Alfadil Haroon, Momen Alnassani, Mahmoud Aljurf, Syed Osman Ahmed, Marwan Shaheen, Amr Hanbli, Naeem Chaudhari and Riad El Fakih
Oncology Centre, King Faisal Specialist Hospital and Research Centre, Riyadh, KSA.
Correspondence to: Alfadil Haroon, MD. Oncology Centre, KFSHRC,
Section of Adult Hematology/HSCT, PO Box 3354, Riyadh, 11471, Saudi
Arabia. E-mail:
halfadil@kfshrc.edu.sa or
fadil_130@hotmail.comRiad El Fakih, MD. Oncology Centre, KFSHRC, Section of Adult Hematology/HSCT. PO Box 3354, Riyadh, 11471, Saudi Arabia. E-mail:
riadfakih@hotmail.com or
relfakih1@kfshrc.edu.sa
Published: September 1, 2020
Received: July 21, 2020
Accepted: August 25, 2020
Mediterr J Hematol Infect Dis 2020, 12(1): e2020070 DOI
10.4084/MJHID.2020.070
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
In
late 2019 the coronavirus disease‐2019 (COVID‐19) pandemic caused by
SARS Coronavirus 2 (SARS‐CoV‐2) started in Wuhan, China. Life has
changed radically since then. Data emerging from the first hit
countries show a tendency for a complicated course and higher mortality
in some subgroups of infected patients. Cancer patients are
immunosuppressed from their disease and the therapy they receive.
Hematopoietic cell transplant (HCT) recipients are a subgroup of
patients that are severely immunocompromised and may be at an even
higher risk of a complicated course during this infection. Reports
describing the course of these patients with COVID-19 disease are
limited. We herein report the onset, progression, and outcome of 11
sequential cases of HCT recipients infected by SARS‐CoV‐2 treated in
our center. The patients' age ranged from 17 to 60 years, the duration
from transplant to infection ranged from day +5 to 192 months, six
patients were post-allo-HCT, four post-auto-HCT, and one had both allo
and auto-HCT. The presenting symptoms were not different from other
viral illnesses. The majority (seven patients) had mild COVID-19 stage,
while 3 had a moderate stage on presentation. None of the patients
required oxygen supplementation nor mechanical ventilation.
|
Introduction
The
coronavirus disease‐2019 (COVID‐19) pandemic caused by SARS Coronavirus
2 (SARS‐CoV‐2), was first noted in Wuhan, China, in December 2019 and
has since spread worldwide. At the time of writing this report,
more than 23 million cases and 800 thousand COVID-19-related deaths
have been confirmed by the world health organization (WHO) (https://covid19.who.int/
accessed on August 24, 2020). Efforts are ongoing to understand every
aspect of the virus, the host, and to develop an effective therapy or
vaccine.[1,2] Although the majority of infected
patients have mild disease, critical illness occurs in about 6.1% of
affected patients. People at risk for severe outcome and death include
those older than 60 years and those with comorbid disease states such
as hypertension (HTN), chronic cardiac disease, chronic respiratory
disease, chronic kidney disease (CKD), cardiovascular disease, diabetes
mellitus (DM), cancer and immunosuppressed patients.[3]
Data emerging about cancer patients show that these patients have an
increased risk of complications and intensive care unit (ICU)
admission.[4] Hematopoietic cell transplant (HCT)
patients are severely immune-compromised, and their course with
COVID-19 is expected to be complicated. We herein report 11 cases of
COVID-19 in post HCT patients.
Case 1
A
36-years-old man was diagnosed with Philadelphia positive B-cell acute
lymphoblastic leukemia (ALL) with no central nervous system (CNS)
involvement in early March 2019. He was treated with daily dasatinib,
weekly vincristine, and dexamethasone twice weekly. He was then treated
with methotrexate and Ara-c and achieved complete molecular remission
by polymerase chain reaction (PCR). Subsequently, he underwent
allogeneic peripheral blood matched sibling HCT with cyclophosphamide
(Cy) and total body irradiation (TBI) myeloablative conditioning (MAC)
in July 2019. The course was complicated by grade II,
steroid-responsive, acute gut graft versus host disease (GVHD), and
cytomegalovirus (CMV) reactivation. He remains in complete molecular
remission, and cyclosporine (CSA) was discontinued in March 2020 (day
237 post HCT). He is currently on prophylactic acyclovir and Bactrim.
Early June 2020, he developed low grade fever of 37.9 Celsius (C) and
fatigue. He tested positive for SARS-Cov2 infection by real‐time
Polymerase Chain Reaction (RT-PCR) on June 11, 2020 (day 323 post HCT).
He did not have any further fever after June 12. He only received as
needed acetaminophen. Repeat nasal SARS-Cov2 PCR on August 20, 2020,
was negative, and his SARS-Cov2 total antibody test was reactive.
Case 2
A
29-years-old man was diagnosed with Philadelphia negative pre B ALL
with no CNS involvement in August 2017. He was treated with a
pediatric-inspired protocol and achieved remission; however, he had
isolated CNS relapse while on maintenance therapy. He received salvage
chemotherapy with fludarabine and Ara- C, and upon remission, he was
consolidated with matched sibling allo-HCT using MAC with Cy/TBI; GVHD
prophylaxis consisted of methotrexate and CSA. His course was
complicated by grade II, steroid-responsive acute gut and skin GVHD as
well as CMV colitis. Day 170 post HCT (June 5, 2020) while he was on
tapering tacrolimus, he developed fever, cough, headache, runny nose
followed by loss of taste and smell. He tested positive by PCR for
SARS-Cov2 infection. Chest x-ray showed small faint ground-glass
opacity in the left lower lung zone. Tacrolimus was stopped, and he was
treated with ceftriaxone, hydroxychloroquine (HCQ), and azithromycin.
He improved after 7days and was discharged. Repeat nasal SARS-Cov2 PCRs
on July 8, 2020, were negative, and his SARS-Cov2 total antibody test
was reactive on July 25, 2020. Unfortunately, on August 7, 2020, he was
diagnosed with relapsed ALL, and currently, he is receiving
blinatumomab salvage.
Case 3
A
60-years-old man was diagnosed with IgG kappa multiple myeloma (MM) in
2002. He received induction therapy followed by autologous HCT with
melphalan MAC in August 2002. He relapsed 15 months after auto-HCT. He
received another induction followed by allo-HCT in 2004 after achieving
deep remission. His conditioning was reduced intensity with
fludarabine/TBI. In 2013 he had a biochemical relapse, so he was
re-induced with bortezomib based therapy followed by donor lymphocyte
infusion (DLI). He had another relapse in December 2019 and has been on
dexamethasone 32 mg weekly since then. In May 2020, he developed fever
and body aches after contact with a confirmed case of COVID-19, and he
tested positive by PCR for SARS-Cov2 infection. He was admitted and
treated symptomatically only and then discharged three days after
admission after symptom-improvement. Repeat nasal SARS-Cov2 PCR on
August 15, 2020, was negative, and his SARS-Cov2 total antibody test
was reactive on August 16, 2020.
Case 4
A
56-years-old woman known to have hypothyroidism, hypertension (HTN),
diabetes mellitus (DM), and atrial fibrillation (AF) was diagnosed with
Multiple myeloma (MM) in November 2013. She achieved partial remission
(PR) after induction and underwent auto-HCT in July 2014 with melphalan
MAC conditioning. She was on bortezomib maintenance, but she progressed
and was started on Revlimid but could not tolerate it. In March 2019,
she was started on carfilzomib and dexamethasone and achieved a near CR
after six cycles. Her therapy was held since December 2019 due to
diarrhea. Late May 2020, she developed malaise, fatigue, headache, and
fatigue. She tested positive by PCR for SARS-Cov2 infection. She was
hospitalized and treated with ceftriaxone (she did not receive HCQ nor
azithromycin due to her comorbidities and the drug-drug interaction
with her home medications). She recovered and was discharged after nine
days of hospitalization. Repeat nasal SARS-Cov2 PCR on August 20, 2020,
was negative, and his SARS-Cov2 total antibody test was reactive.
Case 5
A
58-year-old man known to have morbid obesity, dyslipidemia,
hypothyroidism, and a remote history of pleural Tuberculosis (TB) was
diagnosed with IgG kappa multiple myeloma in July 2015. He received
induction with bortezomib, lenalidomide, and dexamethasone followed by
auto-HCT with melphalan MAC conditioning after achieving a very good
partial response (VGPR). He was maintained on lenalidomide with
dexamethasone, but he relapsed in December 2017 and was treated with
carfilzomib, lenalidomide, and dexamethasone. In late May 2020, while
on therapy, he developed a sore throat, fever, and runny nose. He
tested positive by PCR for SARS-Cov2 infection. He recovered well after
seven days of initial symptoms. Repeat nasal SARS-Cov2 PCR on August
20, 2020, was negative, and his SARS-Cov2 total antibody test was
reactive.
Case 6
A
26-year-old man was diagnosed with classical Hodgkin lymphoma (cHL)
stage IIB in May 2018. He was started on ABVD (Doxorubicin, Bleomycin,
Vinblastine, and Dacarbazine) and escalated to BEACOPP due to
progression after two cycles of ABVD. He received a total of 4 BEACOPP
followed by radiation, but he relapsed in May 2019. He received salvage
ESHAP (Methylprednisolone, Cisplatin, Etoposide, and Cytarabine) with
brentuximab vedotin for three cycles, followed by MAC auto-HCT with
BEAM (carmustine, etoposide, cytarabine, and melphalan) conditioning in
September 2019, after auto-HCT he was on maintenance brentuximab
vedotin. In June 2020, he complained about a loss of smell and tested
positive by PCR for SARS-Cov2 infection. He was treated symptomatically
with no admission to the hospital. His nasal SARS-Cov2 PCR was not
repeated, and his SARS-Cov2 total antibody test was not done.
Case 7
A
21-year-old man was diagnosed with T-cell lymphoid blast crisis with
underlying chronic myeloid leukemia (CML) in May 2019. He was treated
with a pediatric chemotherapy regimen plus tyrosine kinase inhibitor
(TKI) and achieved major molecular remission after induction. He
underwent haploidentical HCT in June 2020 with fludarabine/TBI MAC
conditioning along with rabbit antithymocyte globulin (ATG) and
post-transplant cyclophosphamide with CSA and Mycophenolate Mofetil
(MMF) for GVHD prophylaxis. On day zero of transplant, his donor
(father) tested positive for SARS-Cov2 by PCR. The collection was
postponed for one day in an attempt to find another donor; in the
meantime, the donor was given HCQ and azithromycin. All his other
family members tested positive for SARS-Cov2 by PCR, and the decision
was made to proceed with the initial donor apheresis. SARS-Cov2-PCR was
negative on the apheresis product. The patient's SARS-Cov2-PCR was
negative before admission and on day +1 after transplant. On day +5
post-HCT, the patient's SARS-Cov2-PCR from nasopharyngeal swab came
back positive, but the patient was asymptomatic. He was treated with
azithromycin and HCQ, and on day +10 post-HCT, one dose of tocilizumab
was given secondary to elevated inflammatory markers (ferritin and
interleukin 6). Platelet engrafted on day+9, while neutrophils
engrafted on day+23. On day +12 post-HCT, SARS-Cov2-PCR from
nasopharyngeal swab was repeated and was positive. On day +14, the
post-HCT patient developed culture-negative neutropenic fever with
further elevation in the inflammatory markers; the second dose of
tocilizumab was given, and he was started on meropenem, but he was
still asymptomatic besides fever, and specifically, he did not develop
respiratory symptoms. On day+18, CT chest showed left lung ground-glass
opacities. He then developed hemophagocytic lymphohistiocytosis (HLH)
and macrophage activation syndrome (MAS). He was treated with
dexamethasone and Intravenous immune globulin (IVIG) for four days, and
ruxolitinib was added due to a continuous rise in ferritin. After the
addition of ruxolitinib, his inflammatory markers and liver enzymes
decreased figure (1 and 2). Currently, he fully recovered and is
asymptomatic; however, his repeat nasal SARS-Cov2 PCR on August 5,
2020, was definite, and his SARS-Cov2 total antibody test was
non-reactive.
Case 8
A
17-year-old female patient was diagnosed with Philadelphia positive B
cell ALL in August 2018. She received dasatinib, vincristine, rituximab
and dexamethasone induction and achieved deep molecular remission
followed by haploidentical-HCT from her brother donor using
fludarabine/TBI MAC conditioning in April 2019. Her course was
complicated by falling chimerism and eventually autologous recovery
with donor chimerism reaching zero on day 90 post-HCT. Her
immunosuppression was stopped, and she was started on maintenance
dasatinib. In July 2020, she tested positive for SARS-Cov2 by PCR after
contact with a positive case. She is currently asymptomatic and
continuing on dasatinib and acyclovir. Repeat nasal SARS-Cov2 PCR on
August 20, 2020, was negative, but the SARS-Cov2 total antibody test
was not done.
Case 9
A
35-year-old man was diagnosed with translocation (11;19) acute myeloid
leukemia(AML) in April 2019. He received induction therapy followed by
haploidentical-HCT after achieving remission in May 2019. His
conditioning was thiotepa, fludarabine, and busulfan MAC along with
rabbit ATG, cyclosporine, and MMF for GVHD prophylaxis. His transplant
course was complicated with perianal abscess, CMV reactivation,
collagenous colitis, and acute skin GVHD. In July 2020, while he was on
a tapering dose of prednisone for his GVHD, he presented with fatigue,
fever, and productive cough and tested positive for SARS-Cov2 by PCR.
He had bilateral patchy ground-glass opacities on chest x-ray and was
started on azithromycin, ceftriaxone, and HCQ. Currently, he is doing
well and asymptomatic; repeat nasal SARS-Cov2 PCR on August 7, 2020,
was negative, but the SARS-Cov2 total antibody test was done on July
14, and August 17, 2020, and was non-reactive.
Case 10
A
49-year-old woman was diagnosed with AML with (Fms-like tyrosine
kinase3-Internal tandem duplication) FLT3-ITD positive, Nucleophosmin
(NPM1) positive and Isocitrate dehydrogenase2(IDH2) positive mutations
in mid-June 2019. She received induction therapy with FLT3 inhibitor
and achieved remission. She underwent a matched sibling donor
transplant in November 2019 using MAC busulfan and fludarabine.
Post-HCT, she was on sorafenib maintenance for six months only (stopped
because of recurrent leukopenia). On day +206 post-HCT, she developed a
cough, chest pain, shortness of breath, and sore throat; her SARS-Cov2
PCR from nasopharyngeal swab tested positive. She was hospitalized,
treated symptomatically, and discharged in stable condition after ten
days. Her nasal SARS-Cov2 PCR was not repeated, and her SARS-Cov2 total
antibody test was not done.
Case 11
A
58-year-old woman, known to have DM, HTN, chronic kidney disease,
hypothyroidism, and bronchial asthma, was diagnosed with stage IVB
diffuse large B cell lymphoma (DLBCL), in May 2019. She received six
cycles of reduced dose R-CHOP (Rituximab, Cyclophosphamide,
Doxorubicin, Vincristine, and Prednisone) and achieved complete
remission. She relapsed three months after finishing therapy. She then
received three cycles of Rituximab-ESHAP followed by myeloablative BEAM
auto-transplant after achieving partial remission with RESHAP. Her
transplant was in mid-March 2020. In June 2020, she developed fever and
shortness of breath; she tested positive for SARS-Cov2 by PCR; she
reported contact with a positive case. She was treated with
azithromycin, HCQ, and meropenem and recovered well. Repeat nasal
SARS-Cov2 PCR on August 20, 2020, was negative, but the SARS-Cov2 total
antibody test was not done.
Discussion
COVID-19
is a new disease with no approved therapies (except remdesivir in the
United States and Japan, and tocilizumab in China) and lots of
uncertainties. With uncertainty comes fear and anxiety, especially in
subgroups of immunocompromised patients who may be at an increased risk
of a complicated course during the infection. Efforts are ongoing to
understand the impact of this viral infection in different patients'
subgroups. Patients with cancer, and specifically with hematologic
malignancies, seem to be at a higher risk for complications and death.[4-10]
With more than one and a half million transplant-patients in the world,
and around 90 thousand transplants carried out annually worldwide, a
significant number of these patients are expected to develop this new
viral infection.[11] The outcomes and the course of
the infection in this specific subgroup of patients are unknown. So
far, we were able to find one case report of an AML patient who was
diagnosed with COVID-19 infection eight months after allogeneic
transplant while still on cyclosporine. The patient presented with
fever, sore throat, and runny nose and was treated with
lopinavir/ritonavir, steroids, and immunoglobulins but unfortunately
developed ARDS and respiratory failure and died 22 days after the onset
of symptoms.[12] In another case report, a
59‐year‐old man developed severe COVID-19 infection with ARDS a year
after matched sibling transplant for myelofibrosis. The patient had
significant comorbidities in addition to steroid-refractory cGvHD.
Despite therapy with lopinavir‐ritonavir, he progressed to ARDS and
respiratory failure requiring mechanical ventilation. He received
multiple antibiotics during his hospitalization. He improved
significantly and was discharged after increasing the dose of
ruxolitinib that he was taking for cGvHD (he was on 5 BID and was
increased to 10 mg BID).[13] Additionally, a case series (8 patients) was recently reported from a Spanish pediatric transplant group.[14]
The most common symptom was fever, 5/8 patients had radiographic
abnormalities, 5/8 patients were hospitalized, and two of them needed
intensive care. Six patients received HCQ, and the two patients
admitted to ICU received in addition to HCQ, azithromycin, remdesivir,
lopinavir/ritonavir, tocilizumab, siltuximab, and Anakinra. One of the
ICU patients died of alveolar hemorrhage and the others survived.
We report 11 consecutive patients from our center with post hemopoietic cell transplant COVID-19 infection (table 1).
Patients’ age ranged from 17 to 60; the duration from transplant to
infection ranged from day +5 to 192 months, six patients were
post-allo-HCT, four post-auto-HCT, and one had both allo and auto-HCT.
Three patients were transplanted for ALL, 3 for MM, 2 for AML, 1 for
CML with blast crisis,1 for DLBCL, and one for classic HL. Six patients
had other comorbidities (3 DM, 2 GVHD, and one obesity); only one
patient was a smoker. Seven patients were on some form of therapy
either for their primary disease or for post-transplant
immunosuppression or maintenance (3 on steroids, two on calcineurin
inhibitors, one on brentuximab, and one on dasatinib). The presenting
symptoms were not different from other viral illnesses with six
patients having a fever; four had fatigue, three had a cough, two had
headaches, 3 with shortness of breath, 2 with a runny nose, 1 with loss
of smell and two patients were asymptomatic (screened for having
contact with positive cases). The majority (seven patients) had mild
COVID-19 stage (two of them later progress to moderate stage).
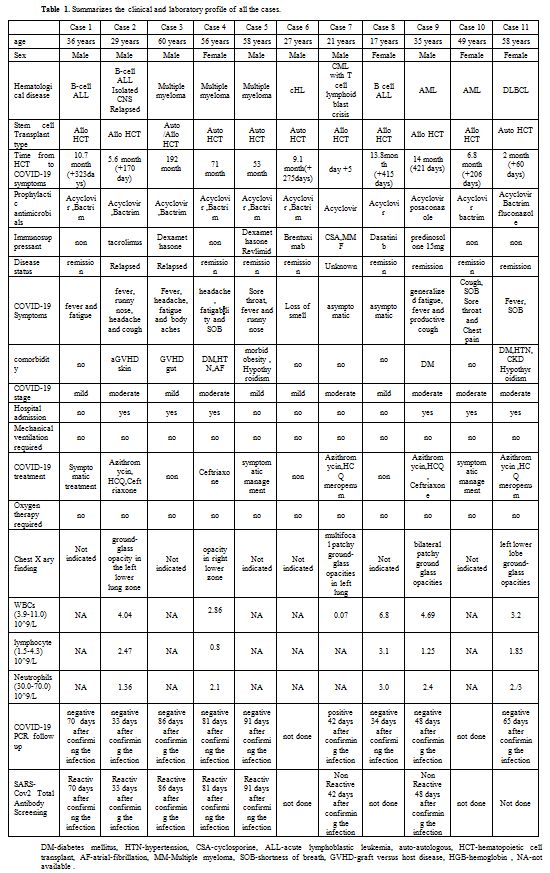 |
Table 1. Summarizes the clinical and laboratory profile of all the cases. |
In
contrast, three had a moderate stage on presentation, six patients were
hospitalized, and five were isolated at home. Six patients did not
receive any therapy other than supportive care; four patients were
treated with a combination of hydroxychloroquine (HCQ), azithromycin,
and either ceftriaxone (two) or meropenem (two), one patient was
treated with ceftriaxone alone. Of note, all the patients were still on
acyclovir prophylaxis as a part of their routine transplant care; seven
were on Bactrim prophylaxis and one on posaconazole. None of the
patients required oxygen supplementation nor mechanical ventilation.
The patient case number 7 tested positive on day +5 of haplo-HCT; his
donor tested positive on the day of donation as well as many other
family members. The harvested product was negative by PCR, and his
nasopharyngeal swab was negative on day +1 but converted to positive on
day +5. We assume that he acquired the infection through transmission
from family members rather than from the apheresis product. A study
from Singapore demonstrated that the virus could be found in the blood
of infected patients in around 8% of the cases.[15]
Recently a donor with a positive PCR from nasopharyngeal swab was used
to donate his sister, and the recipient did not acquire the infection;
the harvested product was tested by PCR and was negative as well.[16]
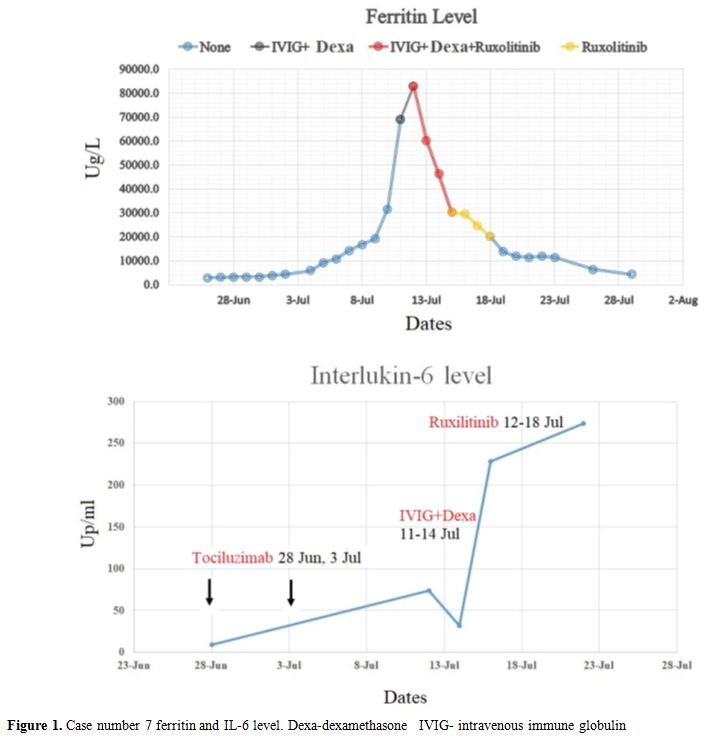 |
Figure 1. Case number 7 ferritin and IL-6 level. Dexa-dexamethasone IVIG- intravenous immune globulin. |
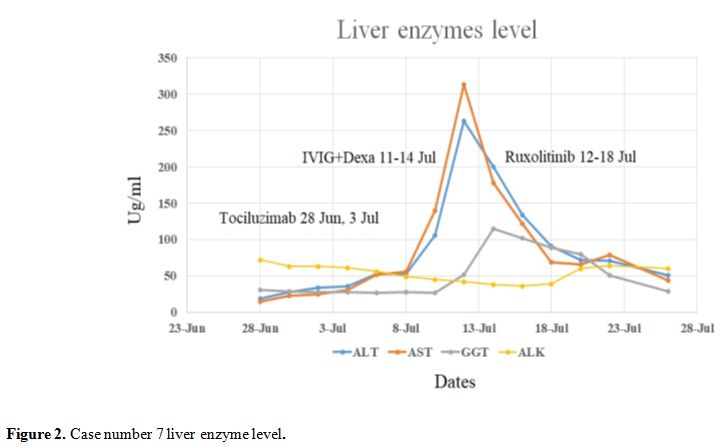 |
Figure 2. Case number 7 liver enzyme level. |
Conclusions
Our
patients surprisingly did extremely well; however, the number of cases
is small, and drawing firm conclusions is impossible. Whether acyclovir
plays any role or not in these patients is unclear; currently, no data
support the use of acyclovir for COVID-19 infection. Transplant
patients are heterogeneous, complex, and their immune reconstitution is
multifactorial and depends on the time from transplant and the presence
of GVHD; these are all crucial variables that can affect the course of
infection in these patients. Large numbers of patients are needed to
delineate the risk factors and variables affecting the outcomes and
better to understand the impact of this virus on transplant patients.
We urge healthcare professionals to report their experience to
understand the effect of COVID-19 on bone marrow transplant recipients
and to serve this subgroup of patients better. Finally, awaiting a
better understanding of this infection, we as providers for this group
of vulnerable patients are urged to take necessary measures to ensure
clear guidance is conveyed to our patients about the importance of
preventive measures until a better understanding or therapy/vaccine of
the disease is in hands.
References
- World Health O. Informal consultation on
prioritization of candidate therapeutic agents for use in novel
coronavirus 2019 infection. January 24 2020. 2020.
- Lurie
N, Saville M, Hatchett R, Halton J. Developing Covid-19 vaccines at
pandemic speed. New England Journal of Medicine 2020;382(21):1969-1973.
https://doi.org/10.1056/NEJMp2005630 PMid:32227757
- Huang
C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X.
Clinical features of patients infected with 2019 novel coronavirus in
Wuhan, China. The lancet 2020;395(10223):497-506. https://doi.org/10.1016/S0140-6736(20)30183-5
- Miyashita
H, Mikami T, Chopra N, Yamada T, Chernyavsky S, Rizk D, Cruz C. Do
patients with cancer have a poorer prognosis of COVID-19? An experience
in New York City. Annals of Oncology 2020. https://doi.org/10.1016/j.annonc.2020.04.006 PMid:32330541 PMCid:PMC7172785
- Guan
WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, Liu XQ, Chen RC, Tang
CL, Wang T and others. Comorbidity and its impact on 1590 patients with
COVID-19 in China: a nationwide analysis. Eur Respir J 2020;55(5). https://doi.org/10.1183/13993003.01227-2020 PMid:32341104 PMCid:PMC7236831
- Miyashita
H, Mikami T, Chopra N, Yamada T, Chernyavsky S, Rizk D, Cruz C. Do
patients with cancer have a poorer prognosis of COVID-19? An experience
in New York City. Ann Oncol 2020. https://doi.org/10.1016/j.annonc.2020.04.006 PMid:32330541 PMCid:PMC7172785
- He
W, Chen L, Chen L, Yuan G, Fang Y, Chen W, Wu D, Liang B, Lu X, Ma Y
and others. COVID-19 in persons with haematological cancers. Leukemia
2020. https://doi.org/10.1038/s41375-020-0836-7 PMid:32332856 PMCid:PMC7180672
- Gavillet M, Klappert JC, Spertini O, Blum S. Acute leukemia in the time of COVID-19. Leukemia Research 2020;92:106353. https://doi.org/10.1016/j.leukres.2020.106353 PMid:32251934 PMCid:PMC7138175
- Wang H, Zhang L. Risk of COVID-19 for patients with cancer. The Lancet Oncology 2020;21(4):e181. https://doi.org/10.1016/S1470-2045(20)30149-2
- Dai
M, Liu D, Liu M, Zhou F, Li G, Chen Z, Zhang Z, You H, Wu M, Zheng Q.
Patients with cancer appear more vulnerable to SARS-COV-2: a
multicenter study during the COVID-19 outbreak. Cancer discovery
2020;10(6):783-791. https://doi.org/10.1158/2159-8290.CD-20-0422 PMid:32345594 PMCid:PMC7309152
- Niederwieser
D, Baldomero H, Atsuta Y, Aljurf M, Seber A, Greinix HT, Koh M, Worel
N, Galeano S, Jaimovich G. One and half million hematopoietic stem cell
transplants (HSCT). Dissemination, trends and potential to improve
activity by telemedicine from the Worldwide Network for Blood and
Marrow Transplantation (WBMT). American Society of Hematology
Washington, DC; 2019. https://doi.org/10.1182/blood-2019-125232
- Huang
J, Lin H, Wu Y, Fang Y, Kumar R, Chen G, Lin S. COVID-19 in
posttransplant patients a report of 2 cases. American Journal of
Transplantation 2020. https://doi.org/10.1111/ajt.15896 PMid:32243697
- Saraceni
F, Scortechini I, Mancini G, Mariani M, Federici I, Gaetani M,
Barbatelli P, Minnucci M, Bagnarelli P, Olivieri A. Severe COVID-19 in
a patient with chronic graft-versus-host disease after hematopoietic
stem cell transplant successfully treated with ruxolitinib. Transplant
Infectious Disease 2020:e13401. https://doi.org/10.1111/tid.13401 PMid:32629531 PMCid:PMC7361240
- Vicent
MG, Martinez AP, Del Castillo MT, Molina B, Sisini L, Moron-Cazalilla
G, Diaz MA. COVID-19 in pediatric hematopoietic stem cell
transplantation: The experience of Spanish Group of Transplant
(GETMON/GETH). Pediatric blood & cancer 2020. https://doi.org/10.1002/pbc.28514 PMid:32573924 PMCid:PMC7361142
- Young
BE, Ong SWX, Kalimuddin S, Low JG, Tan SY, Loh J, Ng O-T, Marimuthu K,
Ang LW, Mak TM. Epidemiologic features and clinical course of patients
infected with SARS-CoV-2 in Singapore. Jama 2020;323(15):1488-1494. https://doi.org/10.1001/jama.2020.3204 PMid:32125362 PMCid:PMC7054855
- Anurathapan
U, Apiwattanakul N, Pakakasama S, Pongphitcha P, Thitithanyanont A,
Pasomsub E, Hongeng S. Hematopoietic stem cell transplantation from an
infected SARS-CoV2 donor sibling. Bone Marrow Transplantation 2020:1-2.
https://doi.org/10.1038/s41409-020-0969-3 PMid:32528121 PMCid:PMC7289075
[TOP]


