M.G. Massaro1, M. Pompili1,4, L.L. Sicignano1, F. Pizzolante1, E. Verrecchia1, F.M. Vecchio3,4, D. Rigante2,4 and R. Manna1,4*
1 Division of
Internal Medicine, Rare Diseases and Periodic Fevers Research Centre,
Fondazione Policlinico A. Gemelli IRCCS, Rome, Italy.
2
Department of Life Sciences and Public Health, Rare Diseases and
Periodic Fevers Research Centre, Fondazione Policlinico A. Gemelli
IRCCS, Rome, Italy.
3 Department of Pathology, Fondazione Policlinico A. Gemelli IRCCS, Rome, Italy.
4 Università Cattolica del Sacro Cuore, Rome, Italy.
Correspondence to: Raffaele Manna, MD, PhD. Institute of Internal
Medicine, Fondazione Policlinico Universitario A. Gemelli IRCCS,
Università Cattolica Sacro Cuore, Rome, Largo A. Gemelli 8, 00168 Rome,
Italy. Tel: +39 06 30159433. Fax: +39 06 35502775. E-mail:
raffaele.manna@policlinicogemelli.it
Published: September 1, 2020
Received: May 30, 2020
Accepted: August 8, 2020
Mediterr J Hematol Infect Dis 2020, 12(1): e2020059 DOI
10.4084/MJHID.2020.059
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Hepatic
involvement in familial Mediterranean fever (FMF) ranges from a
nonspecific increase in liver enzymes to cryptogenic cirrhosis, and the
liver is mostly involved in patients bearing the M694V MEFV mutation in
homozygosis. A 44-year-old Jewish woman with FMF developed nonalcoholic
steatohepatitis during colchicine treatment (2,5 mg per day), confirmed
by both elastography and liver biopsy. Therefore, combined therapy with
the interleukin-1 (IL-1) blocking agent canakinumab (150 mg every four
weeks) and colchicine (at a reduced dose of 1.5 mg per day) was
started. Three months later, transaminases became normal, and after
further six months, there was a marked improvement of liver fibrosis.
IL-1 blockade has the power to halt or mitigate liver involvement in
FMF patients. However, further experience is required to assess its
therapeutic potential in the most severe patients with the hepatic
disease who are partially responsive to long-term prophylaxis with
colchicine.
|
Introduction
Familial Mediterranean fever (FMF) is the oldest and most frequent of all known hereditary periodic fever syndromes:[1]
its febrile attacks (with peaks over 39-40°C) have a length of about
1-3 days and are characterized by self-limited serositis, joint
inflammation and skin manifestations such as erysipelas-like erythema.[2-8] Recurrent polyserositis may be a strongly suggestive clue to diagnose FMF.[9,10]
Secondary renal amyloidosis represents the most ominous complication of
FMF, usually found in 8.6% of cases according to a multicenter study
performed in Turkey.[11] Liver was not considered a
district typically involved in FMF, except for liver amyloidosis, which
might occur and display an aggressive course.[12] To
date, FMF has been linked to a spectrum of liver manifestations ranging
from a mild-to-moderate increase of liver enzymes to cryptogenic
cirrhosis.[13] Colchicine is the mainstay of FMF treatment since 1972,[14,15]
and its efficacy has been largely proved.16 Despite the maximum
tolerated colchicine dose, 5-10% of FMF patients experience more than
one attack monthly[17,18] and are defined as
colchicine non-responders. Interleukin-1 (IL-1) blockade is considered
the gold-standard treatment in refractory FMF, with several reports
having demonstrated both efficacy and safety of anakinra and
canakinumab.[19-22]
We report a young woman
with FMF, undergoing colchicine therapy since the age of 3, who had a
frank liver involvement at both laboratory and histological assessment,
who progressively improved along with anti-IL-1 treatment.
Case Report
A
44-year-old Jewish woman was diagnosed to have FMF at the age of 3
years due to recurrent febrile episodes (until 40°C) lasting less than
48 hours and occurring three times/monthly combined with recurrent
erysipelas-like erythema on the legs, pericardial effusion, recurrent
abdominal pain, and arthromyalgia. The diagnosis was confirmed at a
genetic level, finding the M694V mutation (in homozygosis) in the MEFV
gene. Colchicine was started and gradually increased during early
adulthood, up to an effective dose of 2.5 mg/day, begun at 29 years,
and successfully continued non-stop with good tolerance. The patient
had endometriosis at the age of 25 so that she underwent laparoscopy
with adhesiolysis and removal of multiple endometriotic foci in the
peritoneum, uterus, and annexes. Her more recent medical history was
also characterized by insulin resistance and mixed anxiety-depressive
disorder. For these reasons, she received estrogen-progestin therapy
for about ten years and metformin combined with anti-depressive drugs
(venlafaxine, reboxetine) plus benzodiazepines for about three years.
In
2018, after colchicine use for almost 40 years and 15 years after the
last increase of colchicine dose (to 2,5 mg/die), the serum level of
both transaminases was found abnormal: alanine aminotransferase was
repeatedly over 140 IU/l and aspartate aminotransferase over 90 IU/l.
The general activity of FMF seemed relatively controlled, as serum
amyloid-A (SAA) was 0.74 (n.v. <0.5). Transaminases had been
previously within normal limits at the previous patient's follow-up
evaluations, and no changes were noted due to therapies taken by the
patient. No viral infections could be detected (serology for hepatitis
A-B-C, cytomegalovirus, Epstein-Barr virus, and human immunodeficiency
virus was negative). The patient also denied taking toxic substances,
such as alcohol or illicit drugs. The autoimmunity panel was completely
negative (except for a slight positivity of anti-nuclear antibodies,
1:160). No worsening of FMF typical symptoms was observed during this
period.
The increased level of transaminases was also confirmed
by many tests performed with monthly frequency. Liver ultrasound
assessment revealed standard dimensions, but inhomogeneous
echo-structure as well as moderate steatosis. Neither focal lesions nor
intrahepatic biliary tract abnormalities were documented. Furthermore,
a liver elastography study carried out utilizing a dedicated convex
probe through the "point shear wave" technique (Esaote9 XP) with
multiple sampling (10 areas) on the right lobe revealed an increased
index of elasticity equal to 10.5 kPa. Given the persistently high
transaminases, the patient underwent liver biopsy. Histology showed
mild steatosis with widespread hydropic degeneration of hepatocytes and
centrilobular balloniform activation of CD68+ Kupffer cells, containing
PAS-positive material. This morphological report was compatible with
steatohepatitis (Figure 1).
However, these results were not consistent with colchicine-induced
liver injury, which occurs in cases of drug overdose, characterized by
typical histopathological elements, i.e., anisonucleosis with enlarged
nuclei, multiple nucleoli and frequent mitotic figures arrested in
metaphase,[23] which were not present.
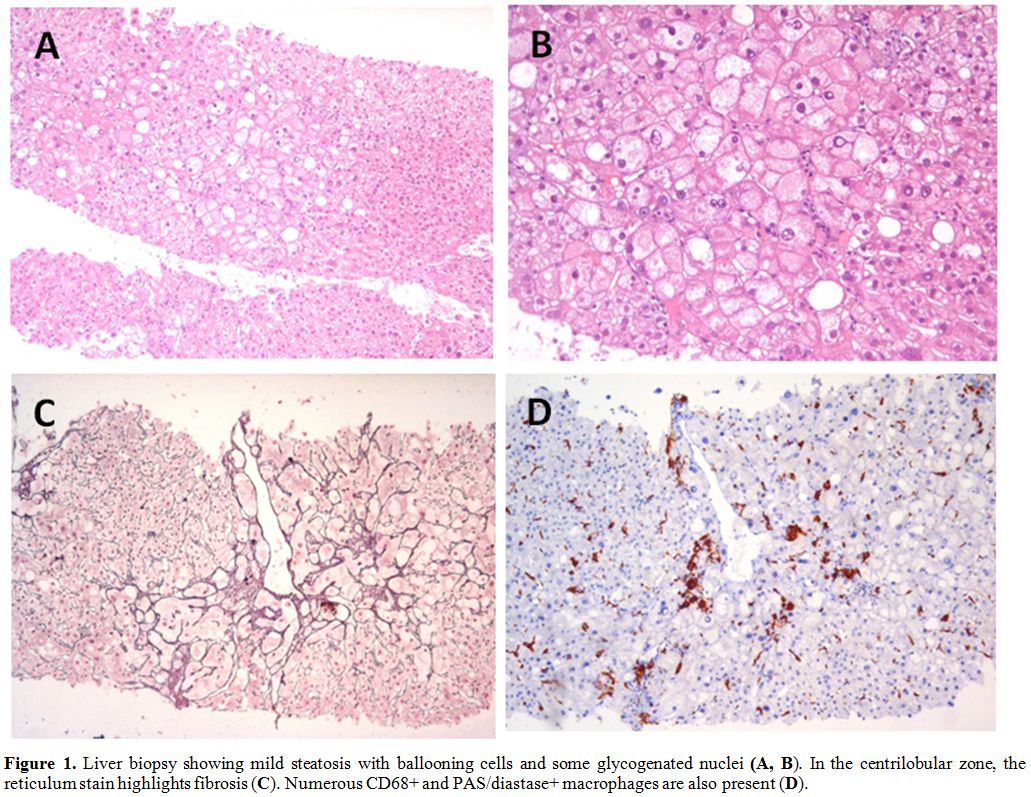 |
Figure
1. Liver biopsy showing mild steatosis with ballooning cells and some glycogenated nuclei (A, B). In the centrilobular zone, the reticulum stain highlights fibrosis (C). Numerous CD68+ and PAS/diastase+ macrophages are also present (D). |
Despite the increase
of transaminases, it was not possible to reduce colchicine dose alone,
as the same patient had presented different disease relapses if
colchicine was reduced to 2 mg/day. For this reason, we decided to
start a combined therapy with canakinumab (150 mg every four weeks) and
piecemeal reduced dose of colchicine (until 1.5 mg/day). Three months
after starting canakinumab, there was a substantial reduction in the
transaminases next to normalization. In addition, liver elastography
performed six months from initiating canakinumab revealed a sound
improvement in the steatosis framework (6.1 kPa versus 10.5 kPa).
To
date, the patient is still receiving the same therapy based on
canakinumab (150 mg every four weeks) and colchicine (1.5 mg/daily),
while transaminases have remained in the standard range. There was no
exacerbation of FMF typical manifestations, and the disease is
currently in remission.
Discussion
FMF is an autoinflammatory disease characterized by recurrent self-limiting episodes of fever and polyserositis.[1,5-7,10]
It is the best-known and most common monogenic fever syndrome, which
shows a preferential ethnic distribution in Turks, Armenians, Jews, and
Arabs.[10,24] This autosomal recessive pathology is caused by mutations in the MEFV
gene, which encodes for pyrin. Pyrin has a relevant role in controlling
the innate immune system and inflammation activation: its functional
abnormality causes aberrant activation of the inflammasome with
overproduction of proinflammatory cytokines, in particular IL-1.[25]
Clinically, the disease is characterized by recurrent inflammation in
the serosal membranes, joints, and skin with long-term complications
such as renal amyloidosis, which might lead to renal failure, if
overlooked.[26]
Untreated FMF is associated
with ongoing persistent inflammation and subsequent accumulation of SAA
in different target-organs such as kidney, but also liver.[1,5-7,10,27,28]
For a long time, the liver was not considered typically involved in
FMF, and AA amyloidosis was considered the only possible culprit in the
case of hepatic involvement.[12] However, an increase of liver enzymes does not occur in the case of amyloidosis,[13] as the most frequent signs of liver AA amyloidosis are an increased level of alkaline phosphatase and hepatomegaly.[29]
To date, liver involvement in FMF has been widely recognized and reported: both types of MEFV mutation and overproduction of IL-1 are probably involved in the damage progression.[30,31]
Experiments in mice have shown that an abundant release of IL-1 causes
inflammation, pyroptosis, and collagen deposition in the liver with
subsequent increase of liver enzymes.[13,32]
Two conflicting studies have enhanced our knowledge about the
connection between FMF and liver involvement, and more between FMF and
nonalcoholic fatty liver disease (NAFLD). The survey conducted by Rimar
et al. enrolled 27 patients with FMF but without a frank metabolic
syndrome and found that 75% of patients had NAFLD. The conclusion of
this study hypothesized a correlation between FMF and NAFLD.[33]
Conversely, the survey conducted by Sarkis et al., which enrolled 52
patients with FMF and 30 healthy controls, showed similar rates of
NAFLD in the FMF population compared to the healthy one.[34]
Indeed, in the study by Rimar et al., FMF patients had fewer risk
factors for NAFLD, and NAFLD was demonstrated by biopsy, which is more
sensitive than ultrasound.[35] A different study
conducted by Tweezer-Zaks et al. documented that M694V homozygosity was
relatively more frequent among FMF patients with NAFLD and nonalcoholic
steatohepatitis.[36]
From a therapeutic point of view, colchicine is the first-choice option for FMF management since 1972.[14,15,37]
The exact mechanism of action underlying colchicine efficacy is not
entirely understood: current evidence suggests that colchicine
downregulates multiple inflammatory pathways and modulates innate
immunity.[38] Colchicine has a narrow therapeutic
range, and hepatotoxicity as a possible consequence of long-term
administration has been shown.[23] In fact, colchicine intoxication with daily doses higher than 5 mg might determine liver toxicity.[13,38]
Of note, the usual doses used to prevent FMF attacks do not seem to
bring about a significant increase in liver enzymes in most cases.[40]
A potentially life-threatening complication of some autoinflammatory
disorders like FMF may be macrophage activation syndrome, characterized
by increased hemophagocytic activity in both bone marrow and liver,
combined with fever and different signs of liver damage.[41,42]
Furthermore, various scores have been created to quantify organ damage
(including liver) or compare disease outcome in patients with
autoinflammatory disorders,[43-45] and some of these have been created explicitly for FMF.[46]
Different
drugs can come to the rescue for FMF patients who are
colchicine-intolerant and non-responders or for those displaying
adverse effects to colchicine. Both anakinra, the IL-1 receptor
antagonist given subcutaneously daily, and canakinumab, the long-acting
specific monoclonal antibody against IL-1β
canakinumab, given subcutaneously every four weeks, can be extremely
active in the management of another autoinflammatory disorder, which is
the cryopyrin-associated periodic syndrome, almost fully mediated by
IL-1.[47] As an inappropriate production of IL-1 also
plays a central role in the pathogenesis of FMF attacks, blocking IL-1
by specific biological anti-IL-1 drugs should be an ideal strategy in
colchicine-resistant patients with FMF.[48,49]
Anakinra and canakinumab have been used in the most difficult-to-treat
patients with FMF, though recently it has emerged that canakinumab is
better-tolerated for less frequent injection-site reactions.[50]
It is remarkable that in our patient canakinumab combined with
colchicine (at a reduced dose) resulted in normalization of
transaminases, in the reduction of fibrosis markers and in a definite
improvement of liver steatosis.
Conclusions
Clinical
studies are needed to confirm the efficacy of anti-IL-1 drugs such as
canakinumab in inducing the regression of liver involvement in FMF
patients. If so, this drug might represent an excellent therapeutic
alternative for all FMF patients with evidence of hepatic disease.
Given the pathogenetic mechanism, underlying liver involvement in FMF,
and considering the mode of action of anti-IL-1 treatments, a
protective effect of IL-1 blockade for the development of liver
complications is conceivable.
References
- Ozdogan H, Ugurlu S. Familial Mediterranean fever. La Presse Med 2019;48(1 Pt 2):e61-e76. https://doi.org/10.1016/j.lpm.2018.08.014 PMid: 30686512
- Livneh
A, Langevitz P. Diagnostic and treatment concerns in familial
Mediterranean fever. Best Pract Res Clin Rheumatol 2000;14:477-98. https://doi.org/10.1053/berh.2000.0089 PMid: 10985982
- Sohar
E, Gafni J, Pras M, et al. Familial Mediterranean fever. A survey of
470 cases and review of the literature. Am J Med 1967;43:227-53. https://doi.org/10.1016/0002-9343(67)90167-2 PMid: 534064
- Tunca
M, Akar S, Onen F, et al. Familial Mediterranean fever (FMF) in Turkey:
results of a nationwide multicenter study. Medicine 2005;84:1-11. https://doi.org/10.1097/01.md.0000152370.84628.0c PMid: 15643295
- Rigante
D. A systematic approach to autoinflammatory syndromes: a spelling
booklet for the beginner. Expert Rev Clin Immunol 2017;13:571-97. https://doi.org/10.1080/1744666X.2017.1280396 PMid: 28064547
- Rigante
D. The broad-ranging panorama of systemic autoinflammatory disorders
with specific focus on acute painful symptoms and hematologic
manifestations in children. Mediterr J Hematol Infect Dis
2018;10:e2018067. https://doi.org/10.4084/mjhid.2018.067 PMid: 30416699
- Manna
R, Rigante D. Familial Mediterranean fever: assessing the overall
clinical impact and formulating treatment plans. Mediterr J Hematol
Infect Dis 2019;11:e2019027. https://doi.org/10.4084/mjhid.2019.027 PMid: 31205631
- Kees
S, Langevitz P, Zemer D, et al. Attacks of pericarditis as a
manifestation of familial Mediterranean fever. Int J Med 1997;90:643-7. https://doi.org/10.1093/qjmed/90.10.643 PMid: 9415347
- Rigante
D, Cantarini L, Imazio M, et al. Autoinflammatory diseases and
cardiovascular manifestations. Ann Med 2011;43:341-6. https://doi.org/10.3109/07853890.2010.547212 PMid: 21284530
- Rigante
D, Frediani B, Galeazzi M, et al. From the Mediterranean to the sea of
Japan: the transcontinental odyssey of autoinflammatory diseases.
Biomed Res Int 2013;2013:485103. https://doi.org/10.1155/2013/485103 PMid: 23971037
- Kasifoglu
T, Bilge SY, Sari I, et al. Amyloidosis and its related factors in
Turkish patients with familial Mediterranean fever: a multi-centre
study. Rheumatology 2014; 53: 741-5. https://doi.org/10.1093/rheumatology/ket400 PMid: 24369413
- Ben-Chetrit
E, Yazici H. The liver in familial Mediterranean fever: is it involved?
Clin Exp Rheumatol 2017; 35 Suppl 108: 108-12. PMid: 28598780
- Fraisse
T, Savey L, Hentgen V, et al. Non-amyloid liver involvement in familial
Mediterranean fever: a systematic literature review. Liver Int 2020 Mar
20 - Epub ahead of print - https://doi.org/10.1111/liv.14445 PMid: 32196885
- Goldfinger SE. Colchicine for familial Mediterranean fever. N Engl J Med 1972; 287: 1302. https://doi.org/10.1056/NEJM197212212872514 PMid: 4636899
- Rigante
D, La Torraca I, Avallone L, et al. The pharmacological basis of
treatment with colchicine in children with familial Mediterranean
fever. Eur Rev Med Pharmacol Sci 2006; 10: 173-8. PMid: 16910346
- Dinarello
CA, Wolfe SM, Goldfinger SE, et al. Colchicine therapy for familial
Mediterranean fever: a double-blind trial. N Engl J Med 1974; 291:
934-7. https://doi.org/10.1056/NEJM197410312911804 PMid: 4606353
- Ozen
S, Demirkaya E, Erer B, et al. EULAR recommendations for the management
of familial Mediterranean fever. Ann Rheum Dis 2016; 75: 644-51. https://doi.org/10.1136/annrheumdis-2015-208690 PMid: 26802180
- Ozen
S, Koné-Paut I, Gül A. Colchicine resistance and intolerance in
familial Mediterranean fever: definition, causes, and alternative
treatments. Semin Arthritis Rheum 2017; 47: 115-20. https://doi.org/10.1016/j.semarthrit.2017.03.006 PMid: 28413100
- Laskari
K, Boura P, Dalekos GN, et al. Long-term beneficial effect of
canakinumab in colchicine-resistant familial Mediterranean fever. J
Rheumatol 2017; 44: 102-9. https://doi.org/10.3899/jrheum.160518 PMid: 28042127
- Ben-Zvi
I, Kukuy O, Giat E, et al. Anakinra for colchicine-resistant familial
Mediterranean fever: a randomized double-blind placebo-controlled
trial. Arthritis Rheumatol 2017; 69: 854-62. https://doi.org/10.1002/art.39995 PMid: 27860460
- De
Benedetti F, Gattorno M, Anton J, et al. Canakinumab for the treatment
of autoinflammatory recurrent fever syndromes N Engl J Med 2018; 378:
1908-19. https://doi.org/10.1056/NEJMoa1706314 PMid: 29768139
- Kacar
M, Savic S, van der Hilst JCH. The efficacy, safety and tolerability of
canakinumab in the treatment of familial Mediterranean fever: a
systematic review of the literature. J Inflamm Res 2020; 13: 141‐9. https://doi.org/10.2147/JIR.S206204 PMid: 32210604
- Abbott CE, Xu R, Sigal SH. Colchicine-induced hepatotoxicity. ACG Case Rep J 2017; 4: e120. https://doi.org/10.14309/crj.2017.120 PMid: 29201931
- Ben-Chetrit E, Touitou I. Familial Mediterranean fever in the world. Arthritis Rheum 2009; 61: 1447-53. https://doi.org/10.1002/art.24458 PMid: 19790133
- Rigante
D. New mosaic tiles in childhood hereditary autoinflammatory disorders.
Immunol Lett 2018; 193: 67-76. https://doi.org/10.1016/j.imlet.2017.11.013 PMid: 29198619
- Rigante
D. A developing portrait of hereditary periodic fevers in childhood.
Expert Opin Orphan Drugs 2018; 6: 47-55. https://doi.org/10.1080/21678707.2018.1406797
- Lidar
M, Doron A, Barzilai A, et al. Erysipelas-like erythema as the
presenting feature of familial Mediterranean fever. J Eur Acad Dermatol
Venereol 2013; 27: 912-5. https://doi.org/10.1111/j.1468-3083.2011.04442.x PMid: 22243424
- van
der Hilst JC, Simon A, Drenth JPH. Hereditary periodic fever and
reactive amyloidosis. Clin Exp Med 2005; 5: 87-98. https://doi.org/10.1007/s10238-005-0071-6 PMid: 16284730
- Ebert
EC, Nagar M. Gastrointestinal manifestations of amyloidosis. Am J
Gastroenterol 2008; 103: 776-87. https://doi.org/10.1111/j.1572-0241.2007.01669.x PMid: 18076735
- Tilg
H, Wilmer A, Vogel W, et al. Serum levels of cytokines in chronic liver
diseases. Gastroenterology 1992; 103: 264-74. https://doi.org/10.1016/0016-5085(92)91122-K PMid: 1612333
- Ludwiczek
O, Vannier E, Moschen A, et al. Impaired counter-regulation of
interleukin-1 by the soluble IL-1 receptor type II in patients with
chronic liver disease. Scand J Gastroenterol 2008; 43: 1360-5. https://doi.org/10.1080/00365520802179925 PMid: 18609176
- Wree
A, Eguchi A, McGeough MD, et al. NLRP3 inflammasome activation results
in hepatocyte pyroptosis, liver inflammation and fibrosis in mice.
Hepatology 2014; 59: 898-910. https://doi.org/10.1002/hep.26592 PMid: 23813842
- Rimar
D, Rosner I, Rozenbaum M, Zuckerman E. Familial Mediterranean fever: an
association with nonalcoholic fatty liver disease. Clin Rheumatol 2011;
30: 987-91. https://doi.org/10.1007/s10067-011-1718-1 PMid: 21360101
- Sarkis
C, Caglar E, Ugurlu S, et al. Nonalcoholic fatty liver disease and
familial Mediterranean fever: are they related? Srp Arh Celok Lek 2012;
140: 589-94. https://doi.org/10.2298/SARH1210589S PMid: 23289274
- Lee
SS, Park SH. Radiologic evaluation of nonalcoholic fatty liver disease.
World J Gastroenterol 2014; 20: 7392-402. https://doi.org/10.3748/wjg.v20.i23.7392 PMid: 24966609
- Tweezer-Zaks
N, Doron-Libner A, Weiss P, et al. Familial Mediterranean fever and
cryptogenic cirrhosis. Medicine (Baltimore) 2007; 86: 355-62. https://doi.org/10.1097/MD.0b013e31815be056 PMid: 18004180
- La
Regina M, Ben-Chetrit E, Gasparyan AY, et al. Current trends in
colchicine treatment in familial Mediterranean fever. Clin Exp
Rheumatol 2013; 31(3 Suppl 77): 41-6. PMid: 24064013
- Leung
YY, Yao Hui LL, Kraus VB. Colchicine-update on mechanisms of action and
therapeutic uses. Semin Arthritis Rheum 2015; 45: 341-50. https://doi.org/10.1016/j.semarthrit.2015.06.013 PMid: 26228647
- Stack
J, Ryan J, McCarthy G. Colchicine: new insights to an old drug. Am J
Ther 2015; 22(5): e151-e157. https://doi.org/10.1097/01.mjt.0000433937.07244.e1 PMid: 24100258
- Terkeltaub
RA, Furst DE, Digiacinto JL, et al. Novel evidence-based colchicine
dose-reduction algorithm to predict and prevent colchicine toxicity in
the presence of cytochrome P450 3A4/P-glycoprotein inhibitors.
Arthritis Rheum. 2011; 63: 2226-2237. https://doi.org/10.1002/art.30389 PMid: 21480191
- Rigante
D, Emmi G, Fastiggi M, et al. Macrophage activation syndrome in the
course of monogenic autoinflammatory disorders. Clin Rheumatol 2015;
34: 1333-9. https://doi.org/10.1007/s10067-015-2923-0 PMid: 25846831
- Stabile
A, Bertoni B, Ansuini V, et al. The clinical spectrum and treatment
options of macrophage activation syndrome in the pediatric age. Eur Rev
Med Pharmacol Sci 2006; 10: 53-9. PMid: 16705949
- Cantarini
L, Iacoponi F, Lucherini OM, et al. Validation of a diagnostic score
for the diagnosis of autoinflammatory diseases in adults. Int J
Immunopathol Pharmacol 2011; 24: 695-702. https://doi.org/10.1177/039463201102400315 PMid: 21978701
- Ter
Haar NM, Annink KV, Al-Mayouf SM, et al. development of the
autoinflammatory disease damage index (ADDI). Ann Rheum Dis 2017; 76:
821-30. https://doi.org/10.1136/annrheumdis-2016-210092 PMid: 27811147
- Ter
Haar NM, van Delft ALJ, Annink KV, et al. In silico validation of the
Autoinflammatory Disease Damage Index. Ann Rheum Dis 2018; 77:
1599-605. https://doi.org/10.1136/annrheumdis-2018-213725 PMid: 30077992
- Pras
E, Livneh A, Balow JE Jr, et al. Clinical differences between North
African and Iraqi Jews with familial Mediterranean fever. Am J Med
Genet 1998; 75(2): 216-219. https://doi.org/10.1002/(SICI)1096-8628(19980113)75:2<216::AID-AJMG20>3.0.CO;2-R PMid: 9450890
- Cantarini
L, Lucherini OM, Frediani B, et al. Bridging the gap between the
clinician and the patient with cryopyrin-associated periodic syndromes.
Int J Immunopathol Pharmacol 2011; 24: 827-36. https://doi.org/10.1177/039463201102400402 PMid: 22230390
- Rigante
D. Phenotype variability of autoinflammatory disorders in the pediatric
patient: a pictorial overview. J Evid Based Med 2020 Jul 6; 13(3). Epub
ahead of print - https://doi.org/10.1111/jebm.12406 PMid: 32627322
- Vitale
A, Insalaco A, Sfriso P, et al. A snapshot on the on-label and
off-label use of the interleukin-1 inhibitors in Italy among
rheumatologists and pediatric rheumatologists: a nationwide multicenter
retrospective observational study. Front Pharmacol 2016; 7: 380. https://doi.org/10.3389/fphar.2016.00380 PMid: 27822185
- van
der Hilst JC, Moutschen M, Messiaen PE, et al. efficacy of anti-IL-1
treatment in familial Mediterranean fever: a systematic review of the
literature. Biologics 2016; 10: 75-80. https://doi.org/10.2147/BTT.S102954 PMid: 27110096