Samir K. Ballas.
Cardeza Foundation for
Hematologic Research, Department of Medicine, Sidney Kimmel Medical
College, Thomas Jefferson University, Philadelphia, PA, USA.
Correspondence to: Samir K. Ballas MD FACP. Cardeza Foundation,
Department of Medicine, Sidney Kimmel Medical College, Thomas Jefferson
University, 1020 Locust Street, Philadelphia, PA 19107. Tel: 856 745
6380, Fax: 856 795 0809. E-Mail:
Samir.ballas@jefferson.edu
Published: September 1, 2020
Received: June 16, 2020
Accepted: August 13, 2020
Mediterr J Hematol Infect Dis 2020, 12(1): e2020064 DOI
10.4084/MJHID.2020.064
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Sickle
pain is the hallmark of sickle cell disease (SCD). It could be acute,
persistent/relapsing, chronic, or neuropathic. Although there is a
general consensus that pain is a major manifestation of SCD, there is a
controversy as to the types of pain and their interrelationship between
acute, chronic, relapsing, persistent, etc. This report first reviews
the general approach to the management of acute vaso-occlusive crisis
(VOC) pain, including education, counseling, pharmacotherapy,
non-pharmacotherapy, and fluid therapy. This is followed by the
presentation of five patients that represent typical issues that are
commonly encountered in the management of patients with SCD. These
issues are: individualized treatment of pain, bilaterality of pain, use
of illicit drugs, tolerance to opioids, opioid-induced hyperalgesia,
and withdrawal syndrome. The clinical aspects and management of each of
these issues are described. Moreover, such complications as tolerance
and withdrawal may persist after discharge and may be mistaken as
chronic pain rather than resolving, persistent or relapsing pain.
|
Introduction
The
hallmark of sickle cell disease (SCD) is the recurrent acute painful
vaso-occlusive crises (VOCs) and the persistent pain (PP) in between
crises in about 50% of adults and 9% of children.[1-3] These types of
pain are unique to patients with SCD and punctuate the quality of their
life with uncertainty, suffering, poor education, poverty,
dysfunctional family life, and dependence on a fragile medical support
system. The frequency, severity, location, and duration of both the
VOCs and PP vary considerably among patients and longitudinally in the
same patient. The reasons for these fluctuations are not well known.[4]
Moreover, most patients present with neither obvious precipitating
factors nor objective signs.[5-7] This state of affairs creates
suspicion among some providers about the authenticity of the VOCs and
the resulting accusations of maladaptive behavior.[8]
The PP
between crises has been labeled as chronic pain by some providers.[1]
By doing so, the uniqueness of sickle cell pain is undermined, and the
patients with SCD are lumped with other chronic pain syndromes in the
general population. This lumping rendered the PP subject to the rules,
regulations, and guidelines for the treatment of chronic pain.[9]
Moreover, since patients with SCD use relatively frequent and large
doses of opioids, they have been assumed to be associated with the
opioid epidemic. Consequently, patients with SCD and pain became often
unfairly undertreated with opioids.
The purpose of this report is
to describe patients with SCD who presented with different pain
characteristics that were addressed and resolved in a manner based on
the changing reality of pain among patients over the dimensions of
space and time.[10]
Overview of Treatment
Education and counseling.
Educating and counseling patients with SCD is a continuous process that
starts when first seen and continues through future follow-ups. I
explain the beneficial and harmful effects of prescribed medications,
including opioids. Prescriptions are given as needed. Vaccines
are administered when required.[11] Patients, parents, and other family
members are instructed on what to expect regarding sickle cell
syndromes by making them aware of the signs and symptoms of VOCs,
infection, acute chest syndrome (ACS), etc. The adoption of good health
habits is reinforced, and the avoidance of situations and factors that
could precipitate a VOC is emphasized.[5,11]
This process of
education and counseling results in a written consent form and
individualized treatment plan with the patient or parents if long-term
opioids are indicated.[12] The agreement lists the patient's rights and
responsibilities, and the treatment plan contains the type, amount, and
route of administration of the opioid in question, including random
drug urine testing.
Pharmacotherapy of Pain.
I use nonsteroidal anti-inflammatory drugs (NSAIDs), short-acting
opioids, and adjuvants to treat acute pain.[13,14] The use of NSAIDS is
limited to patients whose serum creatinine is ≤ 1.0 mg/dL, and they
have no proteinuria or albuminuria. The adjuvants include
antihistamines, antiemetics, laxatives, antidepressants, and
gabapintinoids as needed. I go over the personal side effects of
opioids listed in Table 1. The
use of opioids by patients with SCD is not as problematic as it is in
the general population. A review of data from the Centers for Disease
Control and Prevention (CDC) between 1999 and 2013 showed that less
than 1% of deaths among patients with SCD was due to opioid overdose,
and this low rate of mortality did not change significantly over the
15-year data.[15]
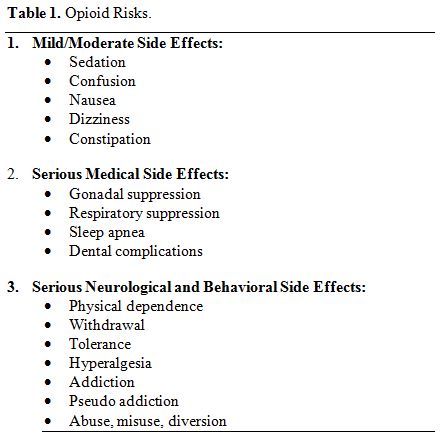 |
Table 1. Opioid Risks. |
Fluid Therapy.
I encourage my patients to use water for oral hydration and avoid soft
drinks as often as possible. Signs and symptoms of dehydration include
dry mouth, tongue, and lips, decreased skin turgor, flat neck veins,
and serum creatinine level higher than steady-state values. I do not
use normal saline for intravenous hydration but use 5% DW or other
crystalloids. I monitor the status of hydration by determining daily
fluid intake and output, daily weight, and check if edema
develops.[16-18] Overhydration, like over blood transfusion, could be
fatal.[19-21]
Beyond Pharmacotherapy.
With the help of our social workers, we address the psychosocial
factors that pertain to each patient and recommend solutions. We also
recommend nonpharmacologic therapies such as meditation, yoga, massage,
relaxation, tai chi, etc.[22,23] Neuropathophysiology of Pain
This was previously reported10 and summarized in Table 2 and Figure 1.[24]
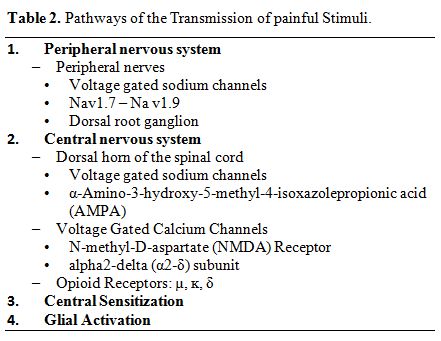 |
Table 2. Pathways of the Transmission of painful Stimuli. |
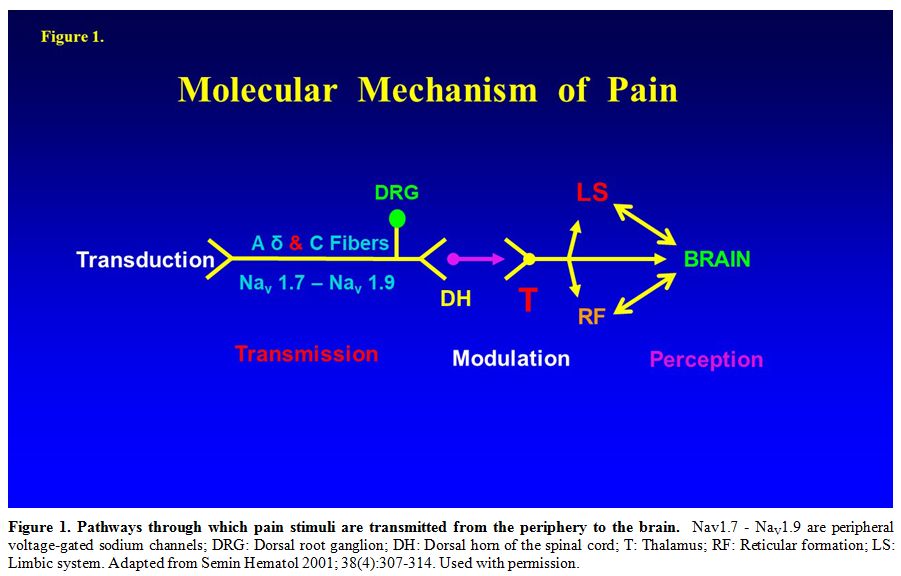 |
Figure 1. Pathways through which pain stimuli are transmitted from the periphery to the brain.
Nav1.7 - NaV1.9 are peripheral voltage-gated sodium channels; DRG:
Dorsal root ganglion; DH: Dorsal horn of the spinal cord; T: Thalamus;
RF: Reticular formation; LS: Limbic system. Adapted from Semin Hematol
2001; 38(4):307-314. Used with permission. |
Patient 1: A woman with Hb SC disease and VOC that went amiss
A
39-year old African American woman known to have hemoglobin (Hb) SC
disease was admitted to the hospital with the diagnosis of VOC. The
pain involved the left shoulder and the upper/lower back and was
constant, sharp and throbbing in nature with an intensity score of 10
on a scale from 0 (no pain) to 10 (most severe pain). Past medical
history was significant for VOCs at a rate of 2-3 VOCs per year with no
pain between VOCs, avascular necrosis (AVN) of the left humeral joint,
pneumonia, urinary tract infection, and a remote history of allergy to
morphine. In the emergency department (ED), she was given meperidine
125 mg intravenously (IV) every two hours. She did not achieve adequate
pain relief after receiving 3 doses of meperidine and, hence, was
admitted to the hospital. The attending provider decided to start her
on patient-controlled analgesia (PCA) pump using morphine lockout dose
1 mg, lockout interval 10 minutes, and a one-hour dose limit of 8 mg
morphine. The patient indicated that she is allergic to morphine and
usually receives meperidine for pain, but she could not give details
about the nature of the allergy to morphine. About 8 hours after
starting the PCA, she experienced hallucinations, disorientation, fever
103 ͦ F, chest oppression, and difficulty breathing with pulse Oximetry
of 86%. She was transferred to the intensive care unit and intubated.
The chest x-ray was normal. She recovered within 24 hours, and
management of pain was resumed with meperidine and was discharged 7
days after admission.
Comments on patient 1.
Management of patients with sickle cell pain should be individualized.
Patients with SCD are authorities on their disease. They know what
helps them most. Accordingly, providers should listen, believe, and
respect patients unless proven otherwise. The selection of a specific
opioid and its dose should be based on the patient's previous
experience. No opioid or a specific dose of an opioid applies to all
patients all the time. Opioids are ligands that bind to receptors and
slow the transmission of painful stimuli along the central nervous
system pathways. The binding to and activation of a specific receptor
by an opioid vary considerably among patients. Opioid receptors are G
protein-coupled with exogenous and endogenous opioids as ligands.[25]
Recent studies[25-27] have revealed a helical structure of the opioid
receptors, which forms pockets in which the corresponding ligand
(opioid) fits snugly. Not all opioids fit snugly into the same
receptor's pocket. This explains why some patients may have better
analgesia with a certain opioid but not with another opioid.
Patient 2: A man with SCA and urine drug screen positive for cannabis and phencyclidine
A
22 year-old-African American man with sickle cell anemia (SCA) whose
past medical history was significant for frequent VOCs that required
hospitalizations > 5 times per year with intermittent pain between
VOCs. Pain during crises was usually constant, sharp, and throbbing in
nature with a score of 8-10/10 and involved the low back, right
shoulder, knees, and legs. Complications of his disease included ACS,
AVN of hips and right shoulder, priapism, and frequent blood
transfusions. In addition, he had asthma as a child and heparin-induced
thrombocytopenia. He refused to take hydroxyurea. Social history was
positive for tobacco, cannabis, and alcohol use. Lab data in the
steady-state included Hb that varied between 8 and 10 g/dL, Hb F 11%,
reticulocyte count 10-15%, WBC count 8 - 14 B/L, normal platelet count,
mildly elevated total bilirubin level and normal hepatic and renal
parameters. Pain management included morphine and ketorolac during
hospitalizations and oxycodone/ acetaminophen (Percocet) as an
outpatient.
When first seen in our center, agreement, and
consent forms that included random urine drug testing were discussed
and signed by the patient and the provider. The first random urine drug
testing done was positive for opiates and cannabinoids. Intensive
counseling indicated that he smoked cannabis because Percocet did not
give him adequate pain relief. The issue was resolved by replacing
Percocet with morphine for the treatment of pain as an outpatient with
the patient affirmation that he will discontinue using cannabis subject
to confirmation by random urine drug testing. Indeed, random urine drug
testing one year later was negative for cannabis and positive for the
opiates (morphine) he was taking. Unfortunately, the random urine drug
testing done later when he was 24 years old was positive for opiates
and phencyclidine.
Another round of counseling revealed that
phencyclidine gave him much better pain relief than morphine used to
do. Accordingly, pain management was modified to use methadone up to 60
mg orally/day instead of morphine and 5% lidocaine patches to apply
over the most painful area for a maximum of 8 hours per day as needed.
Electrocardiogram (EKG) before and after using methadone showed no
prolongation of the QTC interval. Methadone was chosen because, like
phencyclidine, it inhibits the N-methyl-D-aspartate (NMDA) channel but
less severely. This approach resulted in the discontinuation of
phencyclidine and repeating urine drug test at the age of 25 years and
again at the age of 26 years when he was last seen was positive for
opiates only and negative for cannabinoids and phencyclidine.
Comment on patient 2.
This patient is a typical example of opioid tolerance that leads to the
use of illicit drugs. It is defined as reduced potency of the
analgesic effect of an opioid after repeated administration or the need
for higher doses to maintain the same result. It shifts the
dose-response curve to the right (Figure 2A).[28] The binding of an opioid to its receptor generates a series of reactions that could culminate in tolerance, as shown in Figure 3.[29]
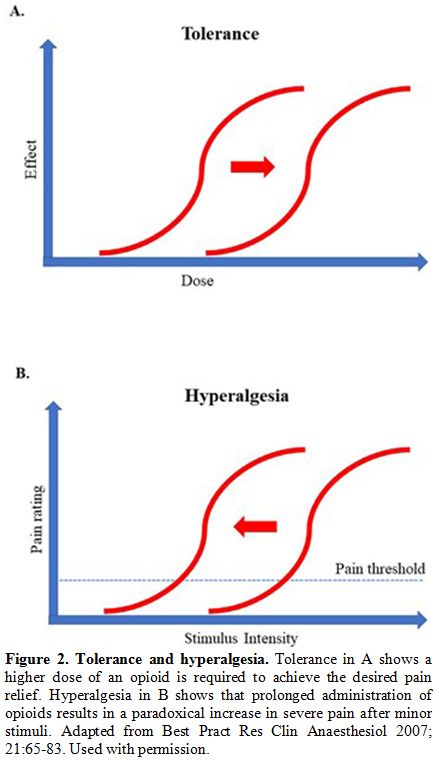 |
Figure 2. Tolerance and hyperalgesia.
Tolerance in A shows a higher dose of an opioid is required to achieve
the desired pain relief. Hyperalgesia in B shows that prolonged
administration of opioids results in a paradoxical increase in severe
pain after minor stimuli. Adapted from Best Pract Res Clin Anaesthesiol
2007; 21:65-83. Used with permission. |
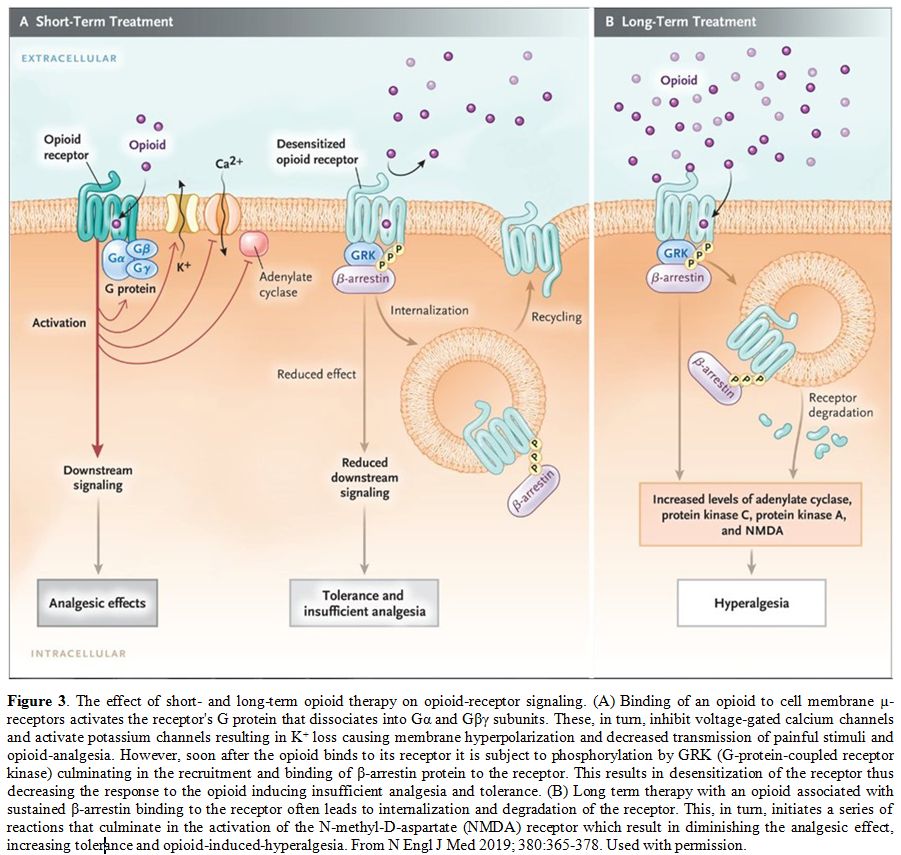 |
Figure 3 The effect of short-
and long-term opioid therapy on opioid-receptor signaling. (A) Binding
of an opioid to cell membrane µ-receptors activates the receptor's G
protein that dissociates into Gα and Gβγ subunits. These, in turn,
inhibit voltage-gated calcium channels and activate potassium channels
resulting in K+ loss causing membrane hyperpolarization and decreased
transmission of painful stimuli and opioid-analgesia. However, soon
after the opioid binds to its receptor it is subject to phosphorylation
by GRK (G-protein-coupled receptor kinase) culminating in the
recruitment and binding of β-arrestin protein to the receptor. This
results in desensitization of the receptor thus decreasing the response
to the opioid inducing insufficient analgesia and tolerance. (B) Long
term therapy with an opioid associated with sustained β-arrestin
binding to the receptor often leads to internalization and degradation
of the receptor. This, in turn, initiates a series of reactions that
culminate in the activation of the N-methyl-D-aspartate (NMDA) receptor
which result in diminishing the analgesic effect, increasing tolerance
and opioid-induced-hyperalgesia. From N Engl J Med 2019; 380:365-378.
Used with permission. |
Recent
studies in mice have shown that tolerance to morphine seems to be
modulated by the gut-microbiome-central nervous system
interactions.[30-32]
Management of opioid tolerance entails the
use of NMDA inhibitors. Actually, the illicit phencyclidine used by
this patient is a potent inhibitor of the NMDA (Figure 4) not only at the level of the spinal cord but in all other tissues and organs and, hence, could be lethal.[33]
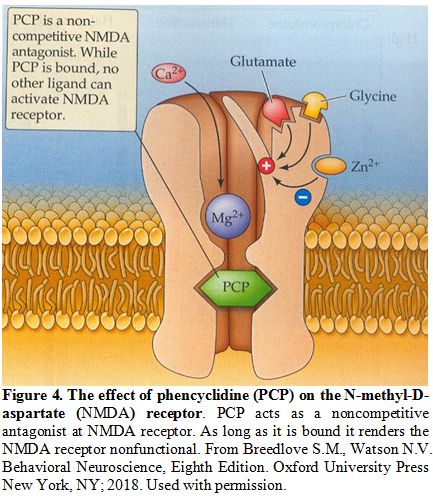 |
Figure 4. The effect of phencyclidine (PCP) on the N-methyl-D-aspartate (NMDA) receptor.
PCP acts as a noncompetitive antagonist at NMDA receptor. As long as it
is bound it renders the NMDA receptor nonfunctional. From Breedlove
S.M., Watson N.V. Behavioral Neuroscience, Eighth Edition. Oxford
University Press New York, NY; 2018. Used with permission.
|
The
NMDA channel is a complex structure.[34] It is both a receptor and a
calcium-gated channel.[35,36] Therapeutic inhibitors of NMDR include
ketamine, clonidine, Lidocaine, dextromethorphan, nitrous oxide, zinc,
and methadone.[29,37,38] More recently, rosuvastatin, B vitamins, and
inhibition of platelet-derived growth factor-β (PDGFR-β) have been
shown to attenuate or eliminate the development of tolerance to
morphine in rats and mice.[39-42] Donica et al. reported that combining
imatinib with a previously ineffective dose of morphine led to complete
pain relief in male Sprague-Dawley rats.[42] In addition, imatinib was
effective in treating sickle cell VOC in patients with chronic myeloid
leukemia and SCA, probably by inhibiting PDGFR-β.[43,44]
Patient 3: A woman with Hb SC and VOC after C-section that became symmetrically bilateral
A
26-year-old pregnant African American woman Hb SC disease had a
Cesarean section (C-section) at week 37 gestation due to signs of fetal
distress with abnormal fetal heart tracing. The surgery was
uneventful, and the fetus survived with a normal APGAR score. Past
medical history was significant for relatively infrequent VOCs (< 2
per year) and splenic sequestration during infancy that did not require
splenectomy. During pregnancy, she took oxycodone 5 mg plus
acetaminophen 325 mg (Percocet) prn for pain. The newborn infant had no
signs/symptoms of neonatal abstinence syndrome. She was advised not to
breastfeed her baby. On the 4th post-operative day, she had a sudden
onset of severe pain, swelling, and tenderness in her right ankle. She
achieved partial relief with morphine, 6 mg IV every 2 hours. About 24
hours later, she had the same severe "mirror image" pain in her left
ankle. Some providers questioned the validity of the symmetrical pain
in the left ankle due to the unlikely possibility of having
vaso-occlusion in such a symmetrical pattern. Physical exam, however,
revealed the presence of similar swelling and tenderness over both
ankles. Better pain relief was achieved by increasing the dose of
morphine to 8 mg IV every 2 hours. She continued to improve gradually
and was discharged with her infant ten days after admission.Comment on patient 3.
This patient illustrates two important issues in SCD: postpartum
breastfeeding and the pathophysiology of the incidence of symmetrical
bilateral pain.Women
with SCD who take opioids during pregnancy must not breastfeed their
infants to prevent newborn withdrawal syndrome that could be fatal.
Codeine used to be considered a safe opioid analgesic for pain during
breastfeeding. This changed after a tragic case report that pertains to
an infant who died at the age of 13 days from morphine poisoning; the
source of morphine was the codeine that the mother was taking. Further
studies showed that the mother was an ultra-rapid metabolizer of
codeine, due to duplication of the CYP2D6 enzyme that metabolized
codeine into morphine.[45,46] The recommendation changed, indicating
that women, in general, must not take opioids during the breastfeeding
period. Symmetrical
bilaterality of pain, such as both hips, both knees, was common in more
than 60% of patients enrolled in the PiSCES study [47] and was also
reported by others.[48] The ankles and feet were the most common
locations of bilaterality. The pathophysiology of this bilaterality is
not known. One possibility is that it is referred pain to a site
different than the original site of pathology.[49] Shunting of the
blood away from the bone marrow, the steal syndrome, is another
possibility.[48] Another explanation of bilaterality is that it is due
to central sensitization at the level of the spinal cord, as described
by Woolf[50] in rats. The convergence of nerve fibers from two different
sites at the same level in the spinal cord is perceived as pain in both
sites (Figure 5).[51]
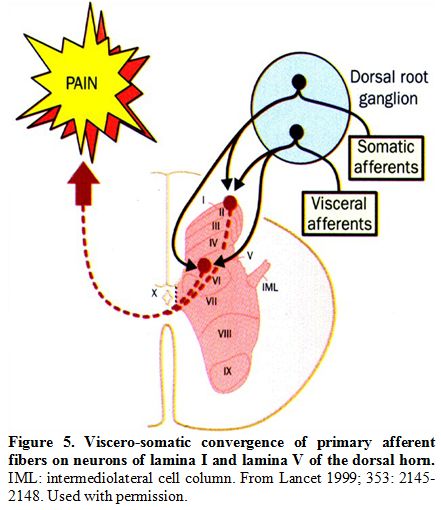 |
Figure 5. Viscero-somatic convergence of primary afferent fibers on neurons of lamina I and lamina V of the dorsal horn. IML: intermediolateral cell column. From Lancet 1999; 353: 2145-2148. Used with permission.
|
Most
recently, bilaterality seems to be due to a phenomenon called
"bioelectric injury mirroring".[52] This extends our knowledge about
the electrophysiology of regenerative response and identifies a novel
communication process via a long-range spread of injury signaling (Figure 6).[52]
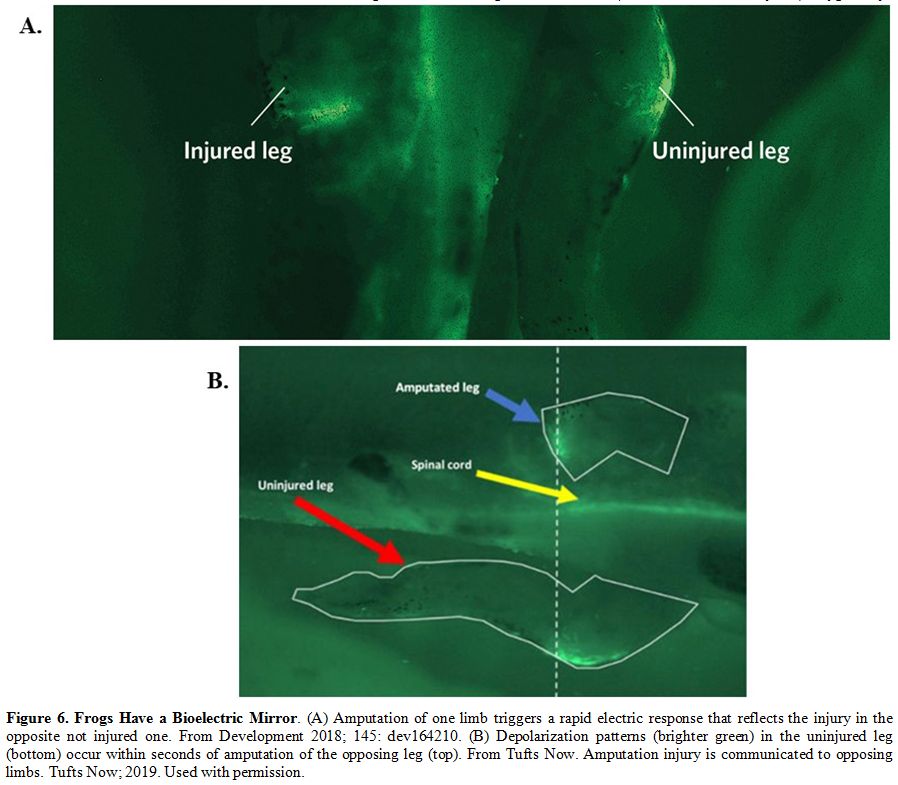 |
Figure 6. Frogs Have a Bioelectric Mirror.
(A) Amputation of one limb triggers a rapid electric response that
reflects the injury in the opposite not injured one. From Development
2018; 145: dev164210. (B) Depolarization patterns (brighter green) in
the uninjured leg (bottom) occur within seconds of amputation of the
opposing leg (top). From Tufts Now. Amputation injury is communicated
to opposing limbs. Tufts Now; 2019. Used with permission.
|
Patient 4: A woman with Hb S-β0-thalassemia whose pain worsened after increasing the dose of morphine
A
29-year-old African American woman with sickle -β0-thalassemia was
admitted to the hospital with VOC involving her low back, chest, and
knees. The pain was typical of her VOCs and was constant and
sharp/throbbing in nature with an intensity score of 10/10. She also
complained of fatigue, malaise, nausea, and vomiting. Past medical history was significant for frequent VOCs (≥ 5 per year) that required treatment in the ED or
in the hospital, cholecystectomy, splenectomy, ACS, repeated blood
transfusions, iron overload, deep vein thrombosis, migraine headache,
urinary tract infection, and C-section at 34 weeks gestation due to
twin pregnancy with both babies in the breech position.Pain
management in this admission included a morphine PCA pump with a basal
rate of 4 mg/h and 1 mg lockout every 10 minutes with a one-hour dose
limit of 10 mg and ibuprofen. Emesis was controlled with ondansetron
IV. Adjuvants included antihistamines and laxatives. She required two
units of RBC transfusion to keep her Hb > 8g/dL. She was also given
heparin for deep vein thrombosis prophylaxis. She continued to complain
of severe pain that required increasing the dose limit of morphine to
16 mg/hour. At the same time, the distribution and the
descriptors of the pain changed; it became worst in her legs and deep
burning in nature. Examination showed severe allodynia where a
superficial touch of her legs caused severe pain, and she avoided
covering her legs with the blanket to prevent pain. The diagnosis of
morphine-induces hyperalgesia was made. The dose of morphine was
gradually decreased and replaced with an equianalgesic dose of
hydromorphone, up to 8 mg iv q2hour. Eventually, adequate relief was
achieved with hydromorphone. She was discharged on the 24th hospital
day on hydromorphone and ibuprofen.Comment on patient 4.
This patient's pain is a typical example of opioid-induced hyperalgesia
(OIH). It is defined as increased sensitivity to pain stimuli
(hyperalgesia) and pain caused by ordinarily nonpainful stimuli
(referred to as allodynia). Typically, hyperalgesia is noted in parts
of the body different from the site of the original pain complaint, and
the descriptors of the pain change with some similarity to certain
aspects of neuropathic pain such as burning sensation. Unlike
tolerance, OIH worsens with higher doses of opioids (Figure 2B).[53-55]The
pathophysiology of OIH is not well understood. A proposed mechanism is
the activation of the NMDA receptor.[33,53] This activation results in
calcium influx, which in turn enhances the excitability of neurons,
which facilitates further transmission of painful stimuli.[33]Studies
in rats showed that morphine hyperalgesia appears to be secondary to
the activation of specific receptors within microglia since the
ablation of the microglia prevented OIH.[56] Figure 7 illustrates how glia interact with neurons and the surrounding blood vessels.[57]
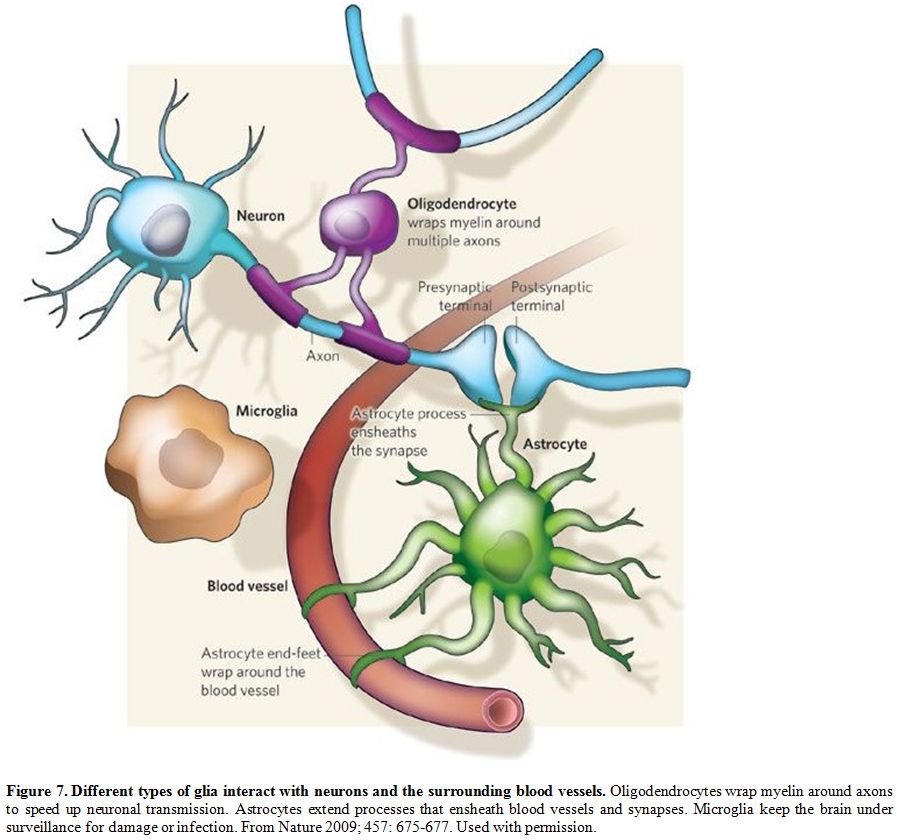 |
Figure 7. Different types of glia interact with neurons and the surrounding blood vessels.
Oligodendrocytes wrap myelin around axons to speed up neuronal
transmission. Astrocytes extend processes that ensheath blood vessels
and synapses. Microglia keep the brain under surveillance for damage or
infection. From Nature 2009; 457: 675-677. Used with permission.
|
Management
of OIH involves weaning from opioids, opioid rotation, and the use of
NMDA inhibitors such as methadone, clonidine, Lidocaine, or ketamine as
needed. Weaning and rotation are usually done together, as was
described in this patient.
Patient 5: A man with SCA and recurrent severe pain between VOCs
A
42-year-old African American man with SCA presented to the ED with
severe diarrhea and nausea/vomiting of five-days duration. These signs
and symptoms were associated with nasal congestion, rhinorrhea, cough,
and severe crampy abdominal pain and a VOC with severe pain involving
his low back, arms, and legs that brought him to the ED. Medications
included Hydroxyurea 1500 mg/day and hydromorphone 4 mg by mouth q 2h
as needed.Other
complications of his SCA included a history of cholecystectomy,
obstructive sleep apnea treated at home with oxygen, AVN of the right
hip that required arthroplasty, and pneumonia.Physical
exam included a temperature of 99.6°F, RR 30/min heart rate 130/min,
pulse oximetry 99% on 2 liters oxygen. The patient was restless,
anxious, and sweaty. Heart and lung exams were normal. A large ulcer
over the left lateral ankle and a smaller ulcer over the right medial
ankle were both clean and healing gradually.Lab
data included Hb 7.7 g/dL, reticulocyte count 7.9%, mean corpuscular
volume (MCV) 120 fL, serum creatinine 1.0 mg/dL and normal serum
electrolytes and liver function tests. A diagnosis of VOC precipitated
by viral gastroenteritis was made and treated accordingly. Cultures of
stools, urine, and blood were all negative. Chest X-ray and EKG were
within normal. Treatment included hydration, opioid analgesia with
hydromorphone up-to 8mg q 2h IV plus hydroxyzine as an adjuvant,
antiemetics with ondansetron, and anti-laxatives with loperamide. He
improved gradually, and after 15 days of hospitalization, he was
discharged on hydromorphone 4 mg by mouth q2h and acetaminophen 325
mg/oxycodone 5 mg three times daily as needed. During
the six months after this hospitalization, he was readmitted about once
every month with similar signs and symptoms. The diagnosis was changed
to gastroenteritis of unknown etiology. The home treatment of pain was
changed up to 16 mg hydromorphone po every 2 hours. At this point, he
was referred to our center for advice.A
detailed review of the history and the physical exam revealed that the
patient had adequate pain relief with hydromorphone used at home for
3-4 days. After that, he gradually developed diarrhea, nausea/vomiting,
running nose, abdominal cramps, followed by typical symptoms of VOC.
This sequence of events recurred before the frequent hospital
admissions mentioned above. This sequence of events was typical of
withdrawal signs and symptoms, and which was treated with clonidine
(0.2 mg three times daily) and methadone (30 mg daily that was
increased gradually to a maximum of 60 mg/day The patient improved
gradually and the gastrointestinal and respiratory signs and symptoms
resolved. He was advised to continue taking clonidine and methadone for
3-4 weeks, after which he will be reevaluated for possible changes.Comment on patient 5.
Withdrawal syndrome is a conglomerate of physical and behavioral signs
and symptoms after the discontinuation or decreasing the dose of an
opioid or other addictive drug. The severity of the symptoms depends on
the drug in question and its dosage. These include yawning, sweating,
lacrimation, rhinorrhea, anxiety, irritability, restlessness, insomnia,
dilated pupils, piloerection, chills, tachycardia, hypertension,
nausea/vomiting, cramping abdominal pains, diarrhea, and muscle aches
and pains.The
incidence and management of withdrawal in SCD have not been well
studied. It is often confused with acute or chronic pain, infection, or
other comorbidities. It is a cause for hospital readmission within
one-two weeks after discharge.[58]Treatment
of severe opioid withdrawal includes methadone plus clonidine either
orally (0.1- 0.2 mg every 4-6 hours prn or by using transdermal
clonidine patch 0.1 mg daily. Other drugs that may be used to treat
withdrawal include buprenorphine plus naloxone
orally.[59,60] Recently, the FDA approved oral lofexidine to treat
the symptoms of withdrawal.[61] Lofexidine is a structural analog of
clonidine. Clinical trials comparing the two medications showed
comparable efficacy, though the severity of adverse events was less
than those with clonidine. This decreased risk for adverse effects
could potentially make lofexidine a safer option for
detoxification.[62-64]
Persistent Sickle Cell Pain
The
word "chronic" in SCD is problematic and subject to different
explanations. Thus, SCD itself is a chronic disease that is usually
symptomatic from childhood through adulthood. Typically,
SCD pain is either acute, which is the hallmark of the disease or
chronic. The latter includes such complications as leg ulcers, AVN of
humeral or femoral heads, and bone infarcts.[65-67]These
chronic pain syndromes are localized and last for months or years.
Recently chronic pain in SCD has been defined as ongoing pain that is
present in at least 50% of days over 3 or 6 months in a single or
multiple locations.[6,68] The problem with this definition is that
during the period of 3- or 6-months, patients with SCD may have been
treated for acute pain in the ED or hospital, thus confounding this
definition. Another definition of chronic pain that was initially
introduced by surgeons is acute pain that becomes chronic. This
definition applies well to post-operative pain that is acute after
surgery but, in some patients, continues in the same operative field
for months or years.[69]Actually,
this definition applies to the chronic complications of SCD. Thus, AVN
of the hips may start acutely but persists for months or years in the
same location. A new definition of chronic pain in SCD is the pain that
persists or occurs between VOCs, the so-called chronic on acute
pain.[70] Although this may occur in disorders other than SCD, it
is not typical of SCD because the sickle pain between VOCs is not the
same: it varies in location, severity, and outcome, as shown in Table 3.
Other patients with chronic pain syndromes such as fibromyalgia,
osteoarthritis, rheumatoid arthritis, low back pain, migraine, etc. are
rarely treated in the ED and rarely require hospital admissions.
Sickle cell disease and sickle cell pain are unique and should be
considered as such.
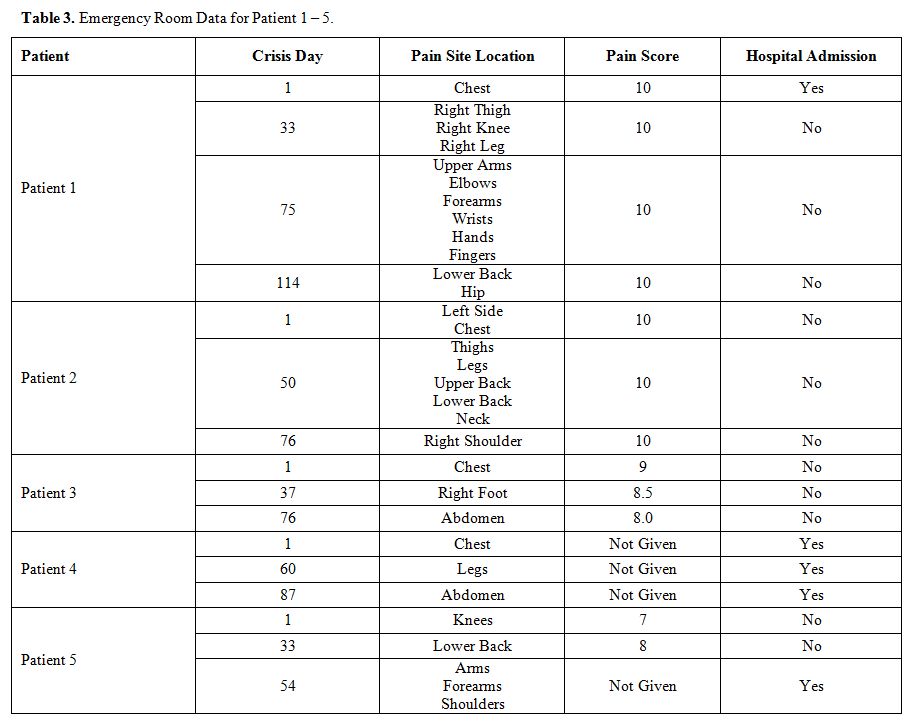 |
Table 3. Emergency Room Data for Patient 1 – 5. |
Patients discharged from the hospital after treatment of uncomplicated VOCs may have:1. No pain.2. Resolving mild pain as the VOC continues to resolve gradually.3.
Persistent pain after discharge that requires continued therapy
with oral analgesics, (due to tolerance, OIH or withdrawal).4. Relapsing pain that occurs after a period with no pain (new mild VOC)I
consider the pain that occurs between VOCs is most likely due to
tolerance to opioids, withdrawal syndrome, OIH, resolving, or acute
relapsing pain. These are specific diagnoses that have specific
recommended treatments rather than treating them as chronic pain.
Accordingly, it seems the use of Buprenorphine/Naloxone is potentially
a good candidate to treat these syndromes as recently reported by
Osunkwo et al.[71]
Summary
The
acute painful VOC is a unique and hallmark clinical entity of SCD.
Recurrent VOCs are not identical but usually vary considerably among
patients and longitudinally in the same patient. This is unlike chronic
pain that tends to be essentially the same from time to time. The
pain between VOCs could be due to tolerance, OIH, withdrawal,
resolving, or relapsing.
References
- Smith WR, Penberthy LT, Bovbjerg VE, McClish DK,
Roberts JD, Dahman B, et al. Daily assessment of pain in adults with
sickle cell disease. Ann Intern Med. 2008;148(2):94-101.
https://doi.org/10.7326/0003-4819-148-2-200801150-00004 PMid:18195334
- Dampier
C, Ely B, Brodecki D, O'Neal P. Characteristics of pain managed at home
in children and adolescents with sickle cell disease by using diary
self-reports. J Pain. 2002;3(6):461-70.
https://doi.org/10.1054/jpai.2002.128064 PMid:14622732
- Ballas
SK, Bauserman RL, McCarthy WF, Castro OL, Smith WR, Waclawiw MA.
Hydroxyurea and acute painful crises in sickle cell anemia: effects on
hospital length of stay and opioid utilization during hospitalization,
outpatient acute care contacts, and at home. J Pain Symptom Manage.
2010;40(6):870-82. https://doi.org/10.1016/j.jpainsymman.2010.03.020
PMid:20864308 PMCid:PMC3005988
- Ballas SK. Sickle Cell Pain, 2nd Edition. Washington DC: International Association for the Study of Pain; 2014.
- Ballas
SK, Gupta K, Adams-Graves P. Sickle cell pain: a critical reappraisal.
Blood. 2012;120(18):3647-56.
https://doi.org/10.1182/blood-2012-04-383430 PMid:22923496
- Yawn
BP, Buchanan GR, Afenyi-Annan AN, Ballas SK, Hassell KL, James AH, et
al. Management of sickle cell disease: summary of the 2014
evidence-based report by expert panel members. JAMA.
2014;312(10):1033-48. https://doi.org/10.1001/jama.2014.10517
PMid:25203083
- Ballas SK, Smith ED. Red
blood cell changes during the evolution of the sickle cell painful
crisis. Blood. 1992;79(8):2154-63.
https://doi.org/10.1182/blood.V79.8.2154.2154
- Pentin
PL. Drug seeking or pain crisis? Responsible prescribing of opioids in
the emergency department. Virtual Mentor. 2013;15(5):410-5.
https://doi.org/10.1001/virtualmentor.2013.15.5.ecas2-1305 PMid:23680561
- Dowell
D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for
Chronic Pain - United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49.
https://doi.org/10.15585/mmwr.rr6501e1 PMid:26987082
- Ballas
SK. Pathophysiology and principles of management of the many faces of
the acute vaso-occlusive crisis in patients with sickle cell disease.
Eur J Haematol. 2015;95(2):113-23. https://doi.org/10.1111/ejh.12460
PMid:25288149
- Ballas SK. More
definitions in sickle cell disease: steady state v base line data. Am J
Hematol. 2012;87(3):338. https://doi.org/10.1002/ajh.22259 PMid:22190068
- Expert
Panel Report. Evidence-Based Management of Sickle Cell Disease Bethesda
MD: National Heart, Lung, and Blood Institute; 2014 [Available from:
http://www.nhlbi.nih.gov/health-pro/guidelines/sickle-cell-disease-guidelines/].
- Ballas
SK. Pain management of sickle cell disease. Hematol Oncol Clin North
Am. 2005;19(5):785-802. https://doi.org/10.1016/j.hoc.2005.07.008
PMid:16214644
- Ballas SK. Update on pain
management in sickle cell disease. Hemoglobin. 2011;35(5):520-9.
https://doi.org/10.3109/03630269.2011.610478 PMid:21910604
- Ruta
NS, Ballas SK. The Opioid Drug Epidemic and Sickle Cell Disease: Guilt
by Association. Pain Med. 2016;17(10):1793-8.
https://doi.org/10.1093/pm/pnw074 PMid:27152018
- Carden
MA, Fay M, Sakurai Y, McFarland B, Blanche S, DiPrete C, et al. Normal
saline is associated with increased sickle red cell stiffness and
prolonged transit times in a microfluidic model of the capillary
system. Microcirculation. 2017;24(5).
https://doi.org/10.1111/micc.12353 PMid:28106307
- Carden
MA, Fay ME, Lu X, Mannino RG, Sakurai Y, Ciciliano JC, et al.
Extracellular fluid tonicity impacts sickle red blood cell
deformability and adhesion. Blood. 2017;130(24):2654-63.
https://doi.org/10.1182/blood-2017-04-780635 PMid:28978568
PMCid:PMC5731085
- Ballas SK. Of pools,
oceans, and the Dead Sea. Blood. 2017;130(24):2578-9.
https://doi.org/10.1182/blood-2017-10-811091 PMid:29242205
- Gardner JW. Death by water intoxication. Mil Med. 2002;167(5):432-4. https://doi.org/10.1093/milmed/167.5.432 PMid:12053855
- Gutmann
FD, Gardner JW. Fatal water intoxication of an Army trainee during
urine drug testing. Mil Med. 2002;167(5):435-7.
https://doi.org/10.1093/milmed/167.5.435
- Serjeant
G. Blood transfusion in sickle cell disease: a cautionary tale. Lancet.
2003;361(9369):1659-60. https://doi.org/10.1016/S0140-6736(03)13293-X
- Ballas
SK. Self-management of sickle cell disease: a new frontier. J Natl Med
Assoc. 2010;102(11):1042-3.
https://doi.org/10.1016/S0027-9684(15)30722-7
- Tanabe
P, Porter J, Creary M, Kirkwood E, Miller S, Ahmed-Williams E, et al. A
qualitative analysis of best self-management practices: sickle cell
disease. J Natl Med Assoc. 2010;102(11):1033-41.
https://doi.org/10.1016/S0027-9684(15)30730-6
- Ballas
SK. Sickle cell disease: current clinical management. Semin Hematol.
2001;38(4):307-14. https://doi.org/10.1016/S0037-1963(01)90024-1
- Waldhoer
M, Bartlett SE, Whistler JL. Opioid receptors. Annu Rev Biochem.
2004;73:953-90.
https://doi.org/10.1146/annurev.biochem.73.011303.073940 PMid:15189164
- Manglik
A, Kruse AC, Kobilka TS, Thian FS, Mathiesen JM, Sunahara RK, et al.
Crystal structure of the micro-opioid receptor bound to a morphinan
antagonist. Nature. 2012;485(7398):321-6.
https://doi.org/10.1038/nature10954 PMid:22437502 PMCid:PMC3523197
- Cox
BM. Recent developments in the study of opioid receptors. Mol
Pharmacol. 2013;83:723-8. https://doi.org/10.1124/mol.112.083279
PMid:23249538
- Koppert W, Schmelz M. The
impact of opioid-induced hyperalgesia for post-operative pain. Best
Pract Res Clin Anaesthesiol. 2007;21(1):65-83.
https://doi.org/10.1016/j.bpa.2006.12.004 PMid:17489220
- Martyn
JAJ, Mao J, Bittner EA. Opioid Tolerance in Critical Illness. N Engl J
Med. 2019;380(4):365-78. https://doi.org/10.1056/NEJMra1800222
PMid:30673555 PMCid:PMC6897318
- Kang M,
Mischel RA, Bhave S, Komla E, Cho A, Huang C, et al. The effect of gut
microbiome on tolerance to morphine mediated antinociception in mice.
Sci Rep. 2017;7:42658. https://doi.org/10.1038/srep42658 PMid:28211545
PMCid:PMC5314392
- Akbarali HI, Dewey WL.
The gut-brain interaction in opioid tolerance. Curr Opin Pharmacol.
2017;37:126-30. https://doi.org/10.1016/j.coph.2017.10.012
PMid:29145012 PMCid:PMC5725258
- Mischel
RA, Dewey WL, Akbarali HI. Tolerance to Morphine-Induced Inhibition of
TTX-R Sodium Channels in Dorsal Root Ganglia Neurons Is Modulated by
Gut-Derived Mediators. iScience. 2018;2:193-209.
https://doi.org/10.1016/j.isci.2018.03.003 PMid:29888757
PMCid:PMC5993194
- Breedlove S.M., Watson N.V. Behavioral Neuroscience, Eighth Edition. Oxford University Press New York, NY; 2018.
- CNS Forum. NMDA Receptor. CNS Forum2002.
- de
Vos JW, Ufkes JG, Kaplan CD, Tursch M, Krause JK, van Wilgenburg H, et
al. L-Methadone and D,L-methadone in methadone maintenance treatment: a
comparison of therapeutic effectiveness and plasma concentrations. Eur
Addict Res. 1998;4(3):134-41. https://doi.org/10.1159/000018936
PMid:9742275
- Wedekind D, Jacobs S, Karg
I, Luedecke C, Schneider U, Cimander K, et al. Psychiatric comorbidity
and additional abuse of drugs in maintenance treatment with L- and
D,L-methadone. World J Biol Psychiatry. 2010;11(2 Pt 2):390-9.
https://doi.org/10.3109/15622970802176487 PMid:20218800
- Zhang
Y, Tao GJ, Hu L, Qu J, Han Y, Zhang G, et al. Lidocaine alleviates
morphine tolerance via AMPK-SOCS3-dependent neuroinflammation
suppression in the spinal cord. J Neuroinflammation. 2017;14(1):211.
https://doi.org/10.1186/s12974-017-0983-6 PMid:29096659 PMCid:PMC5667445
- Swe
KM, Abas AB, Bhardwaj A, Barua A, Nair NS. Zinc supplements for
treating thalassaemia and sickle cell disease. Cochrane Database Syst
Rev. 2013;6:CD009415. https://doi.org/10.1002/14651858.CD009415.pub2
PMid:23807756
- Li Y, Shu Y, Ji Q, Liu J,
He X, Li W. Attenuation of morphine analgesic tolerance by rosuvastatin
in naive and morphine tolerance rats. Inflammation. 2015;38(1):134-41.
https://doi.org/10.1007/s10753-014-0015-y PMid:25261133
- Deng
XT, Han Y, Liu WT, Song XJ. B Vitamins Potentiate Acute Morphine
Antinociception and Attenuate the Development of Tolerance to Chronic
Morphine in Mice. Pain Med. 2017;18(10):1961-74.
https://doi.org/10.1093/pm/pnw358 PMid:28379583
- Wang
Y, Barker K, Shi S, Diaz M, Mo B, Gutstein HB. Blockade of PDGFR-beta
activation eliminates morphine analgesic tolerance. Nat Med.
2012;18(3):385-7. https://doi.org/10.1038/nm.2633 PMid:22344297
PMCid:PMC3296828
- Donica CL, Cui Y, Shi
S, Gutstein HB. Platelet-derived growth factor receptor-beta antagonism
restores morphine analgesic potency against neuropathic pain. PLoS One.
2014;9(5):e97105. https://doi.org/10.1371/journal.pone.0097105
PMid:24820332 PMCid:PMC4018247
- Stankovic
Stojanovic K, Thioliere B, Garandeau E, Lecomte I, Bachmeyer C, Lionnet
F. Chronic myeloid leukaemia and sickle cell disease: could imatinib
prevent vaso-occlusive crisis? Br J Haematol. 2011;155(2):271-2.
https://doi.org/10.1111/j.1365-2141.2011.08670.x PMid:21488859
- Murphy
M, Close J, Lottenberg R, Rajasekhar A. Effectiveness of imatinib
therapy for sickle cell anemia and chronic myeloid leukemia. Am J Med
Sci. 2014;347(3):254-5. https://doi.org/10.1097/MAJ.0000000000000228
PMid:24553361
- Koren G, Cairns J,
Chitayat D, Gaedigk A, Leeder SJ. Pharmacogenetics of morphine
poisoning in a breastfed neonate of a codeine-prescribed mother.
Lancet. 2006;368(9536):704.
https://doi.org/10.1016/S0140-6736(06)69255-6
- Sajantila
A. Editors' pick: codeine toxicity prediction in young infants -
genotype the mothers. Investig Genet. 2012;3(1):24.
https://doi.org/10.1186/2041-2223-3-24 PMid:23186321 PMCid:PMC3528413
- McClish
DK, Smith WR, Dahman BA, Levenson JL, Roberts JD, Penberthy LT, et al.
Pain site frequency and location in sickle cell disease: the PiSCES
project. Pain. 2009;145(1-2):246-51.
https://doi.org/10.1016/j.pain.2009.06.029 PMid:19631468
PMCid:PMC2771372
- Serjeant GR, Chalmers
RM. Current concerns in haematology. 1. Is the painful crisis of sickle
cell disease a "steal" syndrome? J Clin Pathol. 1990;43(10):789-91.
https://doi.org/10.1136/jcp.43.10.789 PMid:1699977 PMCid:PMC502823
- Rathmell
J.P., Fields HL. Pain: pathophysiology and management. In: Longo DL,
Fauci AS, Kasey S, Hauser R, Jameson LS, Loscaizo JL, editors.
Harrison's Principles of Internal Medicine. New York, NY: McGraw Hill;
2012. p. 93-101.
- Woolf CJ. Evidence for
a central component of post-injury pain hypersensitivity. Nature.
1983;306(5944):686-8. https://doi.org/10.1038/306686a0 PMid:6656869
- Cervero F, Laird JM. Visceral pain. Lancet. 1999;353(9170):2145-8. https://doi.org/10.1016/S0140-6736(99)01306-9
- Busse
SM, McMillen PT, Levin M. Cross-limb communication during Xenopus
hindlimb regenerative response: non-local bioelectric injury signals.
Development. 2018;145(19). https://doi.org/10.1242/dev.164210
PMid:30126906
- Youssef F, Pater A, Shehata M. Opioid-induced Hyperalgesia. J Pain Relief. 2015;4:183-5.
- Benjamin
LJ, Payne R. Pain in sickle cell disease: a multidimensional construct.
In: Pace B, editor. Renaissance of Sickle Cell Disease Research in the
Genomic Era. London: Imperial College Press; 2007. p. 99-118.
https://doi.org/10.1142/9781860947964_0007
- de
Montalembert M, Ferster A, Colombatti R, Rees DC, Gulbis B. ENERCA
clinical recommendations for disease management and prevention of
complications of sickle cell disease in children. Am J Hematol.
2011;86(1):72-5. https://doi.org/10.1002/ajh.21865 PMid:20981677
- Ferrini
F, Trang T, Mattioli TA, Laffray S, Del'guidice T, Lorenzo LE, et al.
Morphine hyperalgesia gated through microglia-mediated disruption of
neuronal Cl(-) homeostasis. Nat Neurosci. 2013;16:183-92.
https://doi.org/10.1038/nn.3295 PMid:23292683 PMCid:PMC4974077
- Allen
NJ, Barres BA. Neuroscience: Glia - more than just brain glue. Nature.
2009;457(7230):675-7. https://doi.org/10.1038/457675a PMid:19194443
- Ballas
SK, Lusardi M. Hospital readmission for adult acute sickle cell painful
episodes: frequency, etiology, and prognostic significance. Am J
Hematol. 2005;79(1):17-25. https://doi.org/10.1002/ajh.20336
PMid:15849770
- Burma NE, Kwok CH, Trang
T. Therapies and mechanisms of opioid withdrawal. Pain Manag.
2017;7(6):455-9. https://doi.org/10.2217/pmt-2017-0028 PMid:29125396
- Kenna
GA, Nielsen DM, Mello P, Schiesl A, Swift RM. Pharmacotherapy of dual
substance abuse and dependence. CNS Drugs. 2007;21(3):213-37.
https://doi.org/10.2165/00023210-200721030-00003 PMid:17338593
- NIDA.
FDA approves first medication to reduce opioid withdrawal symptoms
National Institute on Drug Abuse website2018 [Available from:
https://www.drugabuse.gov/news-events/news-releases/2018/05/fda-approves-first-medication-to-reduce-opioid-withdrawal-symptoms].
- Gish
EC, Miller JL, Honey BL, Johnson PN. Lofexidine, an {alpha}2-receptor
agonist for opioid detoxification. Ann Pharmacother. 2010;44(2):343-51.
https://doi.org/10.1345/aph.1M347 PMid:20040696
- Gorodetzky
CW, Walsh SL, Martin PR, Saxon AJ, Gullo KL, Biswas K. A phase III,
randomized, multi-center, double blind, placebo controlled study of
safety and efficacy of lofexidine for relief of symptoms in individuals
undergoing inpatient opioid withdrawal. Drug Alcohol Depend.
2017;176:79-88. https://doi.org/10.1016/j.drugalcdep.2017.02.020
PMid:28527421
- Law FD, Diaper AM,
Melichar JK, Coulton S, Nutt DJ, Myles JS. Buprenorphine/naloxone
versus methadone and lofexidine in community stabilisation and
detoxification: A randomised controlled trial of low dose short-term
opiate-dependent individuals. J Psychopharmacol. 2017;31(8):1046-55.
https://doi.org/10.1177/0269881117711710 PMid:28631527
- Ballas
SK, Talacki CA, Rao VM, Steiner RM. The prevalence of avascular
necrosis in sickle cell anemia: correlation with alpha-thalassemia.
Hemoglobin. 1989;13(7-8):649-55.
https://doi.org/10.3109/03630268908998842 PMid:2634666
- Milner
PF, Kraus AP, Sebes JI, Sleeper LA, Dukes KA, Embury SH, et al. Sickle
cell disease as a cause of osteonecrosis of the femoral head. N Engl J
Med. 1991;325(21):1476-81. https://doi.org/10.1056/NEJM199111213252104
PMid:1944426
- Minniti CP, Eckman J,
Sebastiani P, Steinberg MH, Ballas SK. Leg ulcers in sickle cell
disease. Am J Hematol. 2010;85(10):831-3.
https://doi.org/10.1002/ajh.21838 PMid:20872960 PMCid:PMC2953786
- Dampier
C, Palermo TM, Darbari DS, Hassell K, Smith W, Zempsky W. AAPT
Diagnostic Criteria for Chronic Sickle Cell Disease Pain. J Pain.
2017;18(5):490-8. https://doi.org/10.1016/j.jpain.2016.12.016
PMid:28065813
- Chapman CR, Vierck CJ. The
Transition of Acute Postoperative Pain to Chronic Pain: An Integrative
Overview of Research on Mechanisms. J Pain. 2017;18(4):359.e1-.e38.
https://doi.org/10.1016/j.jpain.2016.11.004 PMid:27908839
- Kent
ML, Tighe PJ, Belfer I, Brennan TJ, Bruehl S, Brummett CM, et al. The
ACTTION-APS-AAPM Pain Taxonomy (AAAPT) Multidimensional Approach to
Classifying Acute Pain Conditions. J Pain. 2017;18(5):479-89.
https://doi.org/10.1016/j.jpain.2017.02.421 PMid:28495013
PMCid:PMC7323793
- Osunkwo I, Veeramreddy
P, Arnall J, Crawford R, Symanowski JT, Olaosebikan R, et al. Use of
Buprenorphine/Naloxone in Ameliorating Acute Care Utilization and
Chronic Opioid Use in Adults with Sickle Cell Disease. Blood. 2019;134
(Suppl 1):790. https://doi.org/10.1182/blood-2019-126589
[TOP]









