Giacomo Marchi1, Alice Vianello1, Ernesto Crisafulli1, Alessio Maroccia1, Stefano Francesco Crinò2, Sara Pecori3, Giulia A Zamboni4, Fulvia Mazzaferri5, Evelina Tacconelli5 and Domenico Girelli1.
1 Department of Medicine, Internal Medicine Unit, University Hospital of Verona, Verona, Italy.
2 Department of Medicine, Gastroenterology and Digestive Endoscopy Unit, University Hospital of Verona, Verona, Italy.
3 Department of Diagnostics and Public Health, Section of Pathology, University Hospital of Verona, Verona, Italy.
4 Department of Diagnostics and Public Health, Section of Radiology, University Hospital of Verona, Verona, Italy.
5 Department of Diagnostics and Public Health, Section of Infectious Disease, University Hospital of Verona, Verona, Italy.
Published: September 1, 2020
Received: May 26, 2020
Accepted: August 7, 2020
Mediterr J Hematol Infect Dis 2020, 12(1): e2020060 DOI
10.4084/MJHID.2020.060
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
COVID-19 is a new pandemic disease whose pathophysiology and clinical description are still not completely defined.
Besides
respiratory symptoms and fever, gastrointestinal (GI) symptoms
(including especially anorexia, diarrhea, and abdominal pain) represent
the most frequent clinical manifestations.
Emerging data point out
that severe SARS-CoV-2 infection causes an immune dysregulation, which
in turn may favor other infections.
Here we describe a patient
with severe COVID-19 pneumonia who developed in the resolving phase
abdominal pain associated with cytomegalovirus (CMV)-induced duodenitis
with bleeding and pancreatitis.
A high level of suspicion toward
multiple infections, including CMV, should be maintained in COVID-19
patients with heterogeneous clinical manifestations.
|
Case Report
A
73-year-old man was referred to the Emergency Department of our
University Hospital because of fever, dry cough, and worsening dyspnea
after contacts with two sons recently diagnosed with COVID-19. No
gastrointestinal (GI) symptoms were present. History revealed
multimorbidity characterized by type 2 diabetes mellitus, hypertension,
atrial fibrillation, multivessel coronary artery disease (requiring
repeated percutaneous angioplasty with stenting two years before), and
a recent diagnosis of primary cutaneous large B-cell lymphoma (PCLBCL)
leg type, localized at the right leg without extra-nodal involvement,
which was treated with local radiotherapy two months before.
The
presenting clinical and imaging picture suggested a severe acute
respiratory syndrome (SARS). The patient was severely hypoxemic
(paO2/FiO2 less than 100), chest X-ray, and computerized tomography
(CT) (Figure 1a) revealed
bilateral interstitial pneumonia. SARS-CoV-2 infection was diagnosed
through polymerase chain reaction on a nasopharyngeal swab, and the
patient was admitted to a dedicated COVID-19 area. High flows of oxygen
and continuous positive airways pressure (CPAP) were started, and the
patient was stimulated to pronation. Electrocardiogram confirmed atrial
fibrillation with a normal QTc interval. Initial blood analysis showed
C-reactive protein 263 mg/L, leukocytosis (16,610/mm3) with neutrophilia (15,940/mm3) and marked lymphocytopenia (260/mm3),
ferritin 487 mcg/L, D-dimer 719 mcg/L, fibrinogen 8.91 g/L, LDH 287
U/L, lactate 2.9 mmol/L. Hemoglobin, platelets, creatinine, and liver
enzymes were in the normal range. Lymphocyte subpopulations analysis
revealed a marked reduction of CD4+ T-cells (100/mm3).
Revision of previous records excluded any prior immunodeficiency.
Empirical treatment was started with hydroxychloroquine 200 mg BID
(after a loading dose of 400 mg BID for the first 24 hours),
lopinavir/ritonavir 100/25 mg BID, methyl-prednisolone 80 mg OD (1
mg/kg) with subsequent tapering, and enoxaparin at therapeutic doses.
Overall, the duration of the antiviral and methyl-prednisolone
treatments was ten days. After an initial critical phase, the
respiratory failure slowly improved so that the patient could be
gradually weaned from CPAP from day 14 after admission.
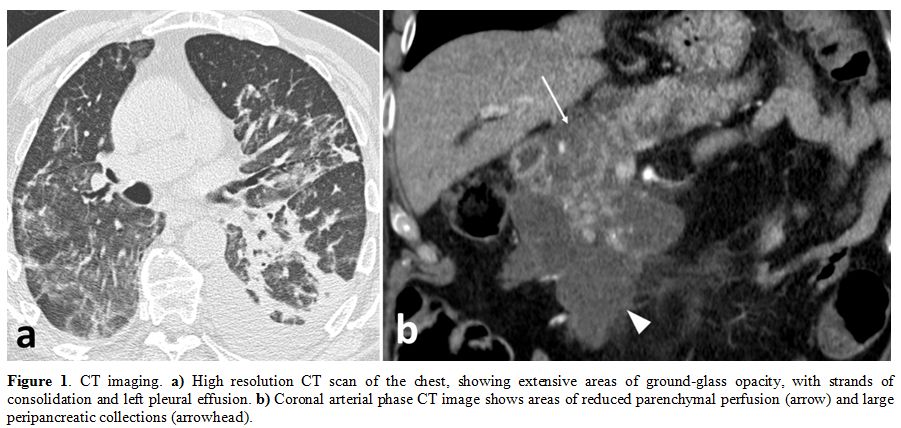 |
Figure
1. CT imaging. a)
High resolution CT scan of the chest, showing extensive areas of
ground-glass opacity, with strands of consolidation and left pleural
effusion. b) Coronal arterial
phase CT image shows areas of reduced parenchymal perfusion (arrow) and
large peripancreatic collections (arrowhead). |
However, on day 18,
the patient developed epigastric pain, melena, hypotension,
tachycardia, and a substantial drop of hemoglobin levels (from 14.5 to
10.6 g/dL). After hemodynamic stabilization with intravenous fluids and
transfusion with two units of red blood cells, an upper endoscopy was
performed, which showed multiple large and confluent ulcers in the
first and second portions of the duodenum (Figure 2a),
suggesting a Cytomegalovirus (CMV) duodenitis. The diagnosis was
confirmed by the positivity of circulating CMV-DNA (6,080 IU/mL titer)
and by duodenal histopathological findings (Figure 2b,c,d).
CMV serologic test showed positive IgG (154 U/mL; positive ≥ 14 U/mL)
and borderline IgM at diagnosis. Helicobacter pylori and Epstein-Barr
encoded RNA were not found on the histologic specimens.
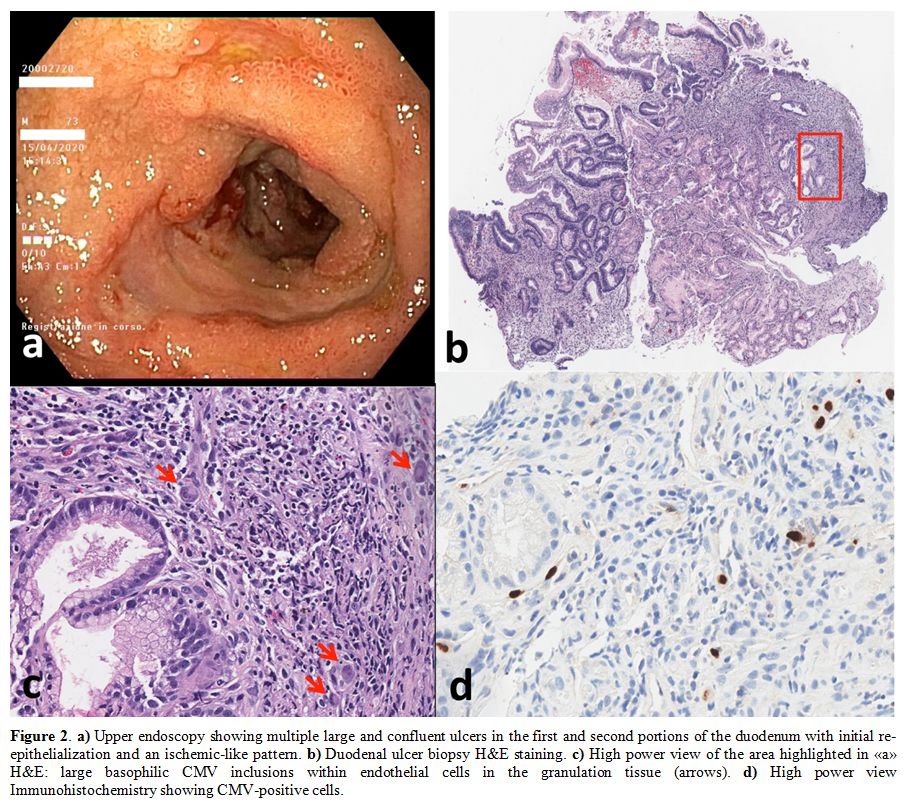 |
Figure 2. a)
Upper endoscopy showing multiple large and confluent ulcers in the
first and second portions of the duodenum with initial
re-epithelialization and an ischemic-like pattern. b) Duodenal ulcer biopsy H&E staining. c) High
power view of the area highlighted in «a» H&E: large
basophilic CMV inclusions within endothelial cells in the granulation
tissue (arrows). d) High power view Immunohistochemistry showing CMV-positive cells. |
Enoxaparin
was immediately stopped, and ganciclovir (5 mg/kg BID) was started. The
patient also underwent a CT-scan of the abdomen, which showed
pancreatitis with a non-homogeneous pattern of the pancreatic head and
peripancreatic fluid collection (Figure 1b).
Pancreatic amylases and lipases were only slightly increased (59 and 63
U/L, respectively). The clinical evolution was favorable, as abdominal
pain disappeared, and pancreatic enzymes returned within the normal
range in few days, allowing early enteral refeeding. The CMV-DNA titer
dropped to 1,320 IU/mL after 12 days of treatment with ganciclovir. On
day 43 from admission, the patient was successfully discharged by the
COVID Unit.
Discussion
Gastrointestinal
(GI) symptoms, including anorexia, diarrhea, and abdominal pain, have
been observed in near one-fourth of COVID-19 patients.[1]
They have been attributed to a direct injury by SARS-CoV-2 on
enterocytes, which highly express the viral receptor
angiotensin-converting enzyme 2 (ACE2),[2,3] although
the direct contribution of the virus to this plethora of symptoms is
uncertain. In small COVID-19 series, GI bleeding,[4] and pancreatitis[5]
have also been reported as possible complications of SARS-CoV-2
infection. Upper endoscopy has been performed rarely in COVID-19
patients, with some retrieving of SARS-CoV-2 genetic materials whose
implications remain uncertain.[6] Nevertheless, defining the pathophysiology of GI clinical manifestations in COVID-19 is critical for appropriate treatment.
Here
we describe a patient who developed a clinically relevant GI bleeding
due to severe duodenitis with multiple ulcers and pancreatitis on day
18 of severe COVID-19 pneumonia. Both duodenitis and pancreatitis are
known as possible complications of CMV infection/reactivation in the
immunocompromised host.[7-11] We could demonstrate
that duodenitis was due to CMV, while some uncertainty remains about
the etiology of pancreatitis, as histology was not feasible.
Nevertheless, no obvious causes of pancreatitis were evident, as CT did
not reveal gallstones, triglycerides were in the normal range, and the
patient had no history of alcohol abuse or recurrent pancreatitis.
Considering the whole clinical picture, the probability of CMV-induced
pancreatitis was quite high in our patient. An emerging feature of
severe COVID-19 patients is represented by secondary immune
dysregulation,[12] heralded by marked lymphocytopenia, which involves all the lymphocyte subtypes, and functional impairment of innate immunity.[13,14] Indeed, in our patient, we observed a marked reduction of lymphocytes, with a nadir of 260/mm3, of which CD4+ T-cells were 100/mm3, CD8+ T-cells were 39/mm3, B-cells were 33/mm3, and NK cells were 82/mm3.
Since the CMV IgG test was positive, a possible explanation is the
reactivation of CMV in an immunocompromised host with severe COVID-19
pneumonia. Corticosteroid treatment may have further contributed to
immunodepression, while it is unlikely that the localized PCLBCL with
no signs of evolution at the moment played a role. Intriguingly, CMV,
and SARS-CoV-2 infections may have potentiated each other, since they
share some innate immunity pathways. For example, patients affected by
COVID-19, besides having a reduced number of NK and CD8+ T cells, also
show functional exhaustion of these cells, with an increased expression
of the inhibitory receptor NKG2A.[15] NKG2A signal
can suppress the cytotoxic activity of NK and CD8+ T cells, and promote
viral spreading during a variety of chronic viral infections, including
CMV.[14] Furthermore, CMV infection can also influence the expression of NKG2A,[16] and it has been linked to the pathogenesis of a number of disorders characterized by immune dysregulation.[17,18]
In
conclusion, to the best of our knowledge, this is the first description
of CMV-associated severe GI complications in a patient with COVID-19
pneumonia. We hypothesize that CMV reactivation may be due to the
marked immune dysregulation during severe COVID-19 pneumonia, which in
turn may be further influenced by the use of immuno-regulatory drugs
(e.g., glucocorticoids, tocilizumab, and others).[19] This complication, potentially treatable, may be overlooked in patients with COVID-19 and secondary immune dysfunction.
References
- Cheung KS, Hung IF, Chan PP, Lung K, Tso E, Liu R,
Ng Y, Chu MY, Chung TW, Tam AR, Yip CC, Leung K-H, Yim-Fong Fung A,
Zhang RR, Lin Y, Cheng HM, Zhang AJ, To KK, Chan K-H, Yuen K-Y, Leung
WK. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus
Load in Fecal Samples from the Hong Kong Cohort and Systematic Review
and Meta-analysis. Gastroenterology 2020, https://doi.org/10.1053/j.gastro.2020.03.065
- Hamming
I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue
distribution of ACE2 protein, the functional receptor for SARS
coronavirus. A first step in understanding SARS pathogenesis. J Pathol
2004;203(2):631-637 https://doi.org/10.1002/path.1570 PMid:15141377 PMCid:PMC7167720
- Gu J, Han B, Wang J. COVID-19: Gastrointestinal Manifestations and Potential Fecal–Oral Transmission. Gastroenterology 2020. https://doi.org/10.1053/j.gastro.2020.02.054
- Tian
Y, Rong L, Nian W, He Y. Review article: gastrointestinal features in
COVID-19 and the possibility of faecal transmission. Aliment Pharmacol
Ther 2020,51(9):843-851. https://doi.org/10.1111/apt.15731 Epub 2020 Mar 31.
- Liu
F, Long X, Zou W, Fang M, Wu W, Li W, Zhang B, Zhang W, Chen X, Xhang
Z. Highly ACE2 Expression in Pancreas May Cause Pancreas Damage After
SARS-CoV-2 Infection. Medrxiv 2020. https://doi.org/10.1101/2020.02.28.20029181
- Beattie
RM, Ashton JJ, Penman ID. COVID-19 and the gastrointestinal tract:
emerging clinical data. Frontline Gastroenterology 2020. https://doi.org/10.1136/flgastro-2020-101507
- Emery VC. Investigation of CMV disease in immunocompromised patients. J Clin Pathol 2001,54:84-88 https://doi.org/10.1136/jcp.54.2.84 PMid:11215290 PMCid:PMC1731357
- Perdan-Pirkmajer
K, Koren-Kranjc M, Tomsic M. A successfully treated pancreatitis caused
by a CMV infection in a lupus patient. Lupus 2011;20(10):1104-5. https://doi.org/10.1177/0961203311398514 PMid:21562021
- Terada
T. Cytomegalovirus-associated severe fatal necrotizing pancreatitis in
a patient with interstitial pneumonitis treated with steroids. An
autopsy case. JOP 2011, 12(2):158-61.
- Kamalkumar
BS, Agarwal SK, Garg P, Dinda A, Tiwari SC. Acute pancreatitis with CMV
papillitis and cholangiopathy in a renal transplant recipient. Clin Exp
Nephrol 2009, 13(4):389-91 https://doi.org/10.1007/s10157-008-0123-9 PMid:19142576
- Sakakibara
Y, Nakazuru S, Kodama Y, Mita E. Acute pancreatitis caused by
cytomegalovirus-associated duodenal papillitis. Annals of
Gastroenterology 2017. https://doi.org/10.20524/aog.2017.0188
- Li H, Liu L, Zhang D, Xu J, Dai H, Tang N, Su X, Cao B. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet 2020. https://doi.org/10.1016/S0140-6736(20)30920-X
- Giamarellos-Bourboulis
EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, et
al. Complex Immune Dysregulation in COVID-19 Patients with Severe
Respiratory Failure. Cell Host & Microbe 2020. https://doi.org/10.1016/j.chom.2020.04.009
- Antonioli
L, Fornai M, Pellegrini C, Blandizzi C. NKG2A and COVID-19: another
brick in the wall. Cellular & Molecular Immunology 2020,
17:672–674. https://doi.org/10.1038/s41423-020-0450-7
- Zheng,
M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes
in COVID-19 patients. Cell Mol Immunol 2020, 17:533–535. https://doi.org/10.1038/s41423-020-0402-2
- Foley
B, Cooley S, Verneris MR, et al (2012) Cytomegalovirus reactivation
after allogeneic transplantation promotes a lasting increase in
educated NKG2C+ natural killer cells with potent function. Blood 2012,
119(11):2665-2674. https://doi.org/10.1182/blood-2011-10-386995
- Halenius A, Hengel H. Human Cytomegalovirus and Autoimmune Disease. BioMed Res Intern 2014. https://doi.org/10.1155/2014/472978
- Jaiswal
S.R., Malhotra P., Mitra D.K., Chakrabarti S.. Focusing on a unique,
innate memory cell population of natural killer cells in the fight
against COVID-19: harnessing the ubiquity of
cytomegalovirus exposure. Mediterr J Hematol
Infect Dis 2020, 12(1): e2020047, https://doi.org/10.4084/MJHID.2020.047
- Sanders
JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic Treatments for
Coronavirus Disease 2019 (COVID-19) A Review. JAMA 2020. https://doi.org/10.1001/jama.2020.6019