost data, we used data from Mainland China.Data used in the model. The detailed data were shown in Table 1.
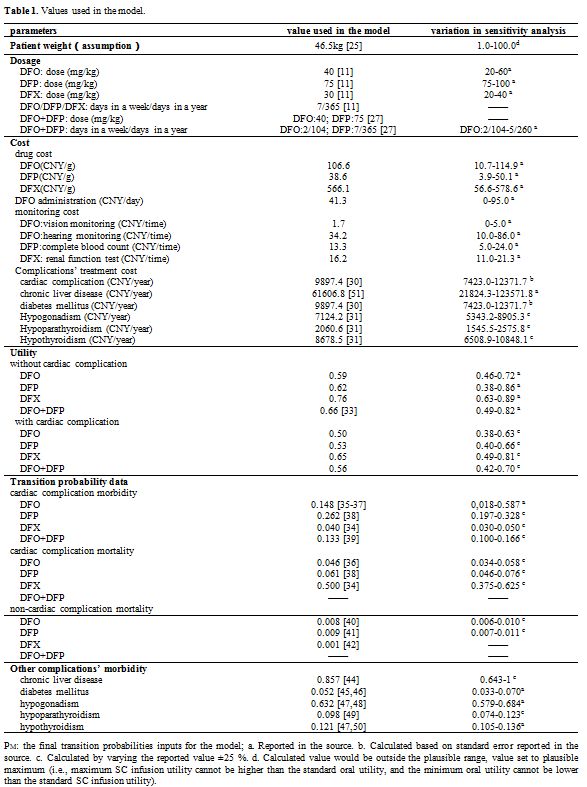 |
Table
1. Values used in the model. |
Cost data:a)
Chelator cost: The chelator cost was calculated according to the
drug cost, chelator dosage, and patient weight. Lau et al. reported
that the mean weight of 381 TM (age ranging from 3 months to 56 years)
was 46.5kg,[25] and it was used in the model. We
searched the official websites of 31 provincial-level administrative
units in Mainland China to obtain the government wholesale acquisition
cost. The average unit cost of drugs was used in the model. The
government official websites showed that there were only brand-name
drugs: Desferal®, Ferriprox®, and Exjade®. Luangasanatip et al.
reported that the cost of the generic version of DFO, USD 0.20 per
gram, was only 9.57% that of the brand-name drug (USD 2.09 per gram).[26]
As a result, we used 10% of the average unit cost of drugs in a
sensitivity analysis. In this study, we assumed that the patient
compliance rate was 100%. The dosage levels and frequencies of the four
iron chelation regimens were based on Chinese guidelines.[11,27]
The average dosage was used in the base-case analysis. The maximum
dosage and minimum dosage were used in the sensitivity analysis.b)
DFO administration cost: We searched the official websites of 31
provincial-level administrative units in Mainland China to obtain the
DFO administration cost. The average cost was used for the base-case
analysis, while the maximum cost was used in the sensitivity analysis.
If DFO patients purchased an infusion pump and used it at home, the DFO
administration was free. Therefore, CNY 0 was used in the sensitivity
analysis.c)
Monitoring cost: Reduction of severe adverse reactions requires
that the vision and hearing of patients on DFO therapy should be
monitored every three months,[28] the blood cell count of patients on DFP therapy should be monitored weekly,[27,29] and the renal function of patients on DFX therapy should be monitored monthly.[27,12]
We searched the official websites of 31 provincial-level administrative
units in Mainland China to obtain those monitoring costs. The average
cost was used for the base-case analysis, while the minimum and maximum
costs were used in the sensitivity analysis.d)
Complications therapy cost: Luangasanatip et al. reported that
the cost of treating iron overload-related cardiac complications in
thalassemia patients was the same as the cost of treating chronic heart
failure complications in patients with diabetes mellitus.[26]
In China, the annual medical cost of treating chronic heart failure
complications in patients with type 2 diabetes mellitus was CNY 9897.37[30]
and the data were used in the model. We used the cost data of
hypogonadism, hypoparathyroidism, and hypothyroidism from Ho et al.[31] The rate was USD 1= CNY 7.0328.[32] Utility data.
Eighteen patients (32 person-time) who were treated at a hospital
between 2015 and 2017 completed an assessment to get their utility
values for DFO (12 person-time), DFP (9 person-time), and DFX (11
person-time) by a time trade-off (TTO) method. Participants were
queried to identify the number of years of life with β-TM treated with
either DFO, DFP, or DFX that they would be willing to trade off for
years of life with perfect health. The average utility was used in the
model. Kuo et al. reported that the utility value for DFO+DFP.[33]
For patients with cardiac disease, their quality of life is estimated
to be approximately 15% less than that of individuals without cardiac
disease.[26]Clinical data. From the systematic review, there was one paper from China reported cardiac complication morbidity and mortality with DFX,[34]
where the morbidity was 7.7%, and mortality was 100% over 2 years.
Three reported cardiac complication morbidity and mortality with DFO
from Asia.[35-37] Ayyub et al. reported cardiac complication morbidity and mortality with DFP from Pakistan,[38]
where the morbidity was 54.5%, and mortality was 16.7% over 3 years.
Tanner et al. paper reported cardiac morbidity with DFO+DFP,[39]
where the morbidity was 13.3% over one year. There was one paper
reported non-cardiac complication mortality with DFO from Iran,[40]
where the mortality was 4.0% over 5 years. However, there were two
papers reported non-cardiac complication mortality from Europe, where
the mortality was 2.6% over 3 years with DFP[41] and 1.0% over 6.9 years with DFX[42] respectively. These were converted into an annual rate for use in the model using actuarial life-table methods;[43]
the mean of different annual rates with the same iron chelation was
used in the model. The morbidity of diabetes mellitus, hypogonadism,
and hypothyroidism were both reported from China,[44-50] and the mean of different morbidity was used in the model.Sensitivity analysis.
One-way sensitivity analysis was performed to investigate the effects
of altering the parameters, including the dosage, costs, utilities, and
transition probabilities, within plausible ranges. To assess the main
drivers of cost-effectiveness, we generated tornado diagrams
representing the one-way sensitivity analysis for each comparison
combination (DFO+DFP versus DFO, DFO+DFP versus DFP, DFO+DFP versus
DFX, DFX versus DFO, DFX versus DFP and DFP versus DFO). A tornado
diagram plotted the results of the effects of the ten most influential
parameters on the outcomes from a sensitivity analysis exercise. These
parameters were ordered such that the most influential parameter is at
the top of the tornado diagram. In addition, all parameters were
simultaneously varied in a probabilistic sensitivity analysis in which
the doses and costs assumed to follow gamma distributions, while the
utilities and transition probabilities were assigned beta
distributions, in line with best practices.[52,53]To
analysis the influence of these key parameters of the one-way
sensitivity analysis, which resulted in an ICER being greater or less
than the payment threshold, we valued and calculated the fraction of
these. Firstly, we valued key parameters according to the influence in
each comparison. The most influential key parameter was valued 10,
decreasing successively, and the least influential key parameter was
valued 1. Secondly, we added up the value of the key parameter. Results
Base-case analysis. The results from the base-case analysis are presented in Table 2.
The ICER results showed that DFP was the most cost-effective treatment
over a 5-year time horizon, followed by DFO, DFX, and DFO+DFP. DFP had
the lowest cost of the four chelation regimens. Additionally, DFX had
the longest QALYs.
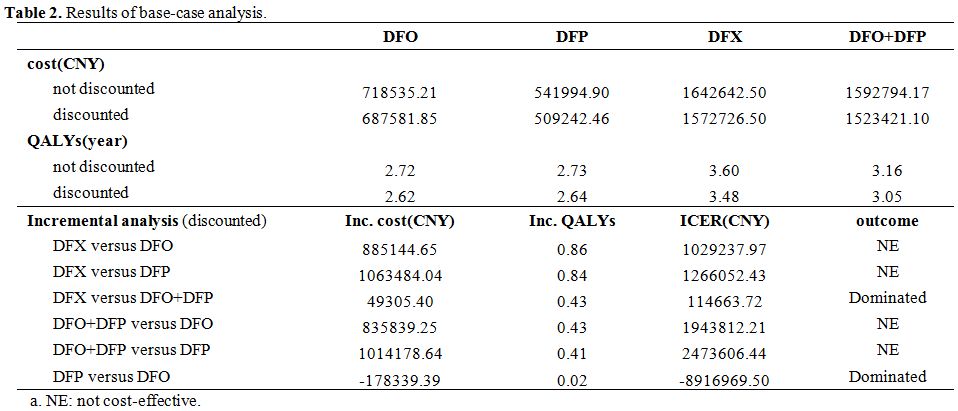 |
Table 2. Results of base-case analysis. |
Sensitivity analysis. One-way sensitivity analysis. The one-way sensitivity analysis results are represented in tornado diagrams in Figure 2.
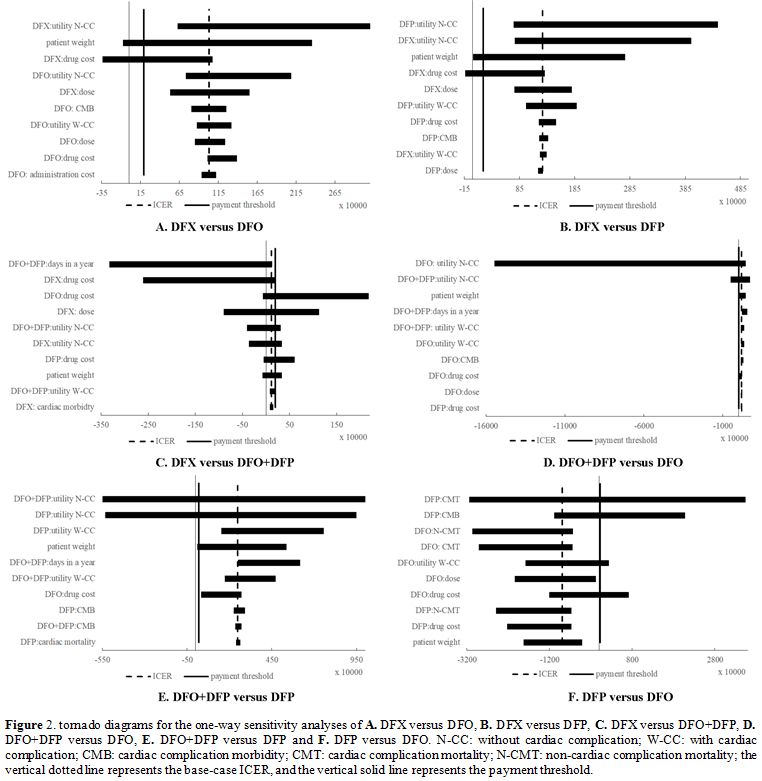 |
Figure 2. Tornado diagrams for the one-way sensitivity analyses of A. DFX versus DFO, B. DFX versus DFP, C. DFX versus DFO+DFP, D. DFO+DFP versus DFO, E. DFO+DFP versus DFP and F.
DFP versus DFO. N-CC: without cardiac complication; W-CC: with cardiac
complication; CMB: cardiac complication morbidity; CMT: cardiac
complication mortality; N-CMT: non-cardiac complication mortality; the
vertical dotted line represents the base-case ICER, and the vertical
solid line represents the payment threshold. |
For
DFX versus DFO (tornado diagram A.), the patient weight and DFX drug
cost resulted in an ICER<payment threshold, indicating that DFO was not
cost-effective.
For DFX versus DFP (tornado diagram B.), the
patient weight and DFX drug cost resulted in an ICER<payment threshold,
indicating that DFP was not cost-effective.
For DFX versus DFO+DFP
(tornado diagram C.), the DFO drug cost, DFX dose, utility without
cardiac complication of DFO+DFP, utility without cardiac complication
of DFX, DFP drug cost, patient weight, utility with cardiac
complication of DFO+DFP resulted in an ICER>payment threshold,
indicating that DFX was not cost-effective.
For DFO+DFP versus DFO
(tornado diagram D.), the utility without cardiac complication of DFO,
utility without cardiac complication of DFO+DFP and patient weight
resulted in an ICER<payment threshold, indicating that DFO was
cost-effective.
For DFO+DFP versus DFP (tornado diagram E.), the
utility without cardiac complication of DFO+DFP, utility without
cardiac complication of DFP, and patient weight resulted in an
ICER<payment threshold, indicating that DFP was cost-effective.
For
DFP versus DFO (tornado diagram F.), the cardiac complication mortality
of DFP, cardiac complication morbidity of DFP, utility with cardiac
complication of DFO, DFO drug cost resulted in an ICER>payment
threshold, indicating that DFP was not cost-effective.
Probabilistic sensitivity analysis. Figure 3 shows
the results of the probabilistic sensitivity analysis, represented by
scatter plots. We simulated 10,000 sets of doses, costs, utilities, and
transition probabilities estimated for each strategy by simultaneously
sampling from the assigned probability distributions of the variables.
At the payment threshold, 193,932.00 CNY/QALY, compared with DFX,
DFO+DFP, and DFO, the likelihood of DFP being cost-effective was
97.32%, 99.43% and 58.04%. Compared with DFX and DFO+DFP, the
likelihood of DFO being cost-effective was 92.84% and 98.01%. Compared
with DFO+DFP, the likelihood of DFX being cost-effective was 53.97%.
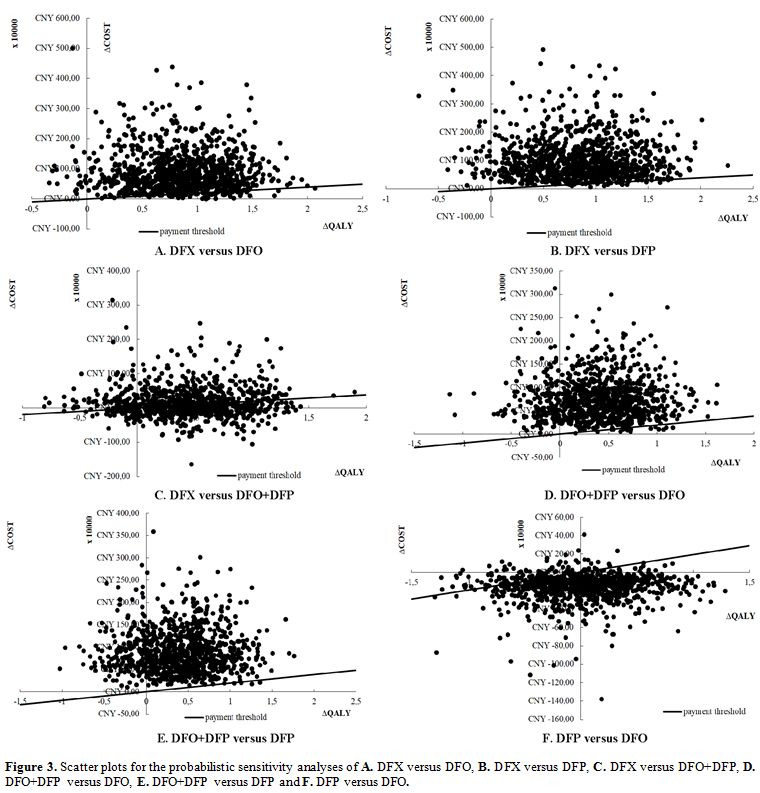 |
Figure 3. Scatter plots
for the probabilistic sensitivity analyses of A. DFX versus DFO, B. DFX
versus DFP, C. DFX versus DFO+DFP, D. DFO+DFP versus DFO, E. DFO+DFP
versus DFP and F. DFP versus DFO. |
The influence of key parameters of the one-way sensitivity analysis. The calculation results of the influence of key parameters of the one-way sensitivity analysis were shown in Table 3.
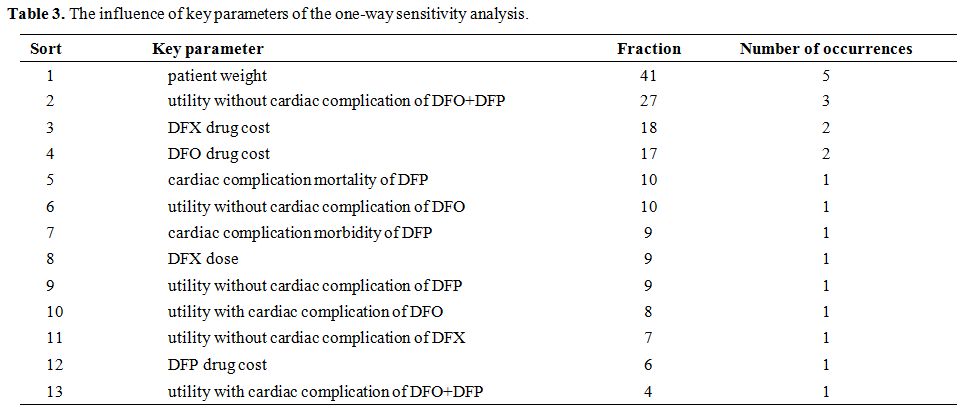 |
Table 3. The influence of key parameters of the one-way sensitivity analysis. |
Discussion
In
this study, an analysis was conducted to evaluate the cost-utility of
the four chelation regimens for β-TM from the Chinese healthcare system
perspective. The results from the base-case analysis indicated that DFP
was the most cost-effective chelation regimen, followed by DFO, DFX,
and DFO+DFP. As a result, using DFP as the primary treatment regimen
for β-TM patients has the potential to result in cost-savings and QALY
gains for the Chinese healthcare system.
A systematic literature review of the cost-effectiveness of the four chelation regimens for β-TM[19]
showed that DFP was the dominant strategy, which was consistent with
the findings of this study. In the systematic literature review, DFX
and DFO were tied as the second most cost-effective treatment regimen,
and we could not judge which one was more cost-effective. In our study,
DFO was the second most cost-effective treatment regimen, and DFP was
the third. The systematic literature review and our study both thought
DFO+DFP was the least cost-effective treatment regimen.
The
one-way sensitivity analysis results and the influence of key
parameters of the one-way sensitivity analysis reported that the
utility without cardiac complication of DFO+DFP, DFX drug cost, DFO
drug cost, cardiac complication mortality of DFP and the utility
without cardiac complication of DFO were important. As a result, we
have some suggestions to save more on costs or to obtain more QALYs.
Firstly, for the Chinese government, DFX and DFO drug cost needs to be
reduced. The available measures included incorporating these drugs into
the National Reimbursement Drug List, negotiating drug prices, imposing
a zero tariff on imported chelators, and encouraging the development of
generic drugs. For Chinese clinicians, the following measures can be
taken: (1) choosing the DFX and DFO that is cheaper or that is
incorporated into the National Reimbursement Drug List to make the drug
cost lower, (2) acting in accordance with patients’ parameters to make
the utility of drugs higher(especially DFO+DFP), and (3) standardizing
the use of chelators to reduce morbidity and mortality (especially DFP).
The
results of the probabilistic sensitivity analysis showed that the
likelihood of DFX being cost-effective compared to DFO+DFP was 63.97%,
and the likelihood of DFP being cost-effective compared to DFO was
58.04%. These findings meant that changes in influential parameters
easily changed the results of DFX versus DFO+DFP and DFP versus DFO.
The key parameters of DFX versus DFO+DFP and of DFP versus DFO were
shown in Figure 2. As a result, clinicians should pay more attention to the key parameters to save more on costs or to obtain more QALYs.
Generally,
one utility is used for oral iron chelation therapy (DFP or DFX), and a
different utility is used for the subcutaneous infusion. However, in
our study, the utility of DFP was different from DFX. The main reason
for the difference was that some patients who used DFP had experienced
severe adverse reactions. In other papers, this difference also
existed. Luangasanatip et al. reported that the utility of DFP and DFX
was 0.61 and 0.85, respectively.[26]
We needed
data on the cardiac complication morbidity, cardiac complication
mortality, and non-cardiac complication mortality of the four chelation
regimens from China (including Mainland China, Hong Kong, Macao, and
Taiwan) to input them into the Markov model. Unfortunately, at the time
of the study, there were not enough clinical data from China. To ensure
the quality of this study, we performed a systematic literature review
and used clinical data from the rest of the world. The clinical data
may vary among different races. Therefore, they may bias the results.
Conclusions
In
this study, DFP was the most cost-effective chelation regimen for β-TM
patients, followed by DFO, DFX, and DFO+DFP. As the most cost-effective
treatment, using DFP as the primary treatment regimen has the potential
to result in cost-savings and QALY gains for the Chinese healthcare
system. To save more on costs or to obtain more QALYs, the Chinese
government should take measures to lower DFX and DFO drug costs, and
Chinese clinicians should choose the cheaper DFX and DFO, increase the
utility of DFO+DFP and reduce mortality and morbidity of DFP. Changes
in key parameters easily affect the results of DFX versus DFO+DFP and
of DFP versus DFO; clinicians should focus on such parameters and
adjust the regimens accordingly.
References
- Origa R. β-thalassemia. Genetics in Medicine. 2017
Jun;19(6):609-619. doi: 10.1038/gim.2016.173. Epub 2016 Nov 3.
https://doi.org/10.1038/gim.2016.173 PMid:27811859
- Ali TT, David JW, Maria DC, Thalassaemia. Lancet 2018; 391: 155-67. https://doi.org/10.1016/S0140-6736(17)31822-6
- Colah
R , Gorakshakar A , Nadkarni A . Global burden, distribution and
prevention of β-thalassemias and hemoglobin E disorders. Expert Review
of Hematology. 2010,3(1):103-117. https://doi.org/10.1586/ehm.09.74 PMid:21082937
- Weatherall,
D. J . The inherited diseases of hemoglobin are an emerging global
health burden[J]. Blood, 2010, 115(22):4331-4336. https://doi.org/10.1182/blood-2010-01-251348 PMid:20233970 PMCid:PMC2881491
- De
Sanctis V., Kattamis C., Canatan D., Soliman A. T., Elsedfy H., Karimi
M., Daar S., Wali Y., YassinM., Soliman N., Sobti P., Al Jaouni S., El
Kholy M., Fiscina B., Angastiniotis M. β-thalassemia distribution in
the old world: an ancient disease seen from a historical standpoint.
Mediterr J Hematol Infect Dis 2017, 9(1): e2017018 https://doi.org/10.4084/mjhid.2017.018 PMid:28293406 PMCid:PMC5333734
- Beijing
Angel Mom Charity Foundation, China Siyuan Foundation for Poverty
Alleviation, China Philanthropy Research Institute of Beijing Normal
University. Blue Paper of Thalassemia in China. 1st Ed. China Social Publishing House.2016.
- Ee
TA, von Riedemann S, Tricta F. Cost-utility of chelators in
transfusion-dependent β-thalassemia major patients: a review of the
pharmacoeconomic literature J. Expert Review of Pharmacoeconomics &
Outcomes Research, 2014, 14(5):651-60. https://doi.org/10.1586/14737167.2014.927314 PMid:24918168
- Cappellini
MD, Cohen A, Porter J, Taher A, Viprakasit V. Guidelines for the
management of transfusion dependent thalassaemia (TDT). 3rd edn.
Nicosia, Cyprus: Thalassaemia International Federation, 2014.
- Hatzipantelis
E S, Karasmanis K, Perifanis V, et al. Combined Chelation Therapy with
Deferoxamine and Deferiprone in β-Thalassemia Major: Compliance and
Opinions of Young Thalassemic Patients. Hemoglobin, 2014,
38(2):111-114. https://doi.org/10.3109/03630269.2013.867407 PMid:24351163
- Delea
TE, Edelsberg J, Sofrygin O, et al. Consequences and costs of
noncompliance with iron chelation therapy in patients with
transfusion-dependent thalassemia: a literature review. Transfusion,
2010, 47(10):1919-1929. https://doi.org/10.1111/j.1537-2995.2007.01416.x PMid:17880620
- Subspecialty
Groups of Hematology, Society of Pediatrics, Chinese Medical
Association; Editorial Board of Chinese Journal of Pediatrics.
Guidelines for the diagnosis and treatment of beta-thalassemia major.
Chinese Journal of Pediatrics. 2018, 56(10):724-729.
- Label for Exjade® revised:06/2010[EB/OL]. http://zy.yaozh.com/instruct/sms201607135/1.jpg. Accessed 14 September 2019.
- Voskaridou
E, Ladis V, Kattamis A, et al. A national registry of
haemoglobinopathies in Greece: deducted demographics, trends in
mortality and affected births. Ann Hematol 2012; 91: 1451-58. https://doi.org/10.1007/s00277-012-1465-7 PMid:22526366
- Pennell
D J , Udelson J E , Arai A E , et al. Cardiovascular function and
treatment in β-thalassemia major: a consensus statement from the
American Heart Association. Circulation, 2013, 128(13):E203-E203. https://doi.org/10.1161/CIR.0b013e31829b2be6 PMid:23775258
- Angelucci
E, Barosi G, Camaschella C, et al. Italian Society of Hematology
practice guidelines for the management of iron overload in thalassemia
major and related disorders. Haematologica, 2008, 93(5):741-752. https://doi.org/10.3324/haematol.12413 PMid:18413891
- Ho
PJ, Tay L, Lindeman R, et al. Australian guidelines for the assessment
of iron overload and iron chelation in transfusion-dependent
thalassaemia major, sickle cell disease and other congenital anaemias.
Internal Medicine Journal, 2011, 41(7):516-524. https://doi.org/10.1111/j.1445-5994.2011.02527.x PMid:21615659
- Paramore
C, Vlahiotis A, Moynihan M, et al. Treatment Patterns and Costs of
Transfusion and Chelation in Commercially-Insured and Medicaid Patients
with Transfusion-Dependent β-Thalassemia. Blood, 2017,130:5635. https://doi.org/10.1016/j.jval.2018.04.1750
- Esmaeilzadeh
F, Azarkeivan A, Emamgholipour S, et al. Economic Burden of Thalassemia
Major in Iran, 2015. Journal of Research in Health Sciences, 2017,
16(3):111.
- Li J., Lin Y., Li X., Zhang
J. Economic evaluation of chelation regimens for β-thalassemia major: a
systematic review. Mediterr J Hematol Infect Dis 2019, 11(1): e2019036.
https://doi.org/10.4084/mjhid.2019.036 PMid:31308912 PMCid:PMC6613630
- People's Bank of China. Current Renminbi Interest Rate Table (October 24, 2015) [EB/OL]. http://www.pbc.gov.cn/zhengcehuobisi/125207/125213/125440/125838/125885/125896/2968988/index.html. Accessed 14 September 2019.
- Liu G E. 2015 China guidelines for pharmacoeconomic evaluations and manual. 1st ed. Beijing: China Science Publishing, 2015.
- National Bureau of Statistics of China. The per capita GDP in 2018[EB/OL]. http://data.stats.gov.cn/easyquery.htm?cn=C01&zb=A0201&sj=2018. Accessed 14 September 2019.
- Jin
J, He Z X, Zhang X H, et al. Endocrine function and fertility in
children with thalassemia. Chinese Journal of Practical Pediatrics
2018, 33(12):977-982.
- Moukalled N.M.,
Bou-Fakhredin R., Taher A.T. Deferasirox: over a decade of experience
in thalassemia. Mediterr J Hematol Infect Dis 2018, 10(1): e2018066,
DOI: 10.4084/mjhid.2018.066 https://doi.org/10.4084/mjhid.2018.066 PMid:30416698 PMCid:PMC6223547
- Lau
E H Y, He XQ, Lee CK, Wu J T. Predicting Future Blood Demand from
Thalassemia Major Patients in Hong Kong. PLoS One. 2013; 8(12): e81846.
Published online 2013 Dec 11. https://doi.org/10.1371/journal.pone.0081846 PMid:24349138 PMCid:PMC3859512
- Luangasanatip
N , Chaiyakunapruk D N , Upakdee N , et al. Iron-Chelating Therapies in
a Transfusion-Dependent Thalassaemia Population in Thailand. Clinical
Drug Investigation, 2011, 31(7):493-505. https://doi.org/10.2165/11587120-000000000-00000 PMid:21627338
- Society
of Hematology, Chinese Medical Association; Branch of Hematology
Physicians, Chinese Medical Doctor Association. Chinese Expert
Consensus on Diagnosis and Treatment of Iron Overload. Chinese Journal
of Hematology. 2011, 32(8):572-574.
- Label for Desferal® revised:03/2017[EB/OL] http://zy.yaozh.com/instruct/20180606sms/a432.pdf. Accessed 14 September 2019.
- US Food and Drug Administration. Label for Ferriprox® revised:02/2015[EB/OL]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/021825s003lbl.pdf. Accessed 14 September 2019
- Hong
Y . Analysis of Complications of Type 2 Diabetes in Patients with
Treatment Costs. Guide of China Medicine, 2015. 13(14):21-22.
- Ho
W L, Chung K P, Yang S S, et al. A pharmaco-economic evaluation of
deferasirox for treating patients with iron overload caused by
transfusion-dependent thalassemia in Taiwan. Journal of the Formosan
Medical Association, 2013, 112(4):221. https://doi.org/10.1016/j.jfma.2011.08.020 PMid:23537869
- The
Monetary Policy Department of The People's Bank of China, On March 18,
2020, China Foreign Exchange Exchange Trading Center was authorized to
announce the announcement of the mid-price of CNY exchange rate[EB/OL].
http://www.pbc.gov.cn/zhengcehuobisi/125207/125217/125925/3990935/index.html. Last retrieved on March 18, 2020.
- Kuo
H T , Tsai M Y , Peng C T , et al. Pilot Study on the "Quality of Life"
as Reflected by Psychosocial Adjustment of Children with Thalassemia
Major Undergoing Iron-Chelating Treatment in Western Taiwan.
Hemoglobin, 2006, 30(2):291-299. https://doi.org/10.1080/03630260600642641 PMid:16798654
- Gao
H Y , Li Q , Chen J J , et al. Curative effects and safety of
deferasirox in treatment of iron overload in children with
β-thalassemia major. Chinese Journal of Contemporary Pediatrics, 2011,
13(7):531.
- Belhoul K M , Bakir M L ,
Kadhim A M , et al. Prevalence of iron overload complications among
patients with b-thalassemia major treated at Dubai Thalassemia Centre.
Annals of Saudi Medicine, 2013, 33(1):18-21. https://doi.org/10.5144/0256-4947.2013.18 PMid:23458935 PMCid:PMC6078582
- Atiq
M, Bana M, Ahmed U S, et al. Cardiac disease in beta-thalassaemia
major: Is it reversible? Singapore Med J, 2006, 47(8):693-696.
- Aleem
A , Al-Momen A K , Al-Harakati M S , et al. Hypocalcemia due to
Hypoparathyroidism in β-Thalassemia Major Patients. Annals of Saudi
Medicine, 1999, 20(5-6):364-366. https://doi.org/10.5144/0256-4947.2000.364 PMid:17264623
- Ayyub
M , Ali W , Anwar M , et al. Efficacy and adverse effects of oral iron
chelator deferiprone (l1, 1,2- dimethyl-3-hydroxypyrid-4-one) in
patients with beta thalassaemia major in Pakistan. Journal of Ayub
Medical College Abbottabad Jamc, 2005, 17(4):12-15.
- Tanner
M A , Galanello R , Dessi C , et al. Combined chelation therapy in
thalassemia major for the treatment of severe myocardial siderosis with
left ventricular dysfunction. Journal of Cardiovascular Magnetic
Resonance, 2008, 10(1):12. https://doi.org/10.1186/1532-429X-10-12 PMid:18298856 PMCid:PMC2289829
- Fadoo
Z , Baña, Ana M, Khurshid M , et al. Cardiac disease in
beta-thalassaemia major: Is it reversible? Singapore Med J, 2006,
47(8):693-696.
- Hoffbrand A V, Al-Refaie
F, Davis B, et al. Long-term trial of deferiprone in 51
transfusion-dependent iron overloaded patients. Blood,1998, 91(1):
295-300. https://doi.org/10.1182/blood.V91.1.295 PMid:9414297
- Casale
M, Filosa A, Ragozzino A, et al. Long-term improvement in cardiac
magnetic resonance in beta-thalassemia major patients treated with
deferasirox extends to patients with abnormal baseline cardiac
function. Am J Hematol. 2018;1-7. https://doi.org/10.1002/ajh.25370 PMid:30489651
- Sun
Y X , Liu Y J , Liu T . Economic evaluation of human papillomavirus
vaccine versus Chinese women aged 18 to 25 for treating cervical
cancer. Chinese Journal of Evidence-Based Medicine, 2017.
- Xu
H G, Fang J P, Huang S L, et al. Evaluation of needle liver biopsy and
pathological features of liver in children with β-thalassemia major.
Chinese Journal of Reproductive Health, 2001, 012(003):123-125,130.
- Zeng
C L, Chen G H, Wei M M, et al. Detection of glycometabolism indexes and
study on the pathogenesis of diabetes mellitus in children with severe
thalassemia. Journal of Xuzhou Medical University 2019, 39(01): 38-41.
- Liang
L Y , Lao W Q , Meng Z , et al. Analysis of the influence of iron
overload in glucose metabolism in thalassemia major patients. Zhonghua
er ke za zhi. Chinese Journal of Pediatrics, 2017, 55(6):419-422.
- Zhang
Y, Chen S Y, Fang M C, et al. The monitoring and analysis of the
endocrine function and growth of patient with β-thalassemia major.
Jilin Medical Journal, 2017,38(12):2239-2241.
- Yang
W J, Wang K, Wu Y F, et al. Clinical Analysis of A Endocrine Detection
in 77 Cases of Thalassemia Major in Guilin City of Guangxi. Guangxi
Medical Journal, 2013, 35(9):1138-1141.
- Huang
Y L, Zhao X Y, Liu S, et al. Evaluation of calcium and phosphorus
metabolism and parathyroid function in children with β-thalassemia
major. Guangxi Medical Journal, 2010, 31(13): 1713-1715.
- Duan
L, Li T, Wang F. Relationship between thyroid function and iron
overload in children with β-thalassemia major. 2020, 28(1): 86-89.
- Yang
S J, Dong H J. A study on direct economic burden of chronic hepatitis B
- relevant diseases. Zhejiang Prev Med, 2015, 27(1):1-9.
- Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics. 2000;17(5):479-500. https://doi.org/10.2165/00019053-200017050-00006 PMid:10977389
- Briggs
AH, Goeree R, Blackhouse G, O'Brien BJ. Probabilistic analysis of
cost-effectiveness models: choosing between treatment strategies for
gastroesophageal reflux disease. Med Decis Making. 2002;22(4):290-308 https://doi.org/10.1177/027298902400448867 PMid:12150595
[TOP]





