Giuseppe Lassandro1, Valentina Palladino1, Anna Amoruso1, Viviana Valeria Palmieri1, Giovanna Russo2 and Paola Giordano1.
1 Department of Biomedical Science and Human Oncology-Pediatric Unit, University of Bari "Aldo Moro," Bari, Italy.
2 Pediatric Hemato-Oncology Unit, Department of Clinical and Experimental Medicine, University of Catania, Catania, Italy.
Correspondence to: Paola Giordano. Department of Biomedical Science and
Human Oncology, Pediatric Unit, University of Bari "Aldo Moro," Bari,
Italy. Tel: +390805592950. E-mail:
paola.giordano@uniba.it
Published: July 1, 2020
Received: May 4, 2020
Accepted: June 13, 2020
Mediterr J Hematol Infect Dis 2020, 12(1): e2020042 DOI
10.4084/MJHID.2020.042
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Human
coronaviruses (HCoVs) commonly cause mild upper-respiratory tract
illnesses but can lead to more severe and diffusive diseases. A variety
of signs and symptoms may be present, and infections can range in
severity from the common cold and sore throat to more serious laryngeal
or tracheal infections, bronchitis, and pneumonia. Among the seven
coronaviruses that affect humans (SARS)-CoV, the Middle East
respiratory syndrome (MERS)-CoV, and the most recent coronavirus
disease 2019 (COVID-19) represent potential life-threatening diseases
worldwide. In adults, they may cause severe pneumonia that evolves in
respiratory distress syndrome and multiorgan failure with a high
mortality rate. Children appear to be less susceptible to develop
severe clinical disease and present usually with mild and aspecific
symptoms similar to other respiratory infections typical of childhood.
However, some children, such as infants, adolescents, or those with
underlying diseases may be more at-risk categories and require greater
caution from clinicians. Available data on pediatric coronavirus
infections are rare and scattered in the literature. The purpose of
this review is to provide to clinicians a complete and updated panel
useful to recognize and characterize the broad spectrum of clinical
manifestations of coronavirus infections in the pediatric age.
|
Introduction
Human
coronaviruses (HCoVs) are a large group of viruses that commonly causes
mild upper-respiratory tract illnesses but can lead to more severe and
diffusive diseases. A variety of signs and symptoms may be present, and
infections can range in severity from the common cold and sore throat
to more serious laryngeal or tracheal infections, bronchitis, and
pneumonia. Coronaviruses are known to circulate in many different
animal species such as mammals and birds that can represent
intermediate hosts and animal reservoirs for human infections.
Coronaviruses, belonging to the family Coronaviridae, are enveloped,
positive-sense, single-stranded RNA (ribonucleic acid) viruses
so-called for their corona- or crown-like surface projections. They are
further classified into four genera: Alpha- and Betacoronavirus
(typical in bats, rodents, civets, and humans), Delta- and
Gammacoronavirus (mainly detected in birds). Their typical sizes range
from 80 to 120 nm. The genome encodes for two nonstructural replicase
polyproteins and four or five structural proteins, including the spike
(S), envelope (E), membrane (M), nucleocapsid (N), and sometimes a
hemagglutinin-esterase protein (HE). The HE protein binds to specific
receptors and guides membrane fusion; the S protein is responsible for
cell entry, the M and E proteins mediate viral assembly process, the
inner N protein develops ribonucleoprotein complexes binding to viral
RNA.[1-5]
To date, seven coronaviruses affect humans: in 1960s HCoV-229E and HCoV-OC43 were firstly reported;[6,7] HCoV-NL63 and HCoV-HKU1 were discovered subsequently in 2004 and 2005, respectively.[8,9]
Additionally, three HCoVs responsible for outbreaks involving high case
fatality rates have been detected in humans in the last two decades:
the severe acute respiratory syndrome (SARS)-CoV, the Middle East
respiratory syndrome (MERS)-CoV and the new coronavirus disease 2019
(COVID-19) (Table 1).
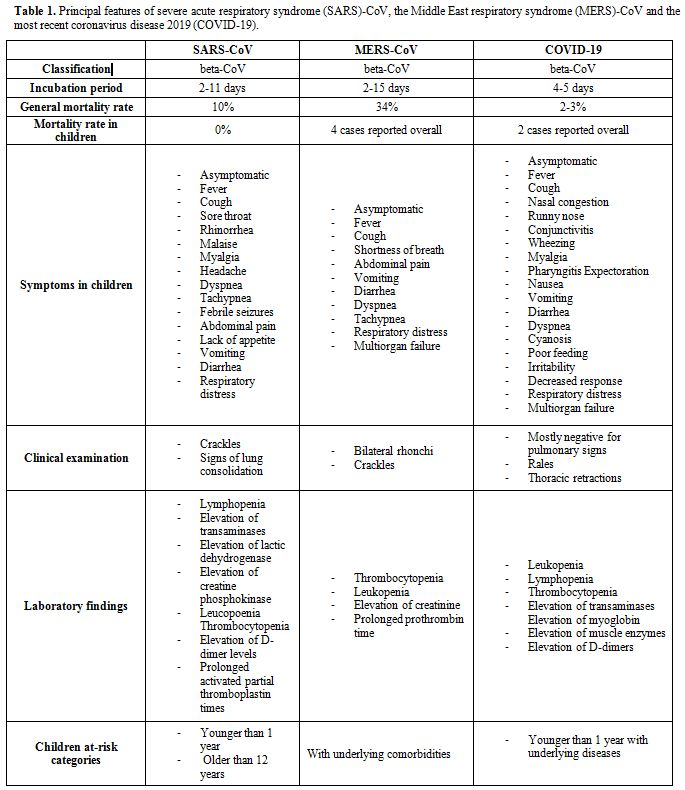 |
Table 1. Principal
features of severe acute respiratory syndrome (SARS)-CoV, the Middle
East respiratory syndrome (MERS)-CoV and the most recent coronavirus
disease 2019 (COVID-19). |
HCoV-OC43,
HCoV-HKU1, SARS, MERS, and COVID-19 belong to beta coronaviruses while
HCoV-229E, HCoV-NL63 belong to alphacoronaviruses.[10-12]
HCoVs can infect all age groups. Generally, children appear to be less
susceptible to coronavirus infections with milder symptoms and a more
favorable clinical course than the adult population. In addition,
coronavirus infections in children often have peculiar clinical
features that differentiate them from those of adults. Available data
on pediatric coronavirus infections are scattered in the literature.
The purpose of this review is to provide to clinicians a complete and
updated panel useful to recognize and characterize the broad spectrum
of clinical manifestations of coronavirus infections in the pediatric
age.
Endemic Coronavirus in Children
Before
SARS and MERS epidemics and the recent COVID-19 pandemic, coronaviruses
used to be considered commonly responsible for mild respiratory
diseases as the common cold. Generally, their median incubation period
is three days. Respiratory droplets are the usual route of
transmission. Hand contamination and transferal from surfaces and
objects are also implicated.[13] Children under the age of 3 years or with cardiac disease appear most frequently affected.[14-16]
The
most common symptoms are rhinorrhea, sore throat, fever, and dry cough,
but there is increasing evidence that coronaviruses are also important
causes of more severe respiratory diseases including bronchitis,
bronchiolitis, asthma exacerbations and pneumonia in children.[17]
About
the detection frequency, the most common strains in alternate seasons
are HCoV-OC43 and HCoV-229E followed by HCoV-NL63, and HCoV-HKU1.[18] Common circulating HCoVs can be isolated from 5% to 13% of children hospitalized for acute respiratory tract infections.[19-22]
Frequently,
respiratory pediatric coronavirus infections are associated with
multiple infections caused by other common viruses, but the clinical
significance of these coinfections is unclear. Coinfections between
coronaviruses and other respiratory viruses such as respiratory
syncytial virus, human metapneumovirus, adenovirus, and influenza or
parainfluenza viruses have been reported in up to 40% of cases.[23-30]
Especially
in younger children, the virus most frequently associated with human
coronaviruses infections is a respiratory syncytial virus (RSV)
probably because of a season overlapping.[31,32]
Among the respiratory infections caused by coronaviruses in children, a
strong association between HCoV-NL63 and croup is also highlighted,[33] whereas HCoV-NL63 and HCoV- HKU1 appear associated with bronchiolitis and wheezing.[34,35]
Although the possible pathogenic role of coronaviruses in pediatric
respiratory infections has been hypothesized, and this has not yet been
confirmed. In several studies a similar prevalence in the detection of
HCoVs in patients with respiratory symptoms compared to healthy
children has been found.[36-39] Moreover, patients
with other underlying medical conditions or immunocompromised appear
more susceptible to developing severe infections than healthy patients.[40-43] Additionally, human coronaviruses are responsible for other common childhood diseases such as acute otitis media[44-47], asthma exacerbations,[48] and conjunctivitis.[8]
They have also been involved in nosocomial infections, especially in
the neonatal intensive care units (NICU). Gagneur et al. in a
prospective study determined the incidence of HCoV-related respiratory
infections in newborns hospitalized in a NICU. Among 64 neonates, seven
positive nasal samples for HCoVs (11%) were detected. All children were
symptomatic. Oxygen and ventilatory support were frequently needed.[49]
Sizun et al. evaluated the clinical role of coronaviruses respiratory
infections in premature newborns. All premature infants infected had
severe respiratory symptoms, including bradycardia, apnea, and
hypoxemia, while chest X-ray revealed diffuse infiltrates.[50]
It has also been shown that coronavirus infections are not only
responsible for respiratory symptoms but can also affect other organs
and systems in children. Several studies have also reported that
respiratory symptoms caused by coronavirus infection may be associated
with central nervous system (CNS) involvement. HCoVs have an intrinsic
capacity to affect neurons and diffuse centrifugally from CNS via the
transneuronal route.[51,52]
Among neurological
symptoms, febrile seizures, convulsions, loss of consciousness,
encephalomyelitis, and encephalitis have been reported.[53-55]
Primarily in 1980, the viral genome was detected post-mortem in the
cerebrospinal fluid of two patients with multiple sclerosis (MS).[56]
Subsequently, the HCoVs neuroinvasion capacity was confirmed in a large
panel of human brain autopsy samples affected by MS and other
neurological diseases.[57] In 2004, Yeah et al.
reported a case of a child with acute disseminated encephalomyelitis in
which the genome of HCoV-OC43 in cerebrospinal fluid was detected.[55]
In 2016, Li et al. demonstrated the presence of anti-CoV IgM
(immunoglobulin M) in 22 (12%) of 183 children with acute encephalitis.[58]
In 2017, a prospective study on 192 children with febrile seizures
demonstrated that coronaviruses were frequently detected.[59]
Additionally,
HCoVs have been implicated as possible causes of many gastrointestinal
disorders in children, and gastrointestinal symptoms have been reported
in several studies in more than 50% of pediatric patients.[28,60,61] Firstly, HCoVs could be associated with neonatal necrotizing enterocolitis.[62] Furthermore, diarrhea, vomiting or other gastrointestinal symptoms have been associated with coronavirus infections.[63-65]
Besides the demonstrated finding in respiratory swabs, all HCoVs can
also be detected in stool samples of patients affected by
gastroenteritis.[60,66] Moreover,
most of the HCoVs found were coinfections with well-known gastroenteric
viruses, including norovirus and rotavirus. HCoVs may also be found
occasionally in healthy children's stool samples.[67]
Although HCoVs have always been associated with respiratory symptoms,
these findings suggest that other systems may also be involved in
children. The absence of serious symptoms may not be coupled with
serological negativity. Therefore, these viruses should be considered
in the differential diagnoses of most of the common diseases of
childhood.
SARS in Children
The
2002–2004 severe acute respiratory syndrome outbreak was a viral
respiratory illness caused by SARS-CoV. The outbreak firstly emerged in
the southern Chinese province of Guangdong in November 2002 and[68] then spread to 29 countries with 8,096 people infected and 774 died.[69]
The
SARS global outbreak was contained in July 2003. Since 2004, there have
not been any known cases of SARS reported anywhere in the world.[70]
Probably, civet cats or bats could be the initial step of the
transmission to humans. Humans to humans infection occurs by
respiratory droplets or direct contact. Healthcare or household
contacts are critical routes of transmission.[71,72]
SARS-CoV infection cases were classified by the World Health Organization (WHO) into suspected, probable, and confirmed (Table 2).[73]
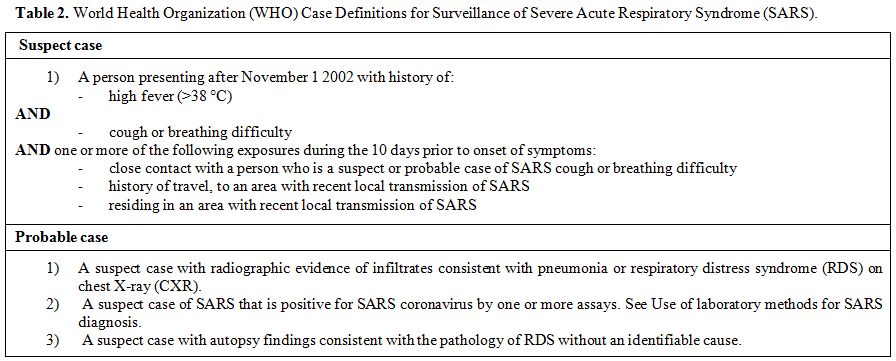 |
Table 2. World Health Organization (WHO) Case Definitions for Surveillance of Severe Acute Respiratory Syndrome (SARS). |
The
median incubation period ranges between 2-11 days. SARS causes atypical
pneumonia, which may progress to respiratory failure. Symptoms include
fever, malaise, myalgia, headache, diarrhea, and rigors. Adults are
more likely to develop severe illness characterized by dyspnea,
lymphopenia, acute respiratory distress syndrome (ARDS), and a fatal
clinical course in 10% of cases. The exact number of children affected
by SARS worldwide is unknown. However, children appear to be less
susceptible to SARS with a lower incidence of the disease and no
reported mortality. The majority of children had documented exposure to
adults with SARS, usually a family member. Most infected children had
previously attended school, but the spread of the infection in the
school environment has not been demonstrated, and this could probably
be linked to lower infectiousness of the virus among children.[74,75]
Children have less severe symptoms than adults, and they rarely need
intensive care. However, subclinical and asymptomatic infections appear
uncommon. Most children reported worldwide were healthy,
previously and underlying conditions were infrequently reported.[75-77]
Usually, children require hospitalization after 3–4 days the onset of
symptoms: fever (90-100%), dry cough (43-80%), sore throat (5-30%),
rhinorrhea (33-60%), malaise and myalgia (10-40%), headache (14-40%)
are common. Dyspnea, tachypnea, and febrile seizures are infrequent.
Aspecific gastrointestinal symptoms, including abdominal pain, appetite
lack, vomiting, and diarrhea, have been reported. Physical examination
at presentation is negative in the majority of children, and chest
auscultation does not reveal significant findings. Moreover, sometimes
crackles or signs of lung consolidation can be detected. As well as the
clinical examination, laboratory findings are not specific in children
with SARS and can be confused with those of other respiratory
infections typical of childhood. Commonly lymphopenia, the elevation of
transaminases, lactic dehydrogenase, and creatine phosphokinase are
detected. Other hematological abnormalities such as leukopenia,
thrombocytopenia, the elevation of D-dimer levels and mildly prolonged
activated partial thromboplastin times are also observed.[78-80]
Circulating interleukin (IL)-1β levels might be increased, resulting in
caspase-1-dependent pathway activation responsible for an exaggerated
and persistent inflammatory response and the consequent respiratory
failure in severe cases.[81] In children, radiological findings are nonspecific and similar to other viral respiratory abnormalities.
Commonly,
the chest X-ray shows ground-glass opacity or focal consolidation.
Linear atelectasis and peribronchial thickening have also been
reported. Computed tomography (CT) shows more extensive airspace
consolidation and ground-glass attenuation than chest X-ray, but it is
performed in selective cases in pediatric age.[78-80,82]
Usually, the clinical course is less severe in children compared to
adults, and few patients require oxygen supplementation and assisted
ventilation but preterm newborns, children younger than one year and
older than 12 years of age have more severe symptoms and are likely to
develop respiratory distress.[78-80] In pediatric
age, SARS infection commonly has a "biphasic" pattern. The first stage
of the disease is characterized by virus replication and clinically by
the onset of symptoms. The second phase is characterized by pulmonary
involvement, which is typically less severe in children than in adults.
Most children will become afebrile within seven days, and they usually
do not progress to respiratory distress, the adult third phase, that is
only reported in a minimal number of cases, commonly among teenagers.[83,84]
In
pregnant women, SARS infection is associated with a high incidence of
spontaneous miscarriage, prematurity, and intrauterine growth
retardation (IUGR). The increased morbidities during pregnancy are
likely to be due to the hypoxic state and circulatory insufficiency
that worsen placental blood flow and cause miscarriage or IUGR.
Significantly, among pregnant women, mortality is 25%.[85]
However, perinatal SARS infections have not been documented. In none
infants born from pregnant women affected, real-time PCR (RT-PCR)
assays and viral cultures conducted on neonatal blood, body secretions
and amniotic fluid were positive for SARS. In infants, no congenital
malformations have been reported. However, in premature newborns,
severe gastrointestinal complications such as jejunal perforation and
necrotizing enterocolitis have been described.[86]
However, it is not known if these neonatal morbidities are related to
prematurity or if maternal infection is a factor that increases their
incidence.
It is unclear why children develop a less serious
disease than adults. Recurrent viral respiratory infections typical of
the pediatric age could be helpful to the immune system in promptly
recognizing and defeating new viral pathogens. Furthermore, the
immaturity of the immune system could be protective because the
inflammatory cascade that causes respiratory failure in adults is more
difficult to activate. Additionally, children generally have fewer
comorbidities than adults.
Children recovered quickly from SARS.
Li et al. assessed the radiological and clinical outcomes of
forty-seven children with SARS after 6 months from diagnosis. All
children were asymptomatic while mild pulmonary abnormalities including
ground-glass opacities and air trappings were found at CT in sixteen
patients.[87]
Although clinical and laboratory
findings of SARS are aspecific in children, certain features can be
useful to distinguish SARS from other respiratory viral infections.
Children with SARS have a lower incidence of rhinorrhea and productive
cough and higher incidence of monocytopenia than children with
influenza.[88] Additionally, serum lactate
dehydrogenase in the presence of a low neutrophil count and low serum
creatine phosphokinase could be suggestive of SARS infection.[89]
SARS
infections in children appear to be a relatively mild and aspecific
disease, and the diagnosis should be accompanied by laboratory
assessment. Although infants and teenagers are more likely to have a
worse clinical course, usually, all pediatric patients recover entirely
without significant long-term sequelae.
Diagnosis of IDA
The
Middle East respiratory syndrome (MERS) is a viral respiratory
infection caused by the MERS-coronavirus (MERS-CoV). The first
identified case occurred in 2012 in Saudi Arabia.[11,90]
Subsequently, a total of 2494 confirmed cases of MERS, including 858
associated deaths with a case–fatality rate of 34% were reported
globally; the majority of these cases were reported from Arabian
Peninsula, and in the Middle East.[91] Currently, MERS is an extremely rare disease: in the last year MERS was signaled only in Saudi Arabia.[92]
MERS-CoV
is a zoonotic virus: dromedary camels are the primary reservoir hosts.
Humans are infected through contact with infected dromedary camels,
animal products, or humans, especially among close contact between
family members and health care workers. MERS-CoV infection cases were
classified by the WHO into suspected, probable, and confirmed (Table 3).[93]
Usually, the mean incubation period ranges from 2 to 15
days. Clinical severity of the disease varies from asymptomatic to
fatal forms, and the impact of asymptomatic spread is unclear. The
infection can cause severe pneumonia, which may progress to ARDS,
respiratory failure, and death, particularly in older people,
immunocompromised patients, and those with chronic diseases. Common
symptoms include fever, cough, and shortness of breath.
Gastrointestinal symptoms (including diarrhea, vomiting, abdominal
pain), pericarditis, septic shock and disseminated intravascular
coagulation have been reported.[94-97] Children
appear to be less susceptible to MERS-CoV infection, and pediatric
cases described in the literature are rare with a low proportion
(0.1%–4%) of infected children.[98-102] Fagbo et al.
demonstrated in a study conducted on 2235 hospitalized children with
respiratory infections that all patients tested were harmful to
MERS-CoV.[102] Khuri-Bulos et al. confirmed the low
incidence of MERS-CoV infection in childhood in a prospective study
conducted in children <2 years of age hospitalized with acute
respiratory symptoms and/or fever. Among these, none of 474 children
tested resulted positive for MERS-CoV.[103]
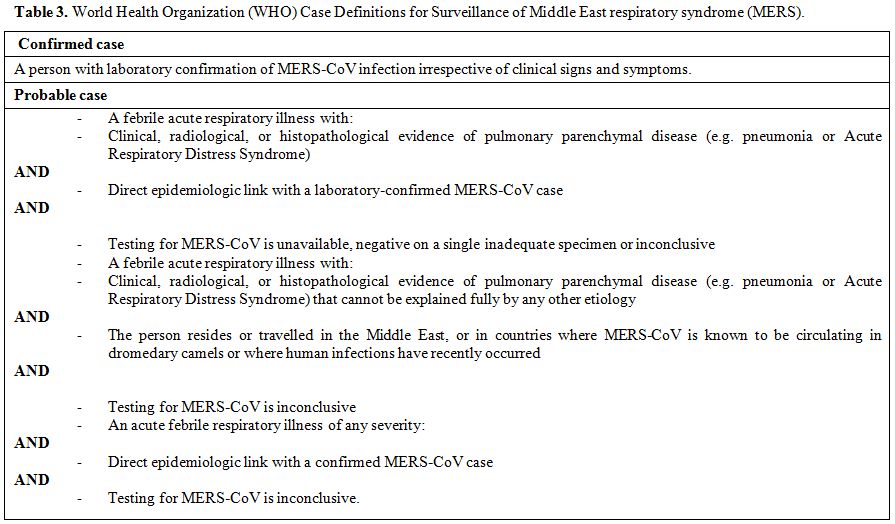 |
Table 2. World Health Organization (WHO) Case Definitions for Surveillance of Middle East respiratory syndrome (MERS). |
In
pediatric age, few cases of MERS CoV infection have been described.
Most of the children were asymptomatic and positive during routine
screening of MERS-CoV. Al-Tawfiq et al. reported a total of 31
pediatric MERS-CoV cases with a mean age of 10 years. Overall, 42% were
asymptomatic, while in symptomatic cases, fever and mild respiratory
symptoms were common.[104] Subsequently, Alfaraj et
al. reported a total of 7 pediatric MERS-CoV cases with a mean age of 8
years. In this case series, common symptoms were fever (57%), cough
(14%), shortness of breath (14%), and gastrointestinal symptoms (28%).
Two (28.6%) patients had abnormal chest radiographic findings with
bilateral infiltration, one (14.3%) required ventilatory support, and
two (28.6%) required supplemental oxygen.[99] Four
with underlying conditions (cystic fibrosis, nephrotic syndrome,
craniopharyngioma, and a right ventricular tumor) had a fatal outcome.
These children developed a critical form of MERS infection complicated
by respiratory and multiorgan failure. Frequently, clinical examination
revealed bilateral rhonchi and crackles while chest X-ray showed
diffuse bilateral infiltrates, ground-glass opacification and pleural
effusion.[105-109] Thrombocytopenia, leukopenia,
increased creatinine and prolonged prothrombin time were the only
laboratory findings reported in literature.[99,105,106]
MERS-CoV
in children is less frequent than adults and appears to be associated
with low mortality unless the patients have underlying comorbidities.
Few cases of MERS-CoV have been reported during pregnancy. A pregnant
woman, aged 39 years, had a stillbirth at approximately five months of
gestation[110] and another woman gave birth to a healthy term baby, but she died after delivery.[107]
In
conclusion, although MERS-CoV represents a clinical concern for the
adult population with a high fatality rate, it remains a sporadic
disease in childhood. Clinicians should learn to recognize and suspect
MERS-CoV infection, as the symptoms and signs are nonspecific, based on
epidemiological criteria to avoid the spread of the disease in patients
at higher risk of worse clinical course.
COVID-19 in Children
The
outbreak of COVID-19 infection (coronavirus disease 2019; previously
2019-nCoV) began in Wuhan, Hubei, China, in December 2019, which then
spread rapidly to other provinces of China and around the world.[111]
On January 30, 2020, the WHO declared the outbreak of a Public Health
Emergency of International Concern and, on March 11, 2020, a pandemic.[112]
As of June 5, 2020, 188 other countries and regions, with more than
6.669.358 confirmed cases, are declared. Among the confirmed cases,
2.904.828 are recovered, and 393.205 died.[113]
Recent genetic analysis suggests the COVID-19 emerged from an animal
source. The full genome sequences showed high homology between
COVID-19, bat coronavirus, and pangolin coronavirus, but further
genetic study is required.
Moreover, according to current evidence, the principal route of transmission of COVID-19 is from human to human.[114,115]
COVID-19 spread between people through respiratory droplets and contact
routes. Droplet transmission occurs when there is close contact with a
person with respiratory symptoms such as coughing or sneezing, who may
spread potentially infectious droplets. Transmission may also occur by
direct contact with infected persons and indirect contact with infected
surfaces or objects. COVID-19 can persist on inanimate objects for days
but can be efficiently inactivated by common disinfectants. Airborne
transmission may be possible when a high risk of aerosolization
procedures are performed, such as endotracheal intubation and
bronchoscopy. The virus is also detected in stool specimens, and
consequently, the feco-oral transmission is also hypothesized.[116-119]
The high transmissibility of COVID-19 may be explained by its
demonstrated presence in the upper respiratory tract of asymptomatic or
presymptomatic subjects with viral loads comparable to those detected
from symptomatic patients. The real proportion of asymptomatic cases is
unclear, ranging from 1% to 78% in different studies. Transmission
from asymptomatic patients infected with COVID-19 most likely
contributed to the rapid and extensive spread of pandemic but further
studies are needed to more accurately estimate the proportion of
genuinely asymptomatic cases and their risk of transmission.[120-126]
COVID-19
has been reported among all age groups. The median incubation period of
COVID-19 infection is 4-5 days with a range up to 24 days.[119,127]
COVID-19 infection case is classified by the WHO into suspected, probable, and confirmed (Table 4).[128]
Clinical severity of the infection varies, ranging from asymptomatic
forms to critical diseases. Common symptoms are fever, dry cough,
malaise, lethargy, shortness of breath, sore throat, and myalgia.
Headache, conjunctivitis, productive cough, and diarrhea are also
described. Mild forms present as a common cold, and severe cases may
worsen in pneumonia that may evolve to ARDS, shock, and multiple organ
dysfunction. More severe clinical pictures are associated with stronger
immune response and with the production of proinflammatory cytokines,
including IL-2, IL-7, IL-10, and tumor necrosis factor- α (TNF-α).
Adverse outcomes are common in elderly patients and those with
underlying diseases. The need for intensive care admission is in 25–30%
of patients. The fatality rate is estimated to range between 2 and 3%.[129-133] About 2% of COVID-19 confirmed cases are children.[124-134,135]
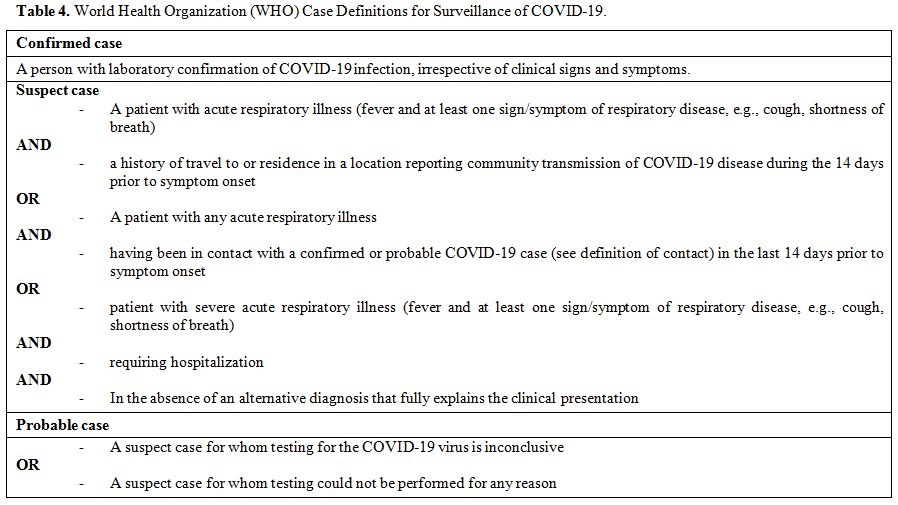 |
Table 4. World Health Organization (WHO) Case Definitions for Surveillance of COVID-19. |
Generally,
children appear to be less likely to develop a severe form of COVID-19
infection, and commonly they have a mild clinical course with a good
prognosis. Few children may evolve into lower respiratory infections.
Probable reasons include having an immune system still immature,
healthier respiratory tract, and less underlying conditions than
adults.[136] Most of them have an infected contact
history with family members. Moreover, children, especially those with
asymptomatic or milder form, may represent significant spreaders.
Pediatric patients appear to be likely as adults to become infected but
are less likely to develop symptoms. However, future studies are needed
to understand the role of children in the transmission of the virus.[137-139]
Current researches show that the median age of infection in pediatric
cases is 6-7 years. In symptomatic cases, symptoms are typical of acute
respiratory infections and frequently included fever (59%) and cough
(46%), which may be accompanied by nasal congestion, runny nose,
conjunctivitis, pharyngitis, wheezing, myalgia, and expectoration. Few
children have an atypical presentation with gastrointestinal
manifestations, including nausea, vomiting, and diarrhea. Low oxygen
saturation of less than 92%, dyspnea, cyanosis, and poor feeding, are
less common than adults. Among infants, symptoms such as irritability,
reduced response, and poor feeding could be the main signs of
infection. Family clustering occurred for all infected infants. Rarely
infants require intensive care or mechanical ventilation or have any
severe complications. Common symptoms of pediatric age are summarized
in figure 1. The majority of children recovers 1–2 weeks after the onset of the disease.
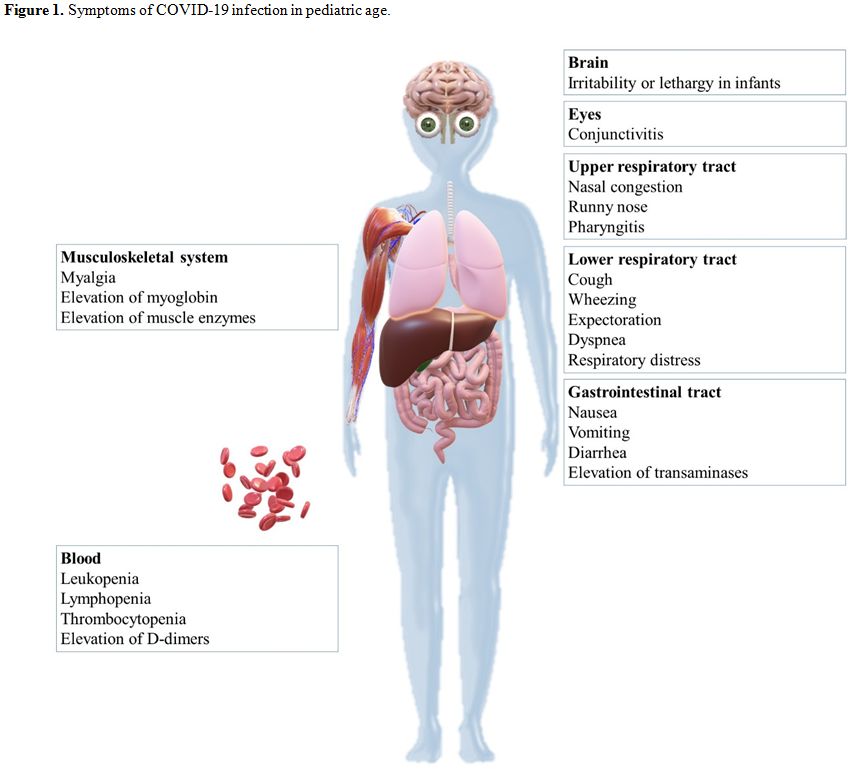 |
Figure 1. Symptoms of COVID-19 infection in pediatric age. |
Regarding
biochemical results, leukopenia and lymphopenia are frequent in
children. Elevation of transaminases, myoglobin, muscle enzymes, and
D-dimers might be seen in severe cases.[140-146]
Dong et al. reported that 94% of 2143 pediatric patients affected by
COVID-19 developed an asymptomatic, mild, or moderate form of
infection. A severe disease characterized by dyspnea, central cyanosis,
and oxygen saturation of less than 92% was reported in 5% of cases. A
critical disease characterized by ARDS and multiple organs failure was
reported in less than 1% of cases.[141] The
prevalence of severe and critical disease appears higher in younger
children, particularly in children aged <1-year-old and in children
with underlying diseases. To date, death was an uncommon event reported
in one 10-month-old infant with intussusception and multiorgan failure
and in one 14-year-old boy.[145,147]
Other
systemic symptoms appear to be related to the infection, but their link
has not yet been demonstrated. Since the outbreak of the pandemic, a
large number of rashes, urticaria, and vasculitis affecting hands and
feet of healthy children and adolescents have been reported as well as
itching, burning, difficulty in joint movements and pain.[142]
Recently,
the relationship between COVID-19 infection and the development of
cardiac diseases in children has been hypothesized. Belhadjer et al.
have reported a large number of febrile children resulted positive for
COVID-19 admitted in intensive care units for acute heart failure
associated with a multisystem inflammatory state. In most of the
children, clinical features appeared similar to those of Kawasaki
syndrome: lasting fever, cutaneous rash, lymphadenopathy, persistent
activation of systemic inflammation and positive response to
intravenous immunoglobulin.[148] Similar clinical features have subsequently been reported in children with COVID-19 positive serology.[149,150]
As
in COVID-19 infection, Kawasaki syndrome is triggered by
proinflammatory cascade activated primarily by innate immunity
response. However, further studies are needed to establish the real
pathogenetic relationship between emerging COVID-19 and Kawasaki-like
syndromes.[151]
Among radiological findings,
ground-glass opacity, mono or bilateral infiltrates, mesh shadows, and
tiny nodules are frequently detected. In severe cases, radiological
alterations are diffused, presenting as a "white lung." However,
radiologic evidence of pneumonia might be absent in 15-20% of children.[139,140,152-156]
In selected cases, lung ultrasound might be useful in the managing and
follow-up of COVID-19 infection. This radiological technique can
precociously identify abnormalities including small pleural effusion
and subpleural consolidation and appear more available then X-ray and
CT.[157-158]
Clinical examination appears
mostly negative for pulmonary signs, and in rare cases, rales and
thoracic retractions have been reported.[154]
Whether pregnant women and children born to affected mothers are more
likely to have a worse outcome is currently unclear. Maternal-infant
vertical transmission has not been documented. Amniotic fluid, cord
blood, neonatal throat swab, and breastmilk samples from newborns
delivered by infected women were tested for COVID-19, and all samples
tested negative.[159] Data on the maternal and
perinatal outcomes of pregnant women infected with COVID-19 is limited.
Most pregnant women with COVID-19 present with fever and coughing.
Severe and critical maternal symptomatology have also been reported,
but no women died. The most common adverse pregnancy outcome is
preterm birth, occurring in 41% of cases while the rate of perinatal
death is 7%, including one case of stillbirth and one neonatal death.
There is no data on miscarriage for COVID-19 occurring during the first
trimester. In more than a third of cases, fetal distress and frequent
admission neonatal intensive care units have been reported.[160,161]
Rarely, cases of COVID-19 positivity in newborns have been reported.
Common symptoms are fever, cough, lethargy, and vomiting milk. Mottled
skin and moderate respiratory distress presented with tachycardia,
tachypnoea, subcostal retractions, and low oxygen saturation are also
described in newborn babies.[162-166] Although it
can be severe in some cases, compared with SARS-CoV and MERS-CoV,
COVID-19 causes less severe disease in children. A recent meta-analysis
shows that children infected with COVID-19 have less fever than that
other epidemic HCoVs.[167]
Despite the rapid
worldwide spread of COVID-19 infection, additional data are needed to
define the severity of the disease in children. The severity of the
symptoms and the mortality rate will be better assessed in the future.
Diagnosis and Treatment of HCoVs Infections
Differential
diagnosis with common viral respiratory infections of childhood, such
as influenza virus, adenovirus, respiratory syncytial virus, and
metapneumovirus, should be considered. In the diagnosis of suspected
cases, epidemiological and clinical criteria must be assessed.[73,93,138]
RT-PCR
represents the gold standard to confirm the diagnosis of HCoVs
infections performed on samples of respiratory secretions.[168-174] The viral load is higher in lower respiratory tract secretion samples than in upper respiratory tract samples.
Therefore,
suspected cases resulted in firstly negative could be re-tested with a
second swab, better if with a low respiratory sampling is performed as
proved for SARS and MERS infection.[175,176]
Currently,
few data have been published about the sensitivity and specificity of
RT-PCR nasopharyngeal swabs for COVID-19. In vitro analyses suggest
that the RT-PCR test is highly specific and sensitive.[177]
In vivo, sensitivity is estimated to be higher than 70% but seems to be
lower for "mild" cases while specificity is close to 100%.[178,179]
Accuracy
of RT-PCR swabs in clinical practice differs depending on the site and
quality of the sample. Taking swabs from children may be more difficult
given the intrusive nature of the procedure and further reduce the
specificity and sensitivity of the test. RT-PCR of bronchoalveolar
lavage fluid appears the most accurate technique of virologic
confirmation, but it may not always be easily collected in all
patients, especially in pediatric age. Although a negative test cannot
currently rule out the disease, further studies are needed to define
the exact specificity and sensitivity of RT-PCR nasopharyngeal swabs.[180,181]
Moreover, RT-PCR appears to be useful in virus detection on stool samples.[116]
To date, serology is not considered a diagnostic method. Although most
patients with COVID-19 appear positive for immunoglobulin-G (IgG)
within 19 days while IgM reaches a peak 20–22 days after symptom onset,
the serological response is not useful for early individuation of
positive patients.[182] Additionally, numerous cross-reactions occur between COVID-19 and common HCoVs,[183]
and protective immunity against COVID-19 is not proved. Despite
its potential role in supporting RT-PCR in the diagnosis of COVID-19,
the clinical and immunological meaning of serology is still unclear.[184]
The
spread of the infection can be prevented if children and family members
were educated about proper hygienic practices and infection control
measures, including regular hand washing, cover the mouth with napkin
or towel when coughing or sneezing, avoid crowded places and contact
with sick people. Children with HCoVs should receive early
supportive therapy and continuous monitoring. Additional oxygen,
caloric, and hydro electrolytic support should be performed if
necessary. Frequent checks of oxygen saturation and hematological,
urinary, and biochemical parameters, including liver, kidney,
myocardial enzymes, and coagulation parameters should be analyzed.
Finally, blood gas analysis and radiological diagnostics of the chest
should be done when necessary. This strategy could be useful in the
prevention of ARDS, multiorgan failure, and other nosocomial infections
possibly treated, if bacterial, with appropriate antibiotics. In
critical cases, mechanical ventilation with endotracheal intubation and
other more invasive interventions, such as blood purification and
extracorporeal membrane oxygenation (EMCO), should be adopted.
Additionally, the use of antiviral drugs in children with severe HCoVs
infections may help to reduce viral load and the duration of symptoms.
However, their safety and real effectiveness have not yet been proven.
Interferon alfa and beta, corticosteroids, lopinavir/ritonavir, and
ribavirin, were used in the treatment of SARS-CoV and MERS- CoV in
adults and children.[75,76,78,185]
However, ribavirin can cause hemolytic anemia and liver dysfunction, as
well as corticosteroids, increase the risk of iatrogenic immune
immunosuppression.[186] To date, there is no
evidence regarding the management and treatment of COVID-19 infection
in children. In addition to supportive therapy, the use of nebulized
interferon-alpha-2b and oral lopinavir/ritonavir together with
corticosteroids for complications and hydroxychloroquine or intravenous
immunoglobulin for severe cases have been suggested.[145,187,188]
Recently, a position paper of the Italian Society of Pediatric
Infectious Disease on the treatment of children with COVID-19 infection
has been published.[189] In asymptomatic or
mild cases, only antipyretic therapy is recommended. In severe or
critical cases, the use of hydroxychloroquine ± azithromycin or
lopinavir/ritonavir must be considered. Immunomodulating therapy with
methylprednisolone or tocilizumab or anakinra must be considered in
case of the simultaneous presence of ARDS or progressive deterioration
of respiratory function, the elevation of proinflammatory biomarkers
and an interval of at least seven days from symptoms onset. Supportive
therapy should include antipyretic therapy, inhalation therapy with
topical steroids and/or bronchodilators and venous thromboembolism
prophylaxis therapy.[189-194]
Discharge from
the hospital is recommended when the patient is without fever for
almost three days, respiratory symptoms have improved, and RT-PCR
samples are negative.[188]
Conclusions
IMost
cases of HCoVs infection in children have clinically mild symptoms and
a relatively short time to resolution. Children seem to have a better
prognosis compared to adults, and death is a sporadic event. However,
some children, such as infants, adolescents, or those with underlying
diseases may be more at-risk categories and require greater caution
from clinicians. Learning to recognize pediatric clinical presentations
often indefinite or similar to other typical infections of this age,
allows clinicians to perform a correct and early diagnosis and prevent
the spread of infections in the general population. Furthermore, the
psychological and social impact of the pandemic outbreak should be
considered, especially in the pediatric age. Moreover, we think it is
necessary to implement innovative clinical tools, such as narrative
medicine, to recognize the burden of disease in children and
caregivers.[195-198] References
- Greenberg SB. Update on Human Rhinovirus and Coronavirus Infections. Semin Respir Crit Care Med. 2016;37:555-71. https://doi.org/10.1055/s-0036-1584797
- Corman VM, Muth D, Niemeyer D, Drosten C. Hosts and Sources of Endemic Human Coronaviruses. Adv Virus Res. 2018;100:163-188. https://doi.org/10.1016/bs.aivir.2018.01.001
- Belouzard
S, Millet JK, Licitra BN, Whittaker GR. Mechanisms of coronavirus cell
entry mediated by the viral spike protein. Viruses. 2012;4:1011-33. https://doi.org/10.3390/v4061011
- Woo
PC, Lau SK, Lam CS, Lau CC, Tsang AK, Lau JH, Bai R, Teng JL, Tsang CC,
Wang M, Zheng BJ, Chan KH, Yuen KY. Discovery of seven novel Mammalian
and avian coronaviruses in the genus deltacoronavirus supports bat
coronaviruses as the gene source of alphacoronavirus and
betacoronavirus and avian coronaviruses as the gene source of
gammacoronavirus and deltacoronavirus. J Virol. 2012;86:3995-4008. https://doi.org/10.1128/JVI.06540-11
- Lau
SK, Woo PC, Li KS, Tsang AK, Fan RY, Luk HK, Cai JP, Chan KH, Zheng BJ,
Wang M, Yuen KY. Discovery of a novel coronavirus, China Rattus
coronavirus HKU24, from Norway rats supports the murine origin of
Betacoronavirus 1 and has implications for the ancestor of
Betacoronavirus lineage A. J Virol. 2015;89:3076-92. https://doi.org/10.1128/JVI.02420-14
- Hamre D, Procknow JJ. A new virus isolated from the human respiratory tract. Proc Soc Exp Biol Med. 1966; 121:190-3. https://doi.org/10.3181/00379727-121-30734
- McIntosh
K, Dees JH, Becker WB, Kapikian AZ, Chanock RM. Recovery in tracheal
organ cultures of novel viruses from patients with respiratory disease.
Proc Natl Acad Sci U S A. 1967;57:933-40. https://doi.org/10.1073/pnas.57.4.933
- van
der Hoek L, Pyrc K, Jebbink MF, Vermeulen-Oost W, Berkhout RJ, Wolthers
KC, Wertheim-van Dillen PM, Kaandorp J, Spaargaren J, Berkhout B.
Identification of a new human coronavirus. Nat Med. 2004;10:368-73. https://doi.org/10.1038/nm1024
- Woo
PC, Lau SK, Chu CM, Chan KH, Tsoi HW, Huang Y, Wong BH, Poon RW, Cai
JJ, Luk WK, Poon LL, Wong SS, Guan Y, Peiris JS, Yuen KY.
Characterization and complete genome sequence of a novel coronavirus,
coronavirus HKU1, from patients with pneumonia. J Virol.
2005;79:884-95. https://doi.org/10.1128/JVI.79.2.884-895.2005
- Drosten
C, Günther S, Preiser W, van der Werf S, Brodt HR, Becker S, Rabenau H,
Panning M, Kolesnikova L, Fouchier RA, Berger A, Burguière AM, Cinatl
J, Eickmann M, Escriou N, Grywna K, Kramme S, Manuguerra JC,
Müller S, Rickerts V, Stürmer M, Vieth S, Klenk HD, Osterhaus AD,
Schmitz H, Doerr HW. Identification of a novel coronavirus in patients
with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967-76.
https://doi.org/10.1056/NEJMoa030747
- Zaki
AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation
of a novel coronavirus from a man with pneumonia in Saudi Arabia. N
Engl J Med. 2012;367:1814-20. https://doi.org/10.1056/NEJMoa1211721
- Salata
C, Calistri A, Parolin C, Palù G. Coronaviruses: a paradigm of new
emerging zoonotic diseases. Pathog Dis. 2019;77:ftaa006. https://doi.org/10.1093/femspd/ftaa006
- Lessler
J, Reich NG, Brookmeyer R, Perl TM, Nelson KE, Cummings DA. Incubation
periods of acute respiratory viral infections: a systematic review.
Lancet Infect Dis. 2009;9:291-300. https://doi.org/10.1016/S1473-3099(09)70069-6
- Cabeça
TK, Granato C, Bellei N. Epidemiological and clinical features of human
coronavirus infections among different subsets of patients. Influenza
Other Respir Viruses. 2013;7:1040-7. https://doi.org/10.1111/irv.12101
- Zhang
SF, Tuo JL, Huang XB, Zhu X, Zhang DM, Zhou K, Yuan L, Luo HJ, Zheng
BJ, Yuen KY, Li MF, Cao KY, Xu L. Epidemiology characteristics of human
coronaviruses in patients with respiratory infection symptoms and
phylogenetic analysis of HCoV-OC43 during 2010-2015 in Guangzhou. PLoS
One. 2018;13:e0191789. https://doi.org/10.1371/journal.pone.0191789
- Friedman
N, Alter H, Hindiyeh M, Mendelson E, Shemer Avni Y, Mandelboim M. Human
Coronavirus Infections in Israel: Epidemiology, Clinical Symptoms and
Summer Seasonality of HCoV-HKU1. Viruses. 2018;10:515. https://doi.org/10.3390/v10100515
- Debiaggi
M, Canducci F, Ceresola ER, Clementi M. The role of infections and
coinfections with newly identified and emerging respiratory viruses in
children. Virol J. 2012;9:247. https://doi.org/10.1186/1743-422X-9-247
- Bouvier
M, Chen WJ, Arnold JC, Fairchok MP, Danaher PJ, Lalani T, Malone L, Mor
D, Ridoré M, Burgess TH, Millar EV. Species-specific clinical
characteristics of human coronavirus infection among otherwise healthy
adolescents and adults. Influenza Other Respir Viruses.
2018;12:299-303. https://doi.org/10.1111/irv.12538
- Jennings
LC, Anderson TP, Werno AM, Beynon KA, Murdoch DR. Viral etiology of
acute respiratory tract infections in children presenting to hospital:
role of polymerase chain reaction and demonstration of multiple
infections. Pediatr Infect Dis J. 2004;23:1003-7. https://doi.org/10.1097/01.inf.0000143648.04673.6c
- van
de Pol AC, Wolfs TF, Jansen NJ, van Loon AM, Rossen JW. Diagnostic
value of real-time polymerase chain reaction to detect viruses in young
children admitted to the paediatric intensive care unit with lower
respiratory tract infection. Crit Care. 2006;10:R61. https://doi.org/10.1186/cc4895
- Raymond
F, Carbonneau J, Boucher N, Robitaille L, Boisvert S, Wu WK, De Serres
G, Boivin G, Corbeil J. Comparison of automated microarray detection
with real-time PCR assays for detection of respiratory viruses in
specimens obtained from children. J Clin Microbiol. 2009;47(3):743-50. https://doi.org/10.1128/JCM.01297-08
- Zhao
Y, Lu R, Shen J, Xie Z, Liu G, Tan W. Comparison of viral and
epidemiological profiles of hospitalized children with severe acute
respiratory infection in Beijing and Shanghai, China. BMC Infect Dis.
2019;19:729. https://doi.org/10.1186/s12879-019-4385-5
- Canducci
F, Debiaggi M, Sampaolo M, Marinozzi MC, Berrè S, Terulla C, Gargantini
G, Cambieri P, Romero E, Clementi M. Two-year prospective study of
single infections and coinfections by respiratory syncytial virus and
viruses identified recently in infants with acute respiratory disease.
J Med Virol. 2008;80:716-23. https://doi.org/10.1002/jmv.21108
- Chiu
SS, Chan KH, Chu KW, Kwan SW, Guan Y, Poon LL, Peiris JS. Human
coronavirus NL63 infection and other coronavirus infections in children
hospitalized with acute respiratory disease in Hong Kong, China. Clin
Infect Dis. 2005;40:1721-9. https://doi.org/10.1086/430301
- Minosse
C, Selleri M, Zaniratti MS, Cappiello G, Spanò A, Schifano E, Lauria
FN, Gualano G, Puro V, Campanini G, Gerna G, Capobianchi MR.
Phylogenetic analysis of human coronavirus NL63 circulating in Italy. J
Clin Virol. 2008;43:114-9. https://doi.org/10.1016/j.jcv.2008.04.015
- Dare
RK, Fry AM, Chittaganpitch M, Sawanpanyalert P, Olsen SJ, Erdman DD.
Human coronavirus infections in rural Thailand: a comprehensive study
using real-time reverse-transcription polymerase chain reaction assays.
J Infect Dis. 2007;196:1321-8. https://doi.org/10.1086/521308
- Huang
SH, Su MC, Tien N, Huang CJ, Lan YC, Lin CS, Chen CH, Lin CW.
Epidemiology of human coronavirus NL63 infection among hospitalized
patients with pneumonia in Taiwan. J Microbiol Immunol Infect.
2017;50:763-770. https://doi.org/10.1016/j.jmii.2015.10.008
- Vabret
A, Mourez T, Gouarin S, Petitjean J, Freymuth F. An outbreak of
coronavirus OC43 respiratory infection in Normandy, France. Clin Infect
Dis. 2003;36:985-9. https://doi.org/10.1086/374222
- Gaunt
ER, Hardie A, Claas EC, Simmonds P, Templeton KE. Epidemiology and
clinical presentations of the four human coronaviruses 229E, HKU1,
NL63, and OC43 detected over 3 years using a novel multiplex real-time
PCR method. J Clin Microbiol. 2010; 48:2940-7. https://doi.org/10.1128/JCM.00636-10
- Davis
BM, Foxman B, Monto AS, Baric RS, Martin ET, Uzicanin A, Rainey JJ,
Aiello AE. Human coronaviruses and other respiratory infections in
young adults on a university campus: Prevalence, symptoms, and
shedding. Influenza Other Respir Viruses. 2018;12:582-590. https://doi.org/10.1111/irv.12563
- Ali
A, Akhund T, Warraich GJ, Aziz F, Rahman N, Umrani FA, Qureshi S, Petri
WA Jr, Bhutta Z, Zaidi AK, Hughes MA. Respiratory viruses associated
with severe pneumonia in children under 2 years old in a rural
community in Pakistan. J Med Virol. 2016;88:1882-90. https://doi.org/10.1002/jmv.24557
- van
der Hoek L, Sure K, Ihorst G, Stang A, Pyrc K, Jebbink MF, Petersen G,
Forster J, Berkhout B, Uberla K. Croup is associated with the novel
coronavirus NL63. Version 2. PLoS Med. 2005;2:e240. https://doi.org/10.1371/journal.pmed.0020240
- van
der Hoek L, Sure K, Ihorst G, Stang A, Pyrc K, Jebbink MF, Petersen G,
Forster J, Berkhout B, Uberla K. Croup is associated with the novel
coronavirus NL63. Version 2. PLoS Med. 2005;2:e240. https://doi.org/10.1371/journal.pmed.0020240
- Ebihara
T, Endo R, Ma X, Ishiguro N, Kikuta H. Detection of human coronavirus
NL63 in young children with bronchiolitis. J Med Virol. 2005;75:463-5. https://doi.org/10.1002/jmv.20289
- Bosis
S, Esposito S, Niesters HG, Tremolati E, Pas S, Principi N, Osterhaus
AD. Coronavirus HKU1 in an Italian pre-term infant with bronchiolitis.
J Clin Virol. 2007;38:251-3. https://doi.org/10.1016/j.jcv.2006.11.014
- Prill
MM, Iwane MK, Edwards KM, Williams JV, Weinberg GA, Staat MA, Willby
MJ, Talbot HK, Hall CB, Szilagyi PG, Griffin MR, Curns AT, Erdman DD;
New Vaccine Surveillance Network. Human coronavirus in young children
hospitalized for acute respiratory illness and asymptomatic controls.
Pediatr Infect Dis J. 2012;31:235-40. https://doi.org/10.1097/INF.0b013e31823e07fe
- Singleton
RJ, Bulkow LR, Miernyk K, DeByle C, Pruitt L, Hummel KB, Bruden D,
Englund JA, Anderson LJ, Lucher L, Holman RC, Hennessy TW. Viral
respiratory infections in hospitalized and community control children
in Alaska. J Med Virol. 2010;82:1282-90. https://doi.org/10.1002/jmv.21790
- Kusel
MM, de Klerk NH, Holt PG, Kebadze T, Johnston SL, Sly PD. Role of
respiratory viruses in acute upper and lower respiratory tract illness
in the first year of life: a birth cohort study. Pediatr Infect Dis J.
2006;25:680-6. https://doi.org/10.1097/01.inf.0000226912.88900.a3
- Shi
T, McLean K, Campbell H, Nair H. Aetiological role of common
respiratory viruses in acute lower respiratory infections in children
under five years: A systematic review and meta-analysis. J Glob Health.
2015;5:010408. https://doi.org/10.7189/jogh.05.010408
- Gerna
G, Campanini G, Rovida F, Percivalle E, Sarasini A, Marchi A, Baldanti
F. Genetic variability of human coronavirus OC43-, 229E-, and NL63-like
strains and their association with lower respiratory tract infections
of hospitalized infants and immunocompromised patients. J Med Virol.
2006;78:938-49. https://doi.org/10.1002/jmv.20645
- Pene
F, Merlat A, Vabret A, Rozenberg F, Buzyn A, Dreyfus F, Cariou A,
Freymuth F, Lebon P. Coronavirus 229E-related pneumonia in
immunocompromised patients. Clin Infect Dis. 2003;37:929-32. https://doi.org/10.1086/377612
- Benites
EC, Cabrini DP, Silva AC, Silva JC, Catalan DT, Berezin EN, Cardoso MR,
Passos SD. Acute respiratory viral infections in pediatric cancer
patients undergoing chemotherapy. J Pediatr (Rio J). 2014;90:370-6. https://doi.org/10.1016/j.jped.2014.01.006
- Fisher
BT, Danziger-Isakov L, Sweet LR, Munoz FM, Maron G, Tuomanen E, Murray
A, Englund JA, Dulek D, Halasa N, Green M, Michaels MG, Madan RP,
Herold BC, Steinbach WJ. A Multicenter Consortium to Define the
Epidemiology and Outcomes of Inpatient Respiratory Viral Infections in
Pediatric Hematopoietic Stem Cell Transplant Recipients. J Pediatric
Infect Dis Soc. 2018;7:275-282. https://doi.org/10.1093/jpids/pix051
- Bulut
Y, Güven M, Otlu B, Yenişehirli G, Aladağ I, Eyibilen A, Doğru S. Acute
otitis media and respiratory viruses. Eur J Pediatr. 2007;166:223-8. https://doi.org/10.1007/s00431-006-0233-x.
- Ubukata
K, Morozumi M, Sakuma M, Takata M, Mokuno E, Tajima T, Iwata S; AOM
Surveillance Study Group. Etiology of Acute Otitis Media and
Characterization of Pneumococcal Isolates After Introduction of
13-Valent Pneumococcal Conjugate Vaccine in Japanese Children. Pediatr
Infect Dis J. 2018;37:598-604. https://doi.org/10.1097/INF.0000000000001956
- Ubukata
K, Morozumi M, Sakuma M, Adachi Y, Mokuno E, Tajima T, Iwata S; AOM
Surveillance Study Group. Genetic characteristics and antibiotic
resistance of Haemophilus influenzae isolates from pediatric patients
with acute otitis media after introduction of 13-valent pneumococcal
conjugate vaccine in Japan. J Infect Chemother. 2019;25:720-726. https://doi.org/10.1016/j.jiac.2019.03.019
- Pitkäranta
A, Jero J, Arruda E, Virolainen A, Hayden FG. Polymerase chain
reaction-based detection of rhinovirus, respiratory syncytial virus,
and coronavirus in otitis media with effusion. J Pediatr.
1998;133:390-4. https://doi.org/10.1016/s0022-3476(98)70276-8
- Zheng
XY, Xu YJ, Guan WJ, Lin LF. Regional, age and
respiratory-secretion-specific prevalence of respiratory viruses
associated with asthma exacerbation: a literature review. Arch Virol.
2018;163:845-853. https://doi.org/10.1007/s00705-017-3700-y
- Gagneur
A, Sizun J, Vallet S, Legr MC, Picard B, Talbot PJ. Coronavirus-related
nosocomial viral respiratory infections in a neonatal and paediatric
intensive care unit: a prospective study. J Hosp Infect. 2002;51:59-64.
https://doi.org/10.1053/jhin.2002.1179
- Sizun
J, Soupre D, Legrand MC, Giroux JD, Rubio S, Cauvin JM, Chastel C, Alix
D, de Parscau L. Neonatal nosocomial respiratory infection with
coronavirus: a prospective study in a neonatal intensive care unit.
Acta Paediatr. 1995;84:617-20. https://doi.org/10.1111/j.1651-2227.1995.tb13710.x
- Perlman
S, Evans G, Afifi A. Effect of olfactory bulb ablation on spread of a
neurotropic coronavirus into the mouse brain. J Exp Med. 1990;
172:1127-32. https://doi.org/10.1084/jem.172.4.1127
- Barthold
SW, de Souza MS, Smith AL. Susceptibility of laboratory mice to
intranasal and contact infection with coronaviruses of other species.
Lab Anim Sci. 1990;40:481-5.
- Hung EC,
Chim SS, Chan PK, Tong YK, Ng EK, Chiu RW, Leung CB, Sung JJ, Tam JS,
Lo YM. Detection of SARS coronavirus RNA in the cerebrospinal fluid of
a patient with severe acute respiratory syndrome. Clin Chem. 2003;
49:2108-9. https://doi.org/10.1373/clinchem.2003.025437
- Lau
KK, Yu WC, Chu CM, Lau ST, Sheng B, Yuen KY. Possible central nervous
system infection by SARS coronavirus. Emerg Infect Dis. 2004;10:342-4. https://doi.org/10.3201/eid1002.030638
- Yeh
EA, Collins A, Cohen ME, Duffner PK, Faden H. Detection of coronavirus
in the central nervous system of a child with acute disseminated
encephalomyelitis. Pediatrics. 2004;113:e73-6. https://doi.org/10.1542/peds.113.1.e73
- Burks
JS, DeVald BL, Jankovsky LD, Gerdes JC. Two coronaviruses isolated from
central nervous system tissue of two multiple sclerosis patients.
Science. 1980;209:933-4.
https://doi.org/10.1126/science.7403860
- Arbour N, Day R, Newcombe J, Talbot PJ. Neuroinvasion by human respiratory coronaviruses. J Virol. 2000;74:8913-21. https://doi.org/10.1128/jvi.74.19.8913-8921.2000
- Li
Y, Li H, Fan R, Wen B, Zhang J, Cao X, Wang C, Song Z, Li S, Li X, Lv
X, Qu X, Huang R, Liu W. Coronavirus Infections in the Central Nervous
System and Respiratory Tract Show Distinct Features in Hospitalized
Children. Intervirology. 2016;59:163-169. https://doi.org/10.1159/000453066.
- Pokorn
M, Jevšnik M, Petrovec M, Steyer A, Mrvič T, Grosek Š, Lusa L, Strle F.
Respiratory and Enteric Virus Detection in Children. J Child Neurol.
2017;32:84-93. https://doi.org/10.1177/0883073816670820
- Esper
F, Ou Z, Huang YT. Human coronaviruses are uncommon in patients with
gastrointestinal illness. J Clin Virol. 2010;48:131-3. https://doi.org/10.1016/j.jcv.2010.03.007
- Vabret
A, Dina J, Gouarin S, Petitjean J, Corbet S, Freymuth F. Detection of
the new human coronavirus HKU1: a report of 6 cases. Clin Infect Dis.
2006;42:634-9. https://doi.org/10.1086/500136
- Chany
C, Moscovici O, Lebon P, Rousset S. Association of coronavirus
infection with neonatal necrotizing enterocolitis. Pediatrics.
1982;69:209-14.
- Vabret A, Mourez T, Dina
J, van der Hoek L, Gouarin S, Petitjean J, Brouard J, Freymuth F. Human
coronavirus NL63, France. Emerg Infect Dis. 2005;11:1225-9. https://doi.org/10.3201/eid1108.050110
- Esposito
S, Bosis S, Niesters HG, Tremolati E, Begliatti E, Rognoni A, Tagliabue
C, Principi N, Osterhaus AD. Impact of human coronavirus infections in
otherwise healthy children who attended an emergency department. J Med
Virol. 2006;78:1609-15. https://doi.org/10.1002/jmv.20745
- Talbot
HK, Crowe JE Jr, Edwards KM, Griffin MR, Zhu Y, Weinberg GA, Szilagyi
PG, Hall CB, Podsiad AB, Iwane M, Williams JV; New Vaccine Surveillance
Network. Coronavirus infection and hospitalizations for acute
respiratory illness in young children. J Med Virol. 2009;81:853-6. https://doi.org/10.1002/jmv.21443
- Jevšnik
M, Steyer A, Pokorn M, Mrvič T, Grosek Š, Strle F, Lusa L, Petrovec M.
The Role of Human Coronaviruses in Children Hospitalized for Acute
Bronchiolitis, Acute Gastroenteritis, and Febrile Seizures: A 2-Year
Prospective Study. PLoS One. 2016;11(5):e0155555. https://doi.org/10.1371/journal.pone.0155555
- Risku
M, Lappalainen S, Räsänen S, Vesikari T. Detection of human
coronaviruses in children with acute gastroenteritis. J Clin Virol.
2010;48:27-30. https://doi.org/10.1016/j.jcv.2010.02.013
- Ruan
YJ, Wei CL, Ee AL, Vega VB, Thoreau H, Su ST, Chia JM, Ng P, Chiu KP,
Lim L, Zhang T, Peng CK, Lin EO, Lee NM, Yee SL, Ng LF, Chee RE,
Stanton LW, Long PM, Liu ET. Comparative full-length genome sequence
analysis of 14 SARS coronavirus isolates and common mutations
associated with putative origins of infection. Lancet. 2003
Ma;361:1779-85. https://doi.org/10.1016/s0140-6736(03)13414-9
- World
Health Organization. Summary of probable SARS cases with onset of
illness from November 1 2002 to July 31 2003. Available at: http://www.who.int/csr/sars/country/table2003_09_23/en/print.html
- SARS (10 Years After). Available at: https://www.cdc.gov/dotw/sars/index.html.
- Peiris
JS, Lai ST, Poon LL, Guan Y, Yam LY, Lim W, Nicholls J, Yee WK, Yan WW,
Cheung MT, Cheng VC, Chan KH, Tsang DN, Yung RW, Ng TK, Yuen KY; SARS
study group. Coronavirus as a possible cause of severe acute
respiratory syndrome. Lancet. 2003;361:1319-25. https://doi.org/10.1016/s0140-6736(03)13077-2
- Lee
N, Hui D, Wu A, Chan P, Cameron P, Joynt GM, Ahuja A, Yung MY, Leung
CB, To KF, Lui SF, Szeto CC, Chung S, Sung JJ. A major outbreak of
severe acute respiratory syndrome in Hong Kong. N Engl J Med.
2003;348:1986-94. https://doi.org/10.1056/NEJMoa030685
- Severe Acute Respiratory Syndrome [Internet]. World Health Organization (WHO). https://www.who.int/csr/sars/casedefinition/en/
- Zhong
NS, Wong GW. Epidemiology of severe acute respiratory syndrome (SARS):
adults and children. Paediatr Respir Rev. 2004;5:270-4. https://doi.org/10.1016/j.prrv.2004.07.011
- Hon
KL, Leung CW, Cheng WT, Chan PK, Chu WC, Kwan YW, Li AM, Fong NC, Ng
PC, Chiu MC, Li CK, Tam JS, Fok TF. Clinical presentations and outcome
of severe acute respiratory syndrome in children. Lancet. 2003;
361:1701-3. https://doi.org/10.1016/s0140-6736(03)13364-8
- Bitnun
A, Allen U, Heurter H, King SM, Opavsky MA, Ford-Jones EL, Matlow A,
Kitai I, Tellier R, Richardson S, Manson D, Babyn P, Read S; Other
Members of the Hospital for Sick Children SARS Investigation Team.
Children hospitalized with severe acute respiratory syndrome-related
illness in Toronto. Pediatrics. 2003;112:e261. https://doi.org/10.1542/peds.112.4.e261
- Lee
PP, Wong WH, Leung GM, Chiu SS, Chan KH, Peiris JS, Lam TH, Lau YL.
Risk-stratified seroprevalence of SARS coronavirus in children residing
in a district with point-source outbreak compared to a low-risk area.
Hong Kong Med J. 2008;14 Suppl 4:17-20.
- Chiu
WK, Cheung PC, Ng KL, Ip PL, Sugunan VK, Luk DC, Ma LC, Chan BH, Lo KL,
Lai WM. Severe acute respiratory syndrome in children: experience in a
regional hospital in Hong Kong. Pediatr Crit Care Med. 2003;4:279-83. https://doi.org/10.1097/01.PCC.0000077079.42302.81
- Leung
CW, Kwan YW, Ko PW, Chiu SS, Loung PY, Fong NC, Lee LP, Hui YW, Law HK,
Wong WH, Chan KH, Peiris JS, Lim WW, Lau YL, Chiu MC. Severe acute
respiratory syndrome among children. Pediatrics. 2004;113:e535-43. https://doi.org/10.1542/peds.113.6.e535
- Bitnun
A, Read S, Tellier R, Petric M, Richardson SE. Severe acute respiratory
syndrome-associated coronavirus infection in Toronto children: a second
look. Pediatrics. 2009;123:97-101. https://doi.org/10.1542/peds.2007-3745
- Ng
PC, Lam CW, Li AM, Wong CK, Cheng FW, Leung TF, Hon EK, Chan IH, Li CK,
Fung KS, Fok TF. Inflammatory cytokine profile in children with severe
acute respiratory syndrome. Pediatrics. 2004;113: e7-14. https://doi.org/10.1542/peds.113.1.e7
- Babyn
PS, Chu WC, Tsou IY, Wansaicheong GK, Allen U, Bitnun A, Chee TS, Cheng
FW, Chiu MC, Fok TF, Hon EK, Gahunia HK, Kaw GJ, Khong PL, Leung CW, Li
AM, Manson D, Metreweli C, Ng PC, Read S, Stringer DA. Severe acute
respiratory syndrome (SARS): chest radiographic features in children.
Pediatr Radiol. 2004;34:47-58. https://doi.org/10.1007/s00247-003-1081-8
- Ng
EK, Ng PC, Hon KL, Cheng WT, Hung EC, Chan KC, Chiu RW, Li AM, Poon LL,
Hui DS, Tam JS, Fok TF, Lo YM. Serial analysis of the plasma
concentration of SARS coronavirus RNA in pediatric patients with severe
acute respiratory syndrome. Clin Chem. 2003;49:2085-8. https://doi.org/10.1373/clinchem.2003.024588
- Peiris
JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL, Law KI, Tang BS, Hon
TY, Chan CS, Chan KH, Ng JS, Zheng BJ, Ng WL, Lai RW, Guan Y, Yuen KY;
HKU/UCH SARS Study Group. Clinical progression and viral load in a
community outbreak of coronavirus-associated SARS pneumonia: a
prospective study. Lancet. 2003; 361:1767-72. https://doi.org/10.1016/s0140-6736(03)13412-5
- Wong
SF, Chow KM, Leung TN, Ng WF, Ng TK, Shek CC, Ng PC, Lam PW, Ho LC, To
WW, Lai ST, Yan WW, Tan PY. Pregnancy and perinatal outcomes of women
with severe acute respiratory syndrome. Am J Obstet Gynecol.
2004;191:292-7. https://doi.org/10.1016/j.ajog.2003.11.019
- Shek
CC, Ng PC, Fung GP, Cheng FW, Chan PK, Peiris MJ, Lee KH, Wong SF,
Cheung HM, Li AM, Hon EK, Yeung CK, Chow CB, Tam JS, Chiu MC, Fok TF.
Infants born to mothers with severe acute respiratory syndrome.
Pediatrics. 2003;112:e254. https://doi.org/10.1542/peds.112.4.e254
- Li AM, Chan CH, Chan DF. Long-term sequelae of SARS in children. Paediatr Respir Rev. 2004;5:296-9. https://doi.org/10.1016/j.prrv.2004.07.012
- Chang
LY, Huang FY, Wu YC, Su IJ, Chiu NC, Chen KT, Wu HS, Lin TH, Peng SF,
Kao CL, Lee CY, Huang LM. Childhood severe acute respiratory syndrome
in Taiwan and how to differentiate it from childhood influenza
infection. Arch Pediatr Adolesc Med. 2004;158:1037-42. https://doi.org/10.1001/archpedi.158.11.1037
- Cheng
FW, Ng PC, Chiu WK, Chu WC, Li AM, Lo KL, Hon EK, Nelson EA, Leung TF,
Ng WH, Wong E, Ip P, Fok TF. A case-control study of SARS versus
community acquired pneumonia. Arch Dis Child. 2005;90:747-9. https://doi.org/10.1136/adc.2004.063446
- Zumla A, Hui DS, Perlman S. Middle East respiratory syndrome. Lancet. 2015;386:995-1007. https://doi.org/10.1016/S0140-6736(15)60454-8
- Middle East Respiratory Syndrome Coronavirus (MERS-CoV) [Internet]. Geneva: World Health Organization (WHO); https://www.who.int/emergencies/mers-cov/en/
- MERS situation update, January 2020. Available at: www.emro.who.int/pandemic-epidemic-diseases/mers-cov/mers-situation-update-january-2020.html.
- Middle East Respiratory Syndrome Coronavirus (MERS-CoV). Case definition. [Internet]. https://www.who.int/csr/disease/coronavirus_infections/case_definition/en/
- Assiri
A, McGeer A, Perl TM, Price CS, Al Rabeeah AA, Cummings DA,
Alabdullatif ZN, Assad M, Almulhim A, Makhdoom H, Madani H, Alhakeem R,
Al-Tawfiq JA, Cotten M, Watson SJ, Kellam P, Zumla AI, Memish ZA; KSA
MERS-CoV Investigation Team. Hospital outbreak of Middle East
respiratory syndrome coronavirus. N Engl J Med. 2013;369:407-16. https://doi.org/10.1056/NEJMoa1306742
- Assiri
A, Al-Tawfiq JA, Al-Rabeeah AA, Al-Rabiah FA, Al-Hajjar S, Al-Barrak A,
Flemban H, Al-Nassir WN, Balkhy HH, Al-Hakeem RF, Makhdoom HQ, Zumla
AI, Memish ZA. Epidemiological, demographic, and clinical
characteristics of 47 cases of Middle East respiratory syndrome
coronavirus disease from Saudi Arabia: a descriptive study. Lancet
Infect Dis. 2013;13:752-61. https://doi.org/10.1016/S1473-3099(13)70204-4
- Memish ZA, Perlman S, Van Kerkhove MD, Zumla A. Middle East respiratory syndrome. Lancet. 2020;395:1063-1077. https://doi.org/10.1016/S0140-6736(19)33221-0
- Guery
B, Poissy J, el Mansouf L, Séjourné C, Ettahar N, Lemaire X, Vuotto F,
Goffard A, Behillil S, Enouf V, Caro V, Mailles A, Che D, Manuguerra
JC, Mathieu D, Fontanet A, van der Werf S; MERS-CoV study group.
Clinical features and viral diagnosis of two cases of infection with
Middle East Respiratory Syndrome coronavirus: a report of nosocomial
transmission. Lancet. 2013;381:2265-72. https://doi.org/10.1016/S0140-6736(13)60982-4
- Fagbo
SF, Garbati MA, Hasan R, AlShahrani D, Al-Shehri M, AlFawaz T, Hakawi
A, Wani TA, Skakni L. Acute viral respiratory infections among children
in MERS-endemic Riyadh, Saudi Arabia, 2012-2013. J Med Virol.
2017;89:195-201. https://doi.org/10.1002/jmv.24632
- Alfaraj
SH, Al-Tawfiq JA, Altuwaijri TA, Memish ZA. Middle East respiratory
syndrome coronavirus in pediatrics: a report of seven cases from Saudi
Arabia. Front Med. 2019;13:126-130. https://doi.org/10.1007/s11684-017-0603-y
- Aleanizy
FS, Mohmed N, Alqahtani FY, El Hadi Mohamed RA. Outbreak of Middle East
respiratory syndrome coronavirus in Saudi Arabia: a retrospective
study. BMC Infect Dis. 2017;17:23. https://doi.org/10.1186/s12879-016-2137-3
- Correction:
Case characteristics among Middle East respiratory syndrome coronavirus
outbreak and non-outbreak cases in Saudi Arabia from 2012 to 2015. BMJ
Open. 2019;9:e011865corr1. https://doi.org/10.1136/bmjopen-2016-011865corr1
- Saeed
AA, Abedi GR, Alzahrani AG, Salameh I, Abdirizak F, Alhakeem R, Algarni
H, El Nil OA, Mohammed M, Assiri AM, Alabdely HM, Watson JT, Gerber SI.
Surveillance and Testing for Middle East Respiratory Syndrome
Coronavirus, Saudi Arabia, April 2015-February 2016. Emerg Infect Dis.
2017;23:682-685. https://doi.org/10.3201/eid2304.161793
- Khuri-Bulos
N, Payne DC, Lu X, Erdman D, Wang L, Faouri S, Shehabi A, Johnson M,
Becker MM, Denison MR, Williams JV, Halasa NB. Middle East respiratory
syndrome coronavirus not detected in children hospitalized with acute
respiratory illness in Amman, Jordan, March 2010 to September 2012.
Clin Microbiol Infect. 2014;20:678-82. https://doi.org/10.1111/1469-0691.12438
- Al-Tawfiq
JA, Kattan RF, Memish ZA. Middle East respiratory syndrome coronavirus
disease is rare in children: An update from Saudi Arabia. World J Clin
Pediatr. 2016;5:391-396. https://doi.org/10.5409/wjcp.v5.i4.391
- Thabet
F, Chehab M, Bafaqih H, Al Mohaimeed S. Middle East respiratory
syndrome coronavirus in children. Saudi Med J. 2015;36:484-6. https://doi.org/10.15537/smj.2015.4.10243
- Memish
ZA, Al-Tawfiq JA, Assiri A, AlRabiah FA, Al Hajjar S, Albarrak A,
Flemban H, Alhakeem RF, Makhdoom HQ, Alsubaie S, Al-Rabeeah AA. Middle
East respiratory syndrome coronavirus disease in children. Pediatr
Infect Dis J. 2014;33:904-6. https://doi.org/10.1097/INF.0000000000000325
- Malik
A, El Masry KM, Ravi M, Sayed F. Middle East Respiratory Syndrome
Coronavirus during Pregnancy, Abu Dhabi, United Arab Emirates, 2013.
Emerg Infect Dis. 2016;22:515-7. https://doi.org/10.3201/eid2203
- Das
KM, Lee EY, Al Jawder SE, Enani MA, Singh R, Skakni L, Al-Nakshabandi
N, AlDossari K, Larsson SG. Acute Middle East Respiratory Syndrome
Coronavirus: Temporal Lung Changes Observed on the Chest Radiographs of
55 Patients. AJR Am J Roentgenol. 2015;205:W267-74. https://doi.org/10.2214/AJR.15.14445
- Das
KM, Lee EY, Enani MA, AlJawder SE, Singh R, Bashir S, Al-Nakshbandi N,
AlDossari K, Larsson SG. CT correlation with outcomes in 15 patients
with acute Middle East respiratory syndrome coronavirus. AJR Am J
Roentgenol. 2015;204:736-42. https://doi.org/10.2214/AJR.14.13671
- Payne
DC, Iblan I, Alqasrawi S, Al Nsour M, Rha B, Tohme RA, Abedi GR, Farag
NH, Haddadin A, Al Sanhouri T, Jarour N, Swerdlow DL, Jamieson DJ,
Pallansch MA, Haynes LM, Gerber SI, Al Abdallat MM; Jordan MERS-CoV
Investigation Team. Stillbirth during infection with Middle East
respiratory syndrome coronavirus. J Infect Dis. 2014;209:1870-2. https://doi.org/10.1093/infdis/jiu068
- Zhu
N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W,
Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao
GF, Tan W, China Novel Coronavirus Investigating and Research Team. A
novel coronavirus from patients with pneumonia in China, 2019. N Engl J
Med. 2020; 382: 727-733. https://doi.org/10.1056/NEJMo.a2001.017
- World
Health Organization. Statement on the second meeting of the
International Health regulations (2005) Emergency Committee regarding
the outbreak of novel coronavirus (2019-nCoV). 2020. https://www.who.int/news-room/detail/30-01-2020-statement-onthe-second-meeting-of-the-international-health-regulations-(2005)-emergencycommittee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov). Published January 31, 2020.
- Coronavirus
COVID-19 Global Cases by the Center for Systems Science and Engineering
(CSSE) at Johns Hopkins University (JHU). Available online: https://www.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6
- Shereen
MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: Origin,
transmission, and characteristics of human coronaviruses. J Adv Res.
2020;24:91-98. https://doi.org/10.1016/j.jare.2020.03.005
- Zhang
T, Wu Q, Zhang Z. Probable Pangolin Origin of SARS-CoV-2 Associated
with the COVID-19 Outbreak. Curr Biol.;30:1346-1351.e2. https://doi.org/10.1016/j.cub.2020.03.022
- Xu
Y, Li X, Zhu B, Liang H, Fang C, Gong Y, Guo Q, Sun X, Zhao D, Shen J,
Zhang H, Liu H, Xia H, Tang J, Zhang K, Gong S. Characteristics of
pediatric SARS-CoV-2 infection and potential evidence for persistent
fecal viral shedding. Nat Med. 2020;26:502-505. https://doi.org/10.1038/s41591-020-0817-4
- Liu
J, Liao X, Qian S, Yuan J, Wang F, Liu Y, Wang Z, Wang FS, Liu L, Zhang
Z. Community Transmission of Severe Acute Respiratory Syndrome
Coronavirus 2, Shenzhen, China, 2020. Emerg Infect Dis. 2020;26. https://doi.org/10.3201/eid2606.200239
- World
Health Organization (2020). Modes of transmission of virus causing
COVID-19: implications for IPC precaution recommendations [online].
Website https://www.who.int/news-room/commentaries/detail/modes-of-transmissionof-virus-causing-covid-19-implications-for-ipc-precautionrecommendations
- Kampf
G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on
inanimate surfaces and their inactivation with biocidal agents. J Hosp
Infect. 2020 Mar;104(3):246-251. https://doi.org/10.1016/j.jhin.2020.01.022
- To
KK, Tsang OT, Leung WS, Tam AR, Wu TC, Lung DC, Yip CC, Cai JP, Chan
JM, Chik TS, Lau DP, Choi CY, Chen LL, Chan WM, Chan KH, Ip JD, Ng AC,
Poon RW, Luo CT, Cheng VC, Chan JF, Hung IF, Chen Z, Chen H, Yuen KY.
Temporal profiles of viral load in posterior oropharyngeal saliva
samples and serum antibody responses during infection by SARS-CoV-2: an
observational cohort study. Lancet Infect Dis. 2020;20:565-574. https://doi.org/10.1016/S1473-3099(20)30196-1
- Arons
MM, Hatfield KM, Reddy SC, Kimball A, James A, Jacobs JR, Taylor J,
Spicer K, Bardossy AC, Oakley LP, Tanwar S, Dyal JW, Harney J, Chisty
Z, Bell JM, Methner M, Paul P, Carlson CM, McLaughlin HP, Thornburg N,
Tong S, Tamin A, Tao Y, Uehara A, Harcourt J, Clark S, Brostrom-Smith
C, Page LC, Kay M, Lewis J, Montgomery P, Stone ND, Clark TA, Honein
MA, Duchin JS, Jernigan JA; Public Health–Seattle and King County and
CDC COVID-19 Investigation Team. Presymptomatic SARS-CoV-2 Infections
and Transmission in a Skilled Nursing Facility. N Engl J Med.
2020;382(22):2081-2090. https://doi.org/10.1056/NEJMoa2008457
- Lai
CC, Liu YH, Wang CY, Wang YH, Hsueh SC, Yen MY, Ko WC, Hsueh PR.
Asymptomatic carrier state, acute respiratory disease, and pneumonia
due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2):
Facts and myths. J Microbiol Immunol Infect. 2020. https://doi.org/10.1016/j.jmii.2020.02.012
- Zou
L, Ruan F, Huang M, Liang L, Huang H, Hong Z, Yu J, Kang M, Song Y, Xia
J, Guo Q, Song T, He J, Yen HL, Peiris M, Wu J. SARS-CoV-2 Viral Load
in Upper Respiratory Specimens of Infected Patients. N Engl J Med.
2020;382:1177-1179. https://doi.org/10.1056/NEJMc2001737
- Wu
Z, McGoogan JM. Characteristics of and Important Lessons From the
Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a
Report of 72 314 Cases From the Chinese Center for Disease Control and
Prevention. JAMA. 2020. https://doi.org/10.1001/jama.2020.2648
- Nishiura
H, Kobayashi T, Miyama T, Suzuki A, Jung SM, Hayashi K, Kinoshita R,
Yang Y, Yuan B, Akhmetzhanov AR, Linton NM. Estimation of the
asymptomatic ratio of novel coronavirus infections (COVID-19). Int J
Infect Dis. 2020;94:154-155. https://doi.org/10.1016/j.ijid.2020.03.020
- The
COVID-19 Task force of the Department of Infectious Diseases and the IT
Service Istituto Superiore di Sanità. Integrated surveillance of
COVID-19 in Italy [Internet]. 2020. Available from: https://www.epicentro.iss.it/en/coronavirus/bollettino/Infografica_24aprile ENG.pdf
- Li
Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY,
Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu
M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y,
Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi
G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. Early
Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected
Pneumonia. N Engl J Med. 2020;382:1199-1207. https://doi.org/10.1056/NEJMoa2001316
- Global surveillance for COVID-19 caused by human infection with COVID-19 virus. Case definition. [Internet]. https://www.who.int/csr/disease/coronavirus_infections/case_definition/en/
- Guan
WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui
DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY,
Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng
P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu
SY, Zhong NS; China Medical Treatment Expert Group for Covid-19.
Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J
Med. 2020:NEJMoa2002032. https://doi.org/10.1056/NEJMoa2002032
- Chen
N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y,
Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical
characteristics of 99 cases of 2019 novel coronavirus pneumonia in
Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. https://doi.org/10.1016/S0140-6736(20)30211-7
- Wang
D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong
Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138
Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in
Wuhan, China. JAMA. 2020:e201585. https://doi.org/10.1001/jama.2020.1585
- Huang
C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng
Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H,
Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical
features of patients infected with 2019 novel coronavirus in Wuhan,
China. Lancet. 2020;395:497-506. https://doi.org/10.1016/S0140-6736(20)30183-5
- Xu
XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma CL, Li SB, Wang HY, Zhang S,
Gao HN, Sheng JF, Cai HL, Qiu YQ, Li LJ. Clinical findings in a group
of patients infected with the 2019 novel coronavirus (SARS-Cov-2)
outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606.
https://doi.org/10.1136/bmj.m606
- Livingston E, Bucher K. Coronavirus Disease 2019 (COVID-19) in Italy. JAMA. 2020. https://doi.org/10.1001/jama.2020.4344
- CDC
COVID-19 Response Team. Severe Outcomes Among Patients with Coronavirus
Disease 2019 (COVID-19) - United States, February 12-March 16, 2020.
MMWR Morb Mortal Wkly Rep. 2020 Mar;69:343-346. https://doi.org/10.15585/mmwr.mm6912e2
- Lee PI, Hu YL, Chen PY, Huang YC, Hsueh PR. Are children less susceptible to COVID-19? J Microbiol Immunol Infect. 2020. https://doi.org/10.1016/j.jmii.2020.02.011
- Choi
SH, Kim HW, Kang JM, Kim DH, Cho EY. Epidemiology and clinical features
of coronavirus disease 2019 in children. Clin Exp Pediatr.
2020;63:125-132. https://doi.org/10.3345/cep.2020.00535
- Lu
X, Zhang L, Du H, Zhang J, Li YY, Qu J, Zhang W, Wang Y, Bao S, Li Y,
Wu C, Liu H, Liu D, Shao J, Peng X, Yang Y, Liu Z, Xiang Y, Zhang F,
Silva RM, Pinkerton KE, Shen K, Xiao H, Xu S, Wong GWK; Chinese
Pediatric Novel Coronavirus Study Team. SARS-CoV-2 Infection in
Children. N Engl J Med. 2020:NEJMc2005073. https://doi.org/10.1056/NEJMc2005073
- Bi
Q, Wu Y, Mei S, et al. Epidemiology and transmission of COVID-19 in
Shenzhen China: analysis of 391 cases and 1,286 of their close
contacts. medRxiv 2020. Available at: https://doi.org/10.1101/2020.03.03.20028423
- Cao
Q, Chen YC, Chen CL, Chiu CH. SARS-CoV-2 infection in children:
Transmission dynamics and clinical characteristics. J Formos Med Assoc.
2020;119:670-673. https://doi.org/10.1016/j.jfma.2020.02.009
- Dong
Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, Tong S. Epidemiology of COVID-19
Among Children in China. Pediatrics. 2020:e20200702. https://doi.org/10.1542/peds.2020-0702
- Mazzotta F., Troccoli T., Bonifazi E. A New Vasculitis at the time of COVID-19. Dermatologica Pediatrica, 2020 April 13. https://www.ejpd.com/images/nuova-vasculite-covid-ENG.pdf
- Cai
J, Xu J, Lin D, Yang Z, Xu L, Qu Z, Zhang Y, Zhang H, Jia R, Liu P,
Wang X, Ge Y, Xia A, Tian H, Chang H, Wang C, Li J, Wang J, Zeng M. A
Case Series of children with 2019 novel coronavirus infection: clinical
and epidemiological features. Clin Infect Dis. 2020:ciaa198. https://doi.org/10.1093/cid/ciaa198
- Chen
ZM, Fu JF, Shu Q, Chen YH, Hua CZ, Li FB, Lin R, Tang LF, Wang TL, Wang
W, Wang YS, Xu WZ, Yang ZH, Ye S, Yuan TM, Zhang CM, Zhang YY.
Diagnosis and treatment recommendations for pediatric respiratory
infection caused by the 2019 novel coronavirus. World J Pediatr. 2020. https://doi.org/10.1007/s12519-020-00345-5
- Wei
M, Yuan J, Liu Y, Fu T, Yu X, Zhang ZJ. Novel Coronavirus Infection in
Hospitalized Infants Under 1 Year of Age in China. JAMA.
2020;323:1313–4. https://doi.org/10.1001/jama.2020.2131.
- Yang P, Liu P, Li D, Zhao D. Corona Virus Disease 2019, a growing threat to children? J Infect. 2020. https://doi.org/10.1016/j.jinf.2020.02.024
- Cui
Y, Tian M, Huang D, Wang X, Huang Y, Fan L, Wang L, Chen Y, Liu W,
Zhang K, Wu Y, Yang Z, Tao J, Feng J, Liu K, Ye X, Wang R, Zhang X, Zha
Y. A 55-Day-Old Female Infant infected with COVID 19: presenting with
pneumonia, liver injury, and heart damage. J Infect Dis. 2020:jiaa113. https://doi.org/10.1093/infdis/jiaa113
- Belhadjer
Z, Méot M, Bajolle F, Khraiche D, Legendre A, Abakka S, Auriau J,
Grimaud M, Oualha M, Beghetti M, Wacker J, Ovaert C, Hascoet S, Selegny
M, Malekzadeh-Milani S, Maltret A, Bosser G, Giroux N, Bonnemains L,
Bordet J, Di Filippo S, Mauran P, Falcon-Eicher S, Thambo JB, Lefort B,
Moceri P, Houyel L, Renolleau S, Bonnet D. Acute heart failure in
multisystem inflammatory syndrome in children (MIS-C) in the context of
global SARS-CoV-2 pandemic. Circulation. 2020. https://doi.org/10.1161/CIRCULATIONAHA.120.048360
- Chiotos
K, Bassiri H, Behrens EM, Blatz AM, Chang J, Diorio C, Fitzgerald JC,
Topjian A, John ARO. Multisystem Inflammatory Syndrome in Children
during the COVID-19 pandemic: a case series. J Pediatric Infect Dis
Soc. 2020:piaa069. https://doi.org/10.1093/jpids/piaa069
- Waltuch
T, Gill P, Zinns LE, Whitney R, Tokarski J, Tsung JW, Sanders JE.
Features of COVID-19 post-infectious cytokine release syndrome in
children presenting to the emergency department. Am J Emerg Med. 2020
May 23:S0735-6757(20)30403-4. https://doi.org/10.1016/j.ajem.2020.05.058
- Ronconi
G, Teté G, Kritas SK, Gallenga CE, Caraffa A, Ross R, Conti P.
SARS-CoV-2, which induces COVID-19, causes kawasaki-like disease in
children: role of proinflammatory and anti-inflammatory cytokines. J
Biol Regul Homeost Agents. 2020 June 1;34(3). https://doi.org/10.23812/EDITORIAL-RONCONI-E-59
- Li
W, Cui H, Li K, Fang Y, Li S. Chest computed tomography in children
with COVID-19 respiratory infection. Pediatr Radiol. 2020. https://doi.org/10.1007/s00247-020-04656-7
- Liu
H, Liu F, Li J, Zhang T, Wang D, Lan W. Clinical and CT imaging
features of the COVID-19 pneumonia: Focus on pregnant women and
children. J Infect. 2020;80:e7-e13. https://doi.org/10.1016/j.jinf.2020.03.007
- Xia
W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in
pediatric patients with COVID-19 infection: Different points from
adults. Pediatr Pulmonol. 2020;55:1169-1174. https://doi.org/10.1002/ppul.24718
- Duan YN, Zhu YQ, Tang LL, Qin J. CT features of novel coronavirus pneumonia (COVID-19) in children. Eur Radiol. 2020. https://doi.org/10.1007/s00330-020-06860-3
- Li
B, Shen J, Li L, Yu C. Radiographic and Clinical Features of Children
with 2019 Novel Coronavirus (COVID-19) Pneumonia. Indian Pediatr.
2020:S097475591600156
- Tung-Chen Y. Lung ultrasound in the monitoring of COVID-19 infection. Clin Med (Lond). 2020:clinmed.2020-0123. https://doi.org/10.7861/clinmed.2020-0123
- Yassa
M, Birol P, Mutlu AM, Tekin AB, Sandal K, Tug N. Lung Ultrasound Can
Influence the Clinical Treatment of Pregnant Women With COVID-19. J
Ultrasound Med. 2020. https://doi.org/10.1002/jum.15367
- Chen
H, Guo J, Wang C, Luo F, Yu X, Zhang W, Li J, Zhao D, Xu D, Gong Q,
Liao J, Yang H, Hou W, Zhang Y. Clinical characteristics and
intrauterine vertical transmission potential of COVID-19 infection in
nine pregnant women: a retrospective review of medical records. Lancet.
2020;395:809-815. https://doi.org/10.1016/S0140-6736(20)30360-3
- Zaigham
M, Andersson O. Maternal and perinatal outcomes with COVID-19: A
systematic review of 108 pregnancies. Acta Obstet Gynecol Scand. 2020. https://doi.org/10.1111/aogs.13867
- Di
Mascio D, Khalil A, Saccone G, Rizzo G, Buca D, Liberati M, Vecchiet J,
Nappi L, Scambia G, Berghella V, D'Antonio F. Outcome of Coronavirus
spectrum infections (SARS, MERS, COVID 1 -19) during pregnancy: a
systematic review and meta-analysis. Am J Obstet Gynecol MFM.
2020:100107. https://doi.org/10.1016/j.ajogmf.2020.100107
- Zeng
LK, Tao XW, Yuan WH, Wang J, Liu X, Liu ZS. [First case of neonate
infected with novel coronavirus pneumonia in China]. Zhonghua Er Ke Za
Zhi. 2020;58:E009. Chinese. https://doi.org/10.3760/cma.j.issn.0578-1310.2020.0009
- Cai
JH, Wang XS, Ge YL, Xia AM, Chang HL, Tian H, Zhu YX, Wang QR, Zeng JS.
[First case of 2019 novel coronavirus infection in children in
Shanghai]. Zhonghua Er Ke Za Zhi. 2020;58:E002. Chinese. https://doi.org/10.3760/cma.j.issn.0578-1310.2020.0002
- Fan
C, Lei D, Fang C, Li C, Wang M, Liu Y, Bao Y, Sun Y, Huang J, Guo Y, Yu
Y, Wang S. Perinatal Transmission of COVID-19 Associated SARS-CoV-2:
Should We Worry? Clin Infect Dis. 2020 Mar 17:ciaa226. https://doi.org/10.1093/cid/ciaa226
- Kamali
Aghdam M, Jafari N, Eftekhari K. Novel coronavirus in a 15-day-old
neonate with clinical signs of sepsis, a case report. Infect Dis
(Lond). 2020;52:427–429. https://doi.org/10.1080/23744235.2020.1747634
- Wang
S, Guo L, Chen L, Liu W, Cao Y, Zhang J, Feng L. A case report of
neonatal COVID-19 infection in China. Clin Infect Dis. 2020 Mar
12:ciaa225. https://doi.org/10.1093/cid/ciaa225
- Chang
TH, Wu JL, Chang LY. Clinical characteristics and diagnostic challenges
of pediatric COVID-19: A systematic review and meta-analysis. J Formos
Med Assoc. 2020:S0929-6646(20)30143-1. https://doi.org/10.1016/j.jfma.2020.04.007
- Vabret
A, Mouthon F, Mourez T, Gouarin S, Petitjean J, Freymuth F. Direct
diagnosis of human respiratory coronaviruses 229E and OC43 by the
polymerase chain reaction. J Virol Methods. 2001;97:59-66. https://doi.org/10.1016/s0166-0934(01)00343-3
- Cheng
PK, Wong DA, Tong LK, Ip SM, Lo AC, Lau CS, Yeung EY, Lim WW. Viral
shedding patterns of coronavirus in patients with probable severe acute
respiratory syndrome. Lancet. 2004;363:1699-700. https://doi.org/10.1016/S0140-6736(04)16255-7
- Chim
SS, Chiu RW, Lo YM. Genomic sequencing of the severe acute respiratory
syndrome-coronavirus. Methods Mol Biol. 2006;336:177-94. https://doi.org/10.1385/1-59745-074-X:177
- Chim
SS, Tong YK, Hung EC, Chiu RW, Lo YM. Genomic sequencing of a SARS
coronavirus isolate that predated the Metropole Hotel case cluster in
Hong Kong. Clin Chem. 2004 Jan;50(1):231-3. https://doi.org/10.1373/clinchem.2003.025536
- Lee
JS, Ahn JS, Yu BS, Cho SI, Kim MJ, Choi JM, Seo SH, Park SS, Seong MW.
Evaluation of a Real-Time Reverse Transcription-PCR (RT-PCR) Assay for
Detection of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) in
Clinical Samples from an Outbreak in South Korea in 2015. J Clin
Microbiol. 2017 Aug;55(8):2554-2555. https://doi.org/10.1128/JCM.00667-17
- Kim
MN, Ko YJ, Seong MW, Kim JS, Shin BM, Sung H. Analytical and Clinical
Validation of Six Commercial Middle East Respiratory Syndrome
Coronavirus RNA Detection Kits Based on Real-Time Reverse-Transcription
PCR. Ann Lab Med. 2016 Sep;36(5):450-6. https://doi.org/10.3343/alm.2016.36.5.450
- Al Johani S, Hajeer AH. MERS-CoV diagnosis: An update. J Infect Public Health. 2016 May-Jun;9(3):216-9. https://doi.org/10.1016/j.jiph.2016.04.005
- Memish
ZA, Al-Tawfiq JA, Makhdoom HQ, Assiri A, Alhakeem RF, Albarrak A,
Alsubaie S, Al-Rabeeah AA, Hajomar WH, Hussain R, Kheyami AM, Almutairi
A, Azhar EI, Drosten C, Watson SJ, Kellam P, Cotten M, Zumla A.
Respiratory tract samples, viral load, and genome fraction yield in
patients with Middle East respiratory syndrome. J Infect Dis.
2014;210:1590-4. https://doi.org/10.1093/infdis/jiu292
- Hung IF, Lau SK, Woo PC, Yuen KY. Viral loads in clinical specimens and SARS manifestations. Hong Kong Med J. 2009;15:20-2
- Corman
VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, Bleicker T,
Brünink S, Schneider J, Schmidt ML, Mulders DG, Haagmans BL, van der
Veer B, van den Brink S, Wijsman L, Goderski G, Romette JL, Ellis J,
Zambon M, Peiris M, Goossens H, Reusken C, Koopmans MP, Drosten C.
Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR.
Euro Surveill. 2020;25:2000045. https://doi.org/10.2807/1560-7917.ES.2020.25.3.2000045
- Ren
X, Liu Y, Chen H, et al. Application and optimization of RT-PCR in
diagnosis of SARS-CoV-2 infection. medRxiv. 2020. Available at: https://www.medrxiv.org/content/10.1101/2020.02.25.20027755v2 Accessed March 22, 2020.
- Arevalo-Rodriguez
I, Buitrago-Garcia D, Simancas-Racines D, et al. False-negative results
of initial RT-PCR assays for covid-19: a systematic review. medRxiv
20066787. 2020 10.1101/2020.04.16.20066787
- Zitek T. The Appropriate Use of Testing for COVID-19. West J Emerg Med. 2020 Apr ;21:470-472. https://doi.org/10.5811/westjem.2020.4.47370
- Watson J, Whiting PF, Brush JE. Interpreting a covid-19 test result. BMJ. 2020;369:m1808. https://doi.org/10.1136/bmj.m1808
- Long
QX, Liu BZ, Deng HJ, Wu GC, Deng K, Chen YK, Liao P, Qiu JF, Lin Y, Cai
XF, Wang DQ, Hu Y, Ren JH, Tang N, Xu YY, Yu LH, Mo Z, Gong F, Zhang
XL, Tian WG, Hu L, Zhang XX, Xiang JL, Du HX, Liu HW, Lang CH, Luo XH,
Wu SB, Cui XP, Zhou Z, Zhu MM, Wang J, Xue CJ, Li XF, Wang L, Li ZJ,
Wang K, Niu CC, Yang QJ, Tang XJ, Zhang Y, Liu XM, Li JJ, Zhang DC,
Zhang F, Liu P, Yuan J, Li Q, Hu JL, Chen J, Huang AL. Antibody
responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020. https://doi.org/10.1038/s41591-020-0897-1
- Che
XY, Qiu LW, Liao ZY, Wang YD, Wen K, Pan YX, Hao W, Mei YB, Cheng VC,
Yuen KY. Antigenic cross-reactivity between severe acute respiratory
syndrome-associated coronavirus and human coronaviruses 229E and OC43.
J Infect Dis. 2005;191:2033-7. https://doi.org/10.1086/430355
- Özçürümez
MK, Ambrosch A, Frey O, Haselmann V, Holdenrieder S, Kiehntopf M,
Neumaier M, Walter M, Wenzel F, Wölfel R, Renz H; COVID-19 Task Force
of theGerman Society for Clinical Chemistry and Laboratory Medicine
(DGKL). SARS-CoV-2 Antibody Testing - Questions to be asked. J Allergy
Clin Immunol. 2020 :S0091-6749(20)30739-9. https://doi.org/10.1016/j.jaci.2020.05.020
- Omrani
AS, Saad MM, Baig K, Bahloul A, Abdul-Matin M, Alaidaroos AY,
Almakhlafi GA, Albarrak MM, Memish ZA, Albarrak AM. Ribavirin and
interferon alfa-2a for severe Middle East respiratory syndrome
coronavirus infection: a retrospective cohort study. Lancet Infect Dis.
2014;14:1090-1095. https://doi.org/10.1016/S1473-3099(14)70920-X
- Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. Version 2. PLoS Med. 2006;3:e343. https://doi.org/10.1371/journal.pmed.0030343
- National
Health Commission of People’s Republic of China. Diagnosis and
treatment of pneumonia caused by novel coronavirus (trial version 4). https://www.nhc.gov.cn/xcs/zheng.cwj/20200.1/42945.63ed3.5b4320.9b31.739bd.0785e.67/files./7a930.91112.67475a99d4.30696.2c8bf.78.pdf.
- Shen
K, Yang Y, Wang T, Zhao D, Jiang Y, Jin R, Zheng Y, Xu B, Xie Z, Lin L,
Shang Y, Lu X, Shu S, Bai Y, Deng J, Lu M, Ye L, Wang X, Wang Y, Gao L;
China National Clinical Research Center for Respiratory Diseases;
National Center for Children's Health, Beijing, China; Group of
Respirology, Chinese Pediatric Society, Chinese Medical Association;
Chinese Medical Doctor Association Committee on Respirology Pediatrics;
China Medicine Education Association Committee on Pediatrics; Chinese
Research Hospital Association Committee on Pediatrics; Chinese
Non-government Medical Institutions Association Committee on
Pediatrics; China Association of Traditional Chinese Medicine,
Committee on Children's Health and Medicine Research; China News of
Drug Information Association, Committee on Children's Safety
Medication; Global Pediatric Pulmonology Alliance. Diagnosis,
treatment, and prevention of 2019 novel coronavirus infection in
children: experts' consensus statement. World J Pediatr. 2020. https://doi.org/10.1007/s12519-020-00343-7
- Treatment of children with COVID-19: position paper of the Italian Society of Pediatric Infectious Disease. Available at: https://www.sitip.org/images/covid19/29042020_protocollo__COVID_english.pdf
- Day M. Covid-19: ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ. 2020;368:m1086. https://doi.org/10.1136/bmj.m1086
- European
Medicines Agency. EMA gives advice on the use of non-steroidal
anti-inflammatories for COVID-19. March 2020. Available at: https://www.ema.europa.eu/en/news/ema-gives-advice-use-non-steroidalanti-inflammatories-covid-19
- Global Initiative for Asthma (GINA). Recommendations for inhaled asthma controller medications. March 19, 2020. Available at: https://ginasthma.org/recommendations-for-inhaled-asthma-controllermedications.
- Tang
N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is
associated with decreased mortality in severe coronavirus disease 2019
patients with coagulopathy. J Thromb Haemost. 2020. https://doi.org/10.1111/jth.14817
- Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov Ther. 2020;14:58-60. 29
- Negri
L, Buzzi A, Aru AB, Cannavò A, Castegnaro C, Fasulo MR, Lassandro G,
Rocino A, Santoro C, Sottilotta G, Giordano P, Mazzucconi MG, Mura R,
Peyvandi F, Delle Fave A. Perceived well-being and mental health
in haemophilia. Psychol Health Med. 2020 Jan 26:1-11. https://doi.org/10.1080/13548506.2020.1717556
- Giordano
P, Lassandro G, di Meo NA, Palladino V, Lovrencic B, Spinelli M, Reale
L, Jankovic M. A Narrative Approach to Describe QoL in Children With
Chronic ITP. Front Pediatr. 2019 May 7;7:163. https://doi.org/10.3389/fped.2019.00163
- Giordano P, Lassandro G, Valente M, Molinari AC, Ieranò P, Coppola A. Pediatr Hematol Oncol. 2014 Nov;31(8):687-702. https://doi.org/10.3109/08880018.2014.930768
- Giordano
P, Lassandro G, Giona F, Jankovic M, Nardi M, Nobili B, Notarangelo LD,
Russo G, Mackensen Sv. ITP-QoL questionnaire for children with immune
thrombocytopenia: Italian version validation's. Pediatr Hematol Oncol.
2014 Sep;31(6):534-47. https://doi.org/10.3109/08880018.2014.915443
TOP]




