High estrogen levels exert direct effects on several haemostatic variables, thus possibly inducing thrombotic risk.
In oocyte donation ART, some patients experience repeated implantation failures as well as biochemical pregnancy, adverse events, which can result in anxiety and depression in women.
It is also essential to consider that maternal age is associated with significantly lower pregnancy rates.[6]
In this study, we decided to identify changes in haemostasis in women undergoing infertility treatment and their relationship with clinical pregnancy outcome.
We prospectively recruited 22 otherwise healthy infertile Caucasian women [median age 42 yrs (41-48)] planning oocyte donation (oocytes from an egg donor used for reproductive purposes) at the Center for Assisted Reproductive Technology, University Hospital, Careggi, Florence, Italy.
We excluded women with inherited and acquired thrombophilia, obesity, diabetes, hypertension, and history of previous atherothrombotic disorders, well-known conditions associated with hypercoagulability.
Before starting hormonal therapy and after two weeks from embryo transfer, blood tests, such as haemoglobin, haematocrit, platelet cell count, prothrombin time, activated partial thromboplastin time (aPTT), Fibrinogen, Factor VIII (FVIII) and von Willebrand Factor antigen (vWF:Ag), were performed in all women. Blood samples were collected from the antecubital vein into 0.109 mol/l trisodium citrate tubes (Vacutainer, Becton Dickinson, New Jersey, USA) in the morning, after overnight fasting. Plasma samples were obtained by centrifuging blood at 2000 × g for 15 min at room temperature for vWF:Ag, Fibrinogen, FVIII. Complete blood cell count was performed by using the Sysmex XE-2100 hematology analyzer (Sysmex, Kobe, Japan). Fibrinogen was assessed by the Clauss clotting method (Siemens, Marburg, Germany). FVIII activity was determined by a coagulation-based assay, with deficient plasma in the presence of Pathromtin (Coagulation Factor VIII, Siemens); vWF:Ag levels were detected by a turbidimetric assay (vWF:Ag, Siemens).
The protocol for fertility treatment includes estrogen and progesterone supplementation, with the goals of mimicking the normal menstrual cycle and allowing for implantation and the maintenance of early pregnancy. According to the Italian National Institute of Health glossary, embryo donation means the transfer of an embryo resulting from gametes that did not originate from the recipient and/or her partner. In contrast, implantation means the attachment and subsequent embryo penetration into the endometrium. This process starts 5 to 7 days after fertilization of the oocyte, usually resulting in the formation of a gestation sac.
All women underwent an endometrial preparation protocol with the oral contraceptive pill with a single depot-dose of a GnRH agonist (triptorelin) (Decapeptyl® 3.75, Ipsen Spa, Milan, Italy) on days 20–21 of the cycle, followed by oral estradiol valerate (Progynova®, Bayer, Milan) 2 mg/day from day 2 to 6 of the menstrual cycle, 4 mg/day from day 7 to 10 and 6 mg/day on day 11 until the embryo transfer. After 11-12 days, when we reached a trilaminar 7 mm endometrium by ultrasound evaluation, progesterone supplement was added with daily 400 mg intravaginal capsules (Progeffik®/Prometrium®) plus progesterone 25 mg IM injection (Pleyris ®) every 12h. All women received thromboprophylaxis with Low Molecular Weight Heparin (LMWH) for the management of ART-related thrombotic risk during infertility treatment.
In order to ascertain pregnancy, the dosage of serum beta HCG was carried out 12 days after the embryo transfer date. Clinical pregnancy was confirmed through ultrasonographic parameters, with visualization of the intrauterine gestational sac and at least one embryo with a heartbeat.
Statistical analysis was performed by using the SPSS (Statistical Package for Social Sciences, Chicago, USA) software for Windows (Version 26.0). Continuous variables were expressed as mean ± SD. The nonparametric Mann-Whitney test for unpaired data was used for comparison of continuous variables according to clinical pregnancy outcome. A p-value <0.05 was considered to indicate statistical significance.
Informed written consent for anonymous data analysis was obtained from all women. The investigation conforms to the principles outlined in the Declaration of Helsinki.
At baseline, all women had circulating FVIII and vWF:Ag concentrations, as well as the other blood tests, within the normal range.
Data after two weeks from embryo transfer showed that only women who had clinical pregnancy, evidenced significantly higher levels of FVIII and vWF:Ag, as well as a shortening of aPTT, likely related to the increase of FVIII (Figure 1). No significant changes in haemoglobin, haematocrit, platelet cell count, prothrombin time and fibrinogen concentration were observed (Table 1).
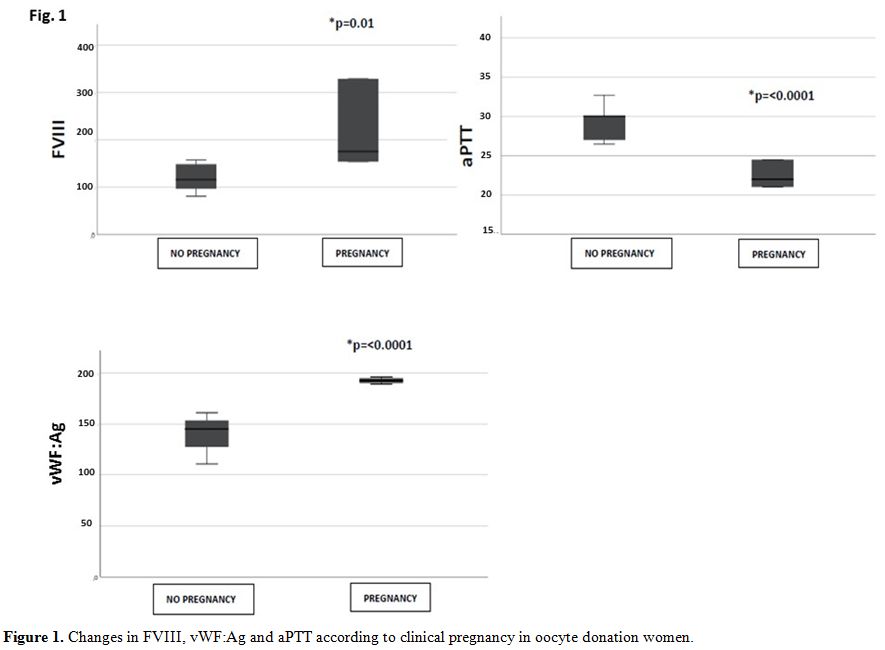 |
Figure 1. Changes in FVIII, vWF:Ag and aPTT according to clinical pregnancy in oocyte donation women. |
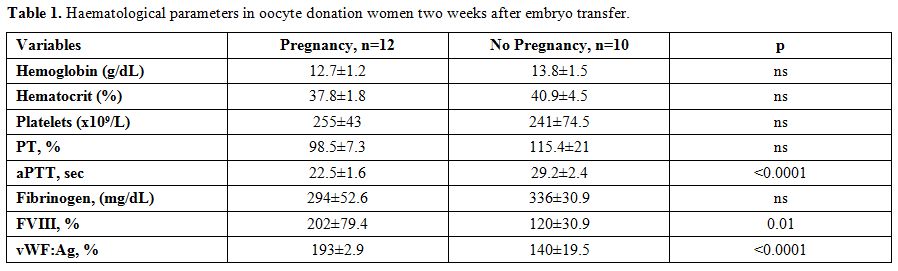 |
Table 1. Haematological parameters in oocyte donation women two weeks after embryo transfer. |
Eleven out of 12 women had positive pregnancy outcomes, and one woman experienced fetal loss after the first trimester of pregnancy.
Local haemostasis at the placental trophoblast implantation is characterized by increased tissue factor expression, triggering changes in all aspects of haemostasis, useful to maintain placental function during normal pregnancy and delivery.[7]
It is noteworthy that both FVIII and vWF:Ag levels start to increase in physiological pregnancy from week 6[8] with a further two- to three-fold increase during the second and third trimester.[7] Moreover, data from ovarian stimulation for autologous procedures show an increase in circulating levels of both factors.[9]
At the best of our knowledge, no information is available concerning these coagulation changes in oocyte donation, in which estrogen and progesterone replacement cycles permit the implantation and maintenance of pregnancy in the absence of ovarian function.
Although preliminary, our findings, which evidence an early increase of these two coagulation proteins, suggest their potential role as early "predictors" of a successful clinical pregnancy in oocyte donation women. This may be intriguing for exploring possible mechanisms responsible for the establishment of a successful pregnancy after oocyte donation. Moreover, circulating FVIII and vWF:Ag levels assessment may be useful in clinical practice to predict the risk of thromboembolic complications associated with assisted reproduction.[10] Finally, it should be interesting to analyse other coagulation parameters and global coagulation tests pregnancy-related to test a similar behaviour.
Due to the small sample of our study, our findings would have to be validated in future studies on larger patient samples.