Maddalena Mazzucchelli, Anna Maria Frustaci, Marina Deodato, Roberto Cairoli and Alessandra Tedeschi.
Department of Haematology, Niguarda Cancer Center, ASST Grande Ospedale Metropolitano Niguarda, Milano.
Published: January 1, 2018
Received: October 19, 2017
Accepted: November 13, 2017
Mediterr J Hematol Infect Dis 2018, 10(1): e2018004 DOI
10.4084/MJHID.2018.004
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Waldenstrom Macroglobulinemia is a rare lymphoproliferative disorder with distinctive clinical features.
Diagnostic
and prognostic characterisation in WM significantly changed with the
discovery of two molecular markers: MYD88 and CXCR4. Mutational status
of these latter influences both clinical presentation and prognosis and
demonstrated therapeutic implications.
Treatment choice in
Waldenstrom disease is strictly guided by patients’ age and
characteristics, specific goals of therapy, the necessity for rapid
disease control, the risk of treatment-related neuropathy, disease
features, the risk of immunosuppression or secondary malignancies and
potential for future autologous stem cell transplantation.
The
therapeutic landscape has expanded during the last years and the
approval of ibrutinib, the first drug approved for Waldenstrom
Macroglobulinemia, represents a significant step forward for a better
management of the disease.
|
Introduction
Waldenstrom
Macroglobulinemia (WM) is a lymphoproliferative disorder characterized
by the proliferation of lymphoplasmacytic elements in the bone marrow
and the presence of monoclonal immunoglobulin M (IgM) gammopathy.[1]
The
World Health Organization (WHO) classification defined WM as
lymphoplasmacytic lymphoma (LPL) secerning IgM proteins, belonging to
the category of Non-Hodgkin B Lymphomas (NHL) with indolent course.[2]
The disease is rare, representing approximately 2% of all cases of non-Hodgkin Lymphoma,[3] and presents distinctive clinical and laboratory features related to the presence of the monoclonal IgM.
Clinical
presentation of WM is extremely heterogeneous, while some signs and
symptoms are secondary to organ infiltration by clonal cells, including
anaemia, lymphoadenopathy and splenomegaly, others are due instead, to
specific immunological and physiochemical features of monoclonal IgM,
such as neuropathy, hyperviscosity, and cryoglobulinemia.[4]
Despite
the indolent disease course sometimes WM may require prompt treatment
to avoid irreparable organ damage or fatal complications, such as in
the case of hyperviscosity syndrome.[5]
Several therapeutic novelties have radically changed MW scenario during the last years.
Furthermore,
the recent discoveries of two mutations, myeloid differentiation
primary response 88 (MYD88) and C-X-C chemokine receptor type 4 (CXCR4)
in WM patients has improved disease characterisation helping to deeper
understand the biology of the disease.[6,7]
In
this review, we describe the main features of WM in the light of the
new findings and current management of the disease including emerging
therapeutic options.
Clinical Presentation
Apart
from the systemic symptoms common to all NHL, MW clinical features can
be secondary to organ involvement, as well paraprotein-related.[4]
The
most frequent clinical sign of bone marrow infiltration is anaemia,
that represents itself as the most common indication for treatment
initiation. Nevertheless, several conditions, other than marrow
replacement, may lead to low haemoglobin level and should be excluded
before starting treatment.[8]
Anaemia may be
related to absolute or functional iron deficiency, that can be
distinguished by low iron saturation despite normal or high serum
ferritin levels. Ciccarelli et al.[9] attributed this event to hepcidin secretion by WM cells; the same findings were confirmed by Treon et al.[10]
who reported an excess of serum hepcidin in WM patients. Taking into
account this evidence, intravenous iron infusion, instead of oral
supplementation can be useful in some selected cases.
Haemolysis
can occur in Waldenstrom. A haemolytic diagnostic workup is necessary
in case of suspected haemolytic anaemia, including cold agglutinin
titres, direct Coombs test, haptoglobin, lactate dehydrogenase and
reticulocyte count.[11]
Similarly to other NHL,
organ infiltration by lymphoid clonal cells can lead to
hepatosplenomegaly, lymphadenopathies and less frequently the
involvement of extranodal tissues.[12]
Notably,
IgM paraprotein itself can be responsible for several clinical
pictures. High IgM serum level, over 4000 mg/dl, represents a risk
factor for symptomatic hyperviscosity syndrome, a particular condition
caused by increased serum viscosity.[13-15] This complication occurs in 5-10% of patients at the time of diagnosis.[16]
In
a recent retrospective study on 825 newly diagnosed WM patients, a
serum IgM level >6000 mg/dl at diagnosis was associated with a
median time to symptomatic hyperviscosity of 3 months, whereas the
median time for patients with serum IgM level of 5000-6000 mg/dl was
approximately three years.[17] These findings may
support the use of serum IgM level >6000 mg/dl as a criterion for
therapy initiation in an otherwise asymptomatic WM patient.
Hyperviscosity
manifestations are heterogeneous and may include spontaneous epistaxis,
ocular and hearing disorder, such as blurred vision, headache, tinnitus
and vertigo. An increase of viscosity involving microcirculation, also
in the central nervous system, can lead to clinical emergencies.[15]
In
case of IgM levels >3000 mg/dl, even in the absence of clinical
manifestations, the funduscopic examination is recommended to reveal
early signs of micro circular damage.[17-18]
IgM related immunological properties can also lead to particular situations.
Type
I and II cryoglobulinemia can clinically emerge with skin alterations
like purpura, ulcers and livedo, especially in the lower extremities.
Moreover, the presence of cryoglobulinemia can also worsen
hyperviscosity manifestations.[19]
IgM
paraprotein related peripheral neuropathies (IgM - PN) are a
heterogeneous group of disorders frequently associated with IgM
monoclonal gammopathies including WM.[20]
The
Last International Workshop on WM (IWWM) consensus panel, identified
six distinct entities of paraprotein-associated neuropathies.[21]
The
presence of anti-MAG antibodies or IgM antibodies directed to other
neural antigens (such as GD1a, GD1b, GM2) can lead to demyelinating and
slowly progressive predominantly distal neuropathy.[22] High titre of anti-GM1 antibodies otherwise, can be associated with a multifocal motor neuropathy.[23]
High titre of antibodies against disialylated gangliosides (GQ1b, GT1a,
GT1b, GD1b, GD2 and GD3) in the presence of neuropathy with
ophthalmoplegia and ataxia may configure CANOMAD (Chronic ataxic
neuropathy with ophthalmoplegia, M-protein, cold agglutinins and
disialosyl ganglioside antibodies) syndrome.[24]
Finally, AL amyloidosis and small fibre neuropathies should always be
considered as a possible cause of a paraproteinaemic neuropathy. In AL
amyloidosis, symptoms are due to direct paraprotein infiltration, and
clinical manifestations are progressive, painful small fibre
predominant length-dependent and typically starting in the feet,
accompanied by an autonomic neuropathy in about 65% of cases.[25]
Small fibre symptoms, presenting as patchy dermatomal sensory
disturbance subsequently coalescing are due to small fibre involvement
of the sensory ganglia.[26]
Other disorders can
be generated from the deposition of IgM-secreting lymphoplasmacytic
elements: amyloidosis is a rare and severe complication in MW. The
organs most commonly involved are kidneys, heart, liver and peripheral
nerves.[27]
Two different and distinctive syndromes are rarely associated to MW.
The
central nervous system involvement, called Bing-Neel syndrome, is a
complication involving almost 1% of patients with WM. Heterogeneous
neurological signs and symptoms may be investigated by the brain and
whole spine imaging and cerebrospinal fluid tests.[28]
Schnitzler
syndrome is a chronic autoimmune urticaria associated with IgM
gammopathy and other rheumatic manifestations, such as recurrent fever,
joint and bone pain, characterized this autoinflammatory disorder.[29]
Diagnosis
Diagnosis
of WM requires the histologic evidence of bone marrow infiltration of
lymphoplasmacytoid elements and the serum presence of monoclonal IgM
gammopathy. The need of at least 10% LPL infiltration as a cut-off to
distinguish WM from IgM monoclonal gammopathy of undetermined
significance (MGUS), was emphasised by the Mayo Clinic consensus.[30]
That is in contrast to the Second International Workshop Criteria that
do not mandate a minimum requirement of the BM involvement to confirm
the diagnosis.[1]
Since the presence of serum IgM
paraprotein itself is not specific and can be highlighted in a variety
of small B-cell lymphoproliferative disorders, such as chronic
lymphocytic leukemia and marginal zone lymphoma (MZL), as well as in
rare cases of IgM myeloma (MM), the diagnosis of WM should be
formulated combining specific histologic features, flow cytometry
parameters and molecular markers.
Bone marrow biopsy shows
lymphoplasmacytic and plasma cells; infiltration can be diffuse,
interstitial or nodular, while purely paratrabecular pattern is
uncommon.[2] Nevertheless, Bassarova et al. described
as distinguishing features of LPL at variance to MZL, the focal
paratrabecular involvement, the presence of lymphoplasmacytoid cells,
Dutcher bodies (P < .001) and the increased numbers of mast cells.[31]
Immuno-phenotype
reveals a clonal population of CD19, CD20, CD22, CD25, CD27, CD38,
CD79a, FCM7 and IgM surface/cytoplasmic IgM positive elements.
Immunohistochemistry demonstrates lymphocytes and lymphoplasmacytic
cells expressing IgM, with kappa or lambda restriction, CD19, CD20,
weak CD22 and CD25. Few cases, 10-20%, can be CD5, CD23 or CD10
positive. Plasma cells in WM are CD38 and CD138 positive but do not
show myelomatous antigen aberrations.[32-34]
There
are no specific chromosomal aberrations associated specifically with
WM. However, the frequency of individual chromosomal abnormalities
differs from that in other lymphoproliferative disorders such as MZL or
CLL.[35]
In particular, 6q deletions and trisomy
4, that seems to be significantly associated with trisomy 18, are
frequent in WM while translocations involving the IGH gene are very
rare.[36] Furthermore, the t(11;14) translocation, recurrent in IgM MM, does not occur in WM.[37] The prognostic value of these abnormalities, especially 6q deletion, is still controversial.[38,39]
In
2012 Treon et al. revealed the presence of a MYD88 L265P mutation in
the majority of patients with WM and this brought new insights in the
diagnosis and treatment of the disease. MYD88 is an adaptor molecule in
Toll-like receptor (TLR) and interleukin 1 receptor (IL-1R) signalling.
Following TLR or IL-1R stimulation, MYD88 is recruited to the activated
receptor complex as a homodimer and its association with IRAK4
activates IRAK1 and IRAK2. Tumor necrosis factor receptor-associated
factor 6 is then activated by IRAK1, leading to nuclear factor kB
(NF-kB) activation via IkBa phosphorylation and neoplastic cell growth
and survival. The MYD88 L265P somatic mutation has been identified in
>90% of WM patients by whole-genome sequencing.[40]
However, the mutation has also been demonstrated in about 10% of MZL
and other lymphoproliferative disorders, so it can't be used as a sole
marker in the distinction of WM. Nevertheless, it is absent in IgM
multiple myeloma and can be used for the differential diagnosis of
these two diseases.[41] Interestingly, the discovery
of MYD88 L265P in IgM MGUS patient may suggest that this mutation could
be an early oncogenic driver playing a role in disease progression to
WM.[42] Recently, Yang and colleagues showed that Bruton tyrosine kinase (BTK) was also activated by MYD88 L265P.[43] The diagnostic role of this mutation has been validated in several studies.[40-50] Recently Hunter et al. identified the first ever reported somatic mutation in human cancer involving CXCR4.[51]
This mutation is present in 30% of WM patients and involves the
C-terminus that contains serine phosphorylation sites which regulate
signalling of CXCR4 by its only known ligand, stromal derived factor-1a
(SDF-1a) (CXCL12). Germline mutations in the C-terminus of CXCR4 in
WHIM patients block receptor internalisation after SDF-1a stimulation
in myeloid cells resulting in persistent CXCR4 activation and bone
marrow myeloid cell trafficking.[52] Two different
types of CXCR4 mutations have been identified: nonsense (CXCR4WHIM/NS)
mutations that truncate the distal 15 to 20 amino acid region, and
frameshift (CXCR4WHIM/FS) mutations that compromise a region of up to
40 amino acids in the C- terminal domain.[51]
The
presence of CXCR4WHIM/NS mutation enhances AKT, ERK, and BTK signalling
and increases cell migration, adhesion, growth, and survival in WM
cells.[46] Other recurrent somatic mutations
described in WM include ARID1A, TRAF3, CD79B, TP53, and MYBBP1A as well
as monoallelic deletions of PRDM2, BTG1, TNFAIP3, and HIVEP2. The
acquisition of most of these mutation/deletions leads to NF-kB
signalling enhancement in response to MYD88 L265P.[51,54,55] Prognosis
Several
publications have been reported with the aim to identify variables that
could be associated with reduced survival in WM patients.[53-60]
Three
population-based studies analysed survival data on large cohorts of
patients. Significant shorter survival resulted to be related to older
age. Nevertheless, a proportion of elderly patients died from causes
unrelated to WM, while disease-specific survival exceeded six years
even for patients > 75 years.[62] Moreover, a significant improvement in survival over time has been reported in patients with WM during the last decade.[63,64]
At
present, the only validated prognostic scoring system for patients with
WM is the International Scoring System for WM (ISSWM) that was proposed
in 2009 for patients requiring treatment. Three groups of patients were
identified by this score: low, intermediate and high risk, showing,
respectively, 87%, 68% and 36% five-year survival rates. The risk
stratification could be identified by five covariates easily testable
in clinical practice: age (> 65), level of beta 2-microglobulin
(β2M> 3 mg/L), anemia (hemoglobin </= 11.5 g/dL),
thrombocytopenia (platelet < 100.000/mmc) and serum monoclonal
protein concentration (IgM > 7 g/dL).[65]
Concerning
molecular markers, the presence or not of MYD88 L265P mutation
demonstrated an impact on survival as patients carrying MYD88 L265P
showed a significant improvement on survival when compared to wild-type
MYD88, thus independently from CXCR4 mutational status.[50]
On
the other hand, CXCR4 mutational status seems to modulate clinical
presentation. In fact, patients with CXCR4 mutations present with a
significantly lower rate of adenopathy, and those with CXCR4 nonsense
mutations have an increased BM disease burden, serum IgM levels, and/or
risk of symptomatic hyperviscosity, while patients with MYD88 mutation
seem to have high BM disease involvement and serum IgM levels.[50,66,67]
Several
reports of familial clustering of patients affected by WM alone or with
other malignancies showed a common predisposition for WM with other
lymphoproliferative diseases. A familial MW was demonstrated in almost
20% of cases by Kristinsson et al.[68] Furthermore,
in a large single-center study, 26% of 924 consecutive patients with WM
had a first- or second-degree relative with either WM or another B-cell
disorder.[69] The diagnosis of familial form
represents an independent marker for disease progression being
associated with a 1.3-fold increased risk of death compared to sporadic
WM, with an increasing hazard ratio for each additional relative with a
lymphoproliferative disorder (defined as WM, NHL, MM, CLL, or MGUS).[70]
From a clinical point of view, greater BM involvement and baseline IgM
level were observed in familial compared to sporadic WM, while no
difference was noted in cytogenetic abnormalities or lymph node or
spleen involvement.[71]
While a report described a younger age at diagnosis of WM in the familial cases,[71] this observation was not confirmed in subsequent studies.[72]
In a single institution study, familial WM was associated with inferior
response rates to rituximab-combination regimens and shorter
time-to-next therapy (TTNT) than the sporadic cases. Furthermore,
time-to-progression (TTP) in familial WM was significantly shorter (21
vs 45 months for sporadic). However, superior outcomes with
bortezomib-containing regimens were observed in patients with familial
WM, regarding overall and major response rates and TTNT.[73]
Treatment
Similarly to other indolent lymphomas, treatment is also indicated for WM only in case of symptomatic disease.
The
Last Consensus on treatment initiation criteria has been recently
published by the Eighth International Workshop on Waldenstrom
Macroglobulinemia (IWWM).
Clinical and laboratory conditions defining symptomatic disease are listed in table 1.
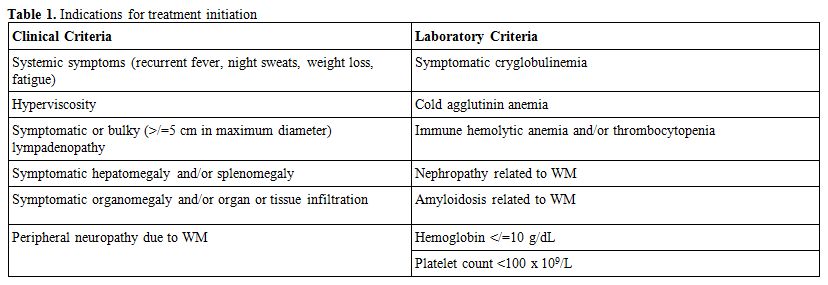 |
Table 1. Indications for treatment initiation |
Notably,
some particular situations require urgent therapeutic approach, in
particular symptomatic hyperviscosity should be considered as a
clinical emergency; plasmapheresis is indicated in such cases to reduce
IgM protein and consequently the risk of permanent organ impairment.
However, the benefit of this procedure is time-limited so
plasmapheresis should be rapidly followed by an acting systemic
treatment.[74]
Disease-specific characteristics at the time of progression should guide treatment choice.
Given
the rarity of WM, most of the current treatment regimens have been
adopted from data derived from phase 2 studies and less often from
prospective trials addressed to WM as well as to other indolent B-cell
lymphomas including Waldenstrom.
Treatment response criteria and classification are reported in table 2.
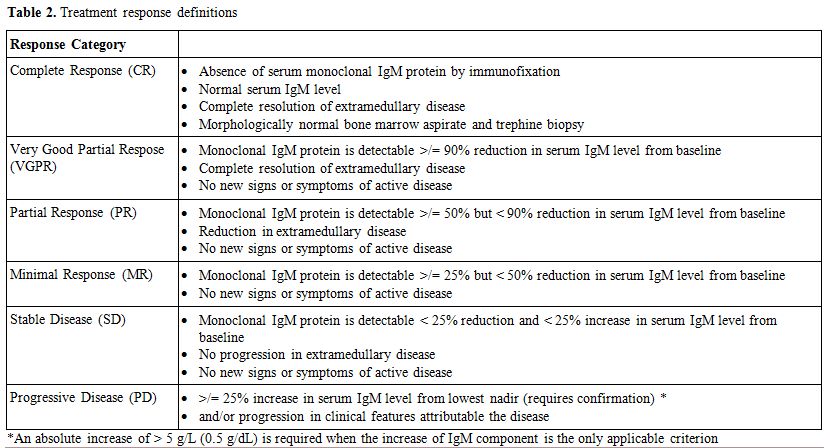 |
Table 2. Treatment response definitions |
Treatment Naïve.
Treatment choice should take into account patients age and
characteristics, specific goals of therapy, necessity for rapid disease
control, risk of treatment-related neuropathy, immunosuppression,
secondary malignancies, and potential for future autologous stem cell
transplantation (ASCT).
In the elderly population with comorbidities, single-agent treatment may be considered a suitable approach.
CD20 that is exclusively expressed in B-cells is a suitable therapeutic target for B-cell malignancies, including WM.[75]
Rituximab is a chimeric anti-CD20 MoAb and has been widely used as a
single agent in WM. Two schedules have been evaluated for Rituximab
single agent in WM, leading to an overall response rate (ORR) of 18-40%
as standard regimen (375 mg/mq for a 4-week cycle) and 35-65% as
extended course (375 mg/mq for additional 4 weeks administered 8 weeks
apart). Furthermore, PFS resulted in 33% for a standard schedule with a
median follow up of 15,7 months and maximum 89,5% for median follow up
of 29 months for the extended course. It is noteworthy that the pitfall
of these studies is the small number of patients.[76-79]
Notably,
the median time to response with rituximab monotherapy is seven months,
so such a slow time of action makes this drug unsuitable for patients
with the urgency of treatment. Furthermore, the possible occurrence of
“IgM flare”, defined as the transient increase of IgM serum level,
typically occurring after 1 to 4 months following rituximab infusion,
could even worsen some WM symptoms secondary to hyperviscosity. In the
presence of IgM levels >4000 mg/dl plasmapheresis should be
considered to prevent flare before rituximab administration.[74]
On the other hand, single-agent rituximab represents a valid option in
the presence of immunologic disorders related to MW, such as
symptomatic cryoglobulinemia, haemolytic anaemia or isolated IgM
related peripheral neuropathy.[74,80]
A recent publication demonstrated a significant clinical improvement in
almost half of the patients with anti-MAG antibody neuropathy treated
with Rituximab in monotherapy.[81]
Ofatumumab is
a fully human IgG1-type anti-CD20 MoAb. Its ability to bind to both the
small and large loop of the membrane antigen CD20 allows a prolonged
dissociation rate. Compared to rituximab, ofatumumab is able to produce
a more significant CDC activity with a similar antibody-dependent
cellular cytotoxicity activity. Ofatumumab has been approved for the
treatment of Chronic Lymphocytic Leukemia,[82] but
its activity as a single agent has also been tested by Furman and
colleagues in 37 WM patients, including nine naive treatment cases.
Following the first infusion, ofatumumab was administered at 1000 or
2000 mg weekly for four infusions. Almost 60% of patients obtained a
response with 35% achieving at least a PR.[83]
Despite the fact that rituximab infusion-related toxicity can be a
concern, leading to therapy interruption in a proportion of cases,
Castillo et al. reported the successful administration of ofatumumab in
22 patients who discontinued a previous treatment with rituximab due to
intolerance.[84]
In the past, oral chlorambucil
was a commonly used agent, resulting in at least a partial response in
75% of patients; despite this fact, a complete response was rare. A
randomised trial comparing two different dosing schedules found no
difference in terms of response and survival.[85]
In
2013, three years followed up from a large randomized study comparing
single agent chlorambucil to fludarabine in 414 patients with
previously untreated progressive WM, was published.[86]
Fludarabine
compared to chlorambucil, resulted in a high although not statistically
significant response rate. Nevertheless, fludarabine treatment led to a
significantly improved PFS and duration of response (median 36.3 and
38.3 months, respectively, versus 27.1 and 19.9 months, respectively
with chlorambucil). Median OS as well, was not reached in the
fludarabine arm versus 69.8 months in the chlorambucil arm. Although a
higher incidence of grade 3-4 neutropenia was observed among patients
treated with fludarabine, second neoplasms, including hematologic
malignancies, were significantly more frequent in the chlorambucil arm
with a 6-year cumulative incidence rate of 20.6% versus 3.7% in the
fludarabine arm.
Considering the slow response to chlorambucil
treatment and the possible risk of secondary malignancies and
myelodysplastic syndromes, this therapy should be reserved for elderly
patients, not in need of rapid disease control.
As in other
lymphoproliferative disorders rituximab is mostly used in combination
treatment and specifically associated in WM with several
chemotherapeutic agents, including alkylators, purine analogues,
bendamustine, and proteasome inhibitors.
The combination of
rituximab, cyclophosphamide and dexamethasone (DRC), was explored in
2007 by Dimopoulos et al. This regimen demonstrated to be highly
effective in WM patients showing 83% ORR, thus including 7% of patients
achieving CR. Long-term follow up of this study was published in 2015;
with a median follow up of 8 years, median PFS was 35 months and median
time to next treatment (TTNT) resulted in 51 months.
The
combination showed to be well tolerated with only 9% of patients
experiencing grade 3 or 4 neutropenia, and with limited long-term
toxicity. Considering the favourable toxic profile and patients’
outcome, DRC is regarded as a suitable combination treatment in the
first line. However considering the long median time to response of 4.1
this regimen does not allow rapid disease control.[87-88]
Three studies evaluated the efficacy of fludarabine-based regimens as primary treatment in WM. Treon and colleagues[89]
explored the combination of rituximab and fludarabine (FR) in 43
patients, including 27 treatment naive. Overall, 96.3% of patients
showed a response which was 86% excluding minor response; these results
were similar in treatment-naive compared to previously treated cases
(16/43 patients). Notably, with a median follow up of 40.3 months, two
years PFS was 67%. The addition of cyclophosphamide to FR[90]
led to 79.1% ORR (MR 74%) in 43 patients; most of them (65%) received
FCR as first-line treatment. Responses were durable; at a median follow
up of 38.8 months PFS was not reached, and two years overall survival
was 88.4%. Souchet et al.[91] in 2016 published the
results of a retrospective study with fludarabine, cyclophosphamide and
rituximab (FCR) offered to 27 naive treatment patients. Overall
response rate and three years PFS resulted in 88% and 96%,
respectively. Cladribine combined with rituximab was administered in 29
patients (55% treatment naive) with symptomatic WM. The ORR rate
observed was 89.6% in the whole population (93% in naive treatment
cases) without any difference between newly or pretreated patients.
Therapy was overall well tolerated as no major infections were reported
and no patients developed transformation to high-grade NHL nor
myelodysplasia. With a median follow-up of 50 months, four patients
relapsed; median time to treatment failure was not reached, but only
the lower limit of its 95% confidence interval was estimated at 60.3
months.[92]
Nevertheless, despite high efficacy
regarding response rate, proportion of major responses and response
duration, purine-analogues based combinations should be avoided as
first-line treatment in younger patients due to the significant
incidence of toxicity, risk of long-term secondary malignancies and the
impact on stem cell harvest. On the other hand, in older patients,
myelosuppression is the primary concern with these agents.
Bendamustine
and rituximab association (BR) is at present one of the most common
regimens used in the first-line treatment of WM patients. In phase III
large trial, the German study group on indolent lymphomas compared BR
vs rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone
(R-CHOP).[93] Overall response rate was similar
between the two regimens; nevertheless, BR demonstrated to be superior
to RCHOP in terms of PFS (69.5 vs 28.1 months) and tolerability. Taking
into account this evidence, BR was designated as the first-line choice
in different international guidelines and expert recommendations;[72,78]
thus also considering the possibility to modulate the schedule of
administration in elderly patients or case of renal impairment.[94]
Combination
strategies with proteasome inhibitors are also effective in WM.
Bortezomib, dexamethasone and rituximab (BDR) combination has been
tested in WM treatment-naive patients by Treon and colleagues obtaining
96% ORR, and PFS of 78.3% in median follow up of 22.8 months.[95]
On long-term analysis, median PFS was reached at 66 months. Moreover,
treatment was rapidly effective (median time o response 1.4 months) and
so, deliverable to patients with urgent need of IgM drop. Nevertheless,
the primary concern of bortezomib administered with the twice-week
schedule was the high rate of discontinuation (60%) due to neuropathy.[92,93]
A
significant reduction of neurological toxicity was obtained with an
alternative bortezomib administration explored by Ghobrial et. al.
Weekly administration of bortezomib, in fact, led to 88% ORR and 79%
one-year event-free survival (EFS) with no grade 3-4 neuropathies
reported.[97] The same bortezomib weekly schedule was
employed in the BDR regimen, except the first cycle in which bortezomib
was administered twice a week, allowing to obtain a low rate of
neurological complication (7% grade >/= 3) and discontinuation (8%)
due to neurological side effects. Efficacy (ORR 85%) and duration of
response (median PFS of 42 months) resulted slightly inferior to
previous studies.[98]
Table 3 summarises main regimens employed in previously untreated patients.
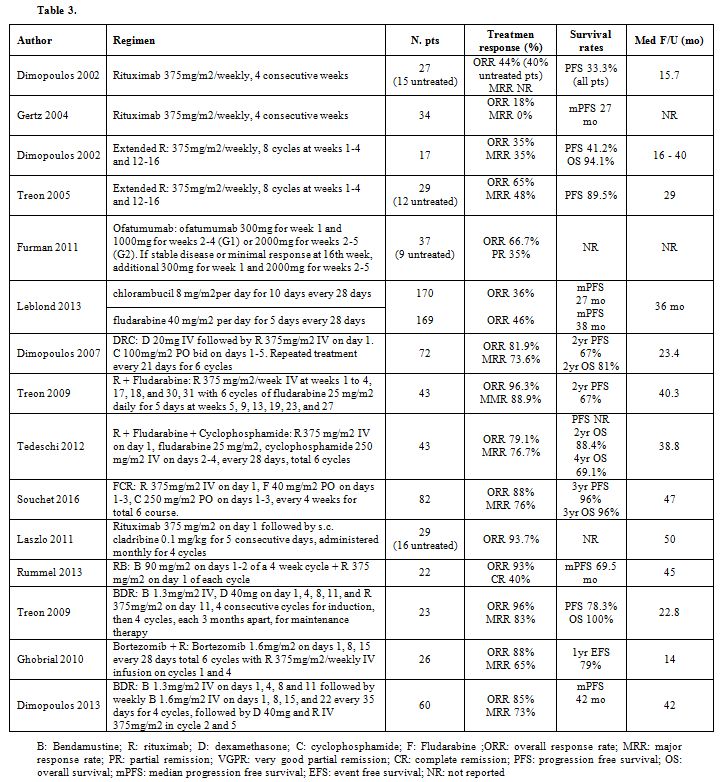 |
Table 3 |
The
last follow-up from this research has been published recently and
showed, after a median follow-up of 86 months, a PFS of 43 months,
while OS was still not reached.[99]
The role of rituximab as maintenance needs to be established as, in the
only published study on this topic, prolonged administration of
rituximab seemed to extend PFS and OS despite a more pronounced
incidence of upper respiratory tract infections and immunoglobulin
reduction.[100]
Salvage Treatment. Given the fact that WM is incurable, almost all patients will relapse after initial therapy.
Type
of therapy used at the time of relapse is determined by the response to
initial therapy and again patient and disease characteristics. There is
a general consensus, as for other lymphoproliferative disorders, to
repeat the original treatment according to response duration. In
symptomatic patients relapsing > 3 years after initial therapy, the
same procedure can be repeated.[30]
On the other hand, for relapse occurring < 3 years after initial treatment, an alternative regimen should be administered.
Similarly
to first-line treatment, in the setting of relapse and refractory WM
patients too, single-agent rituximab showed to be effective. The
response rate with rituximab administered for 4 or 8 cycles ranged
between 31 and 60%; DOR was comparable to that reported in previously
untreated setting and was prolonged by the extended schedule.
Nevertheless, these experiences are limited to a restricted number of
patients.[74,77,98]
The
efficacy and tolerability of bendamustine were evaluated in 2
retrospective studies addressed to WM. Treon et al. reported 83% ORR
and a median PFS of 13 months in 30 patients treated with bendamustine
with or without the addition of rituximab.[102]
Treatment was overall well tolerated, although prolonged
myelosuppression occurred in patients who received previous PA therapy.
Tedeschi et al. reported the outcome of 71 patients treated with BR
with bendamustine given 50/70/90 mg.[103] The ORR
was 90% with the great majority of cases obtaining an MR; responses
were durable as, at a median follow up of 19 months, PFS was still not
reached. It is noteworthy that, despite higher bendamustine dose
correlated with better response quality, the achievement of CR/VGPR did
not statistically impact on survival. Median time to IgM halving was
three months.
No significant toxicities were recorded with almost 70% of patients completing the planned six courses.
Although
bendamustine-based regimens have been widely used in
lymphoproliferative disorders, some concerns about safety are emerging.
In general, severe skin reaction is a known risk associated with
bendamustine, so that recommended preventive measures for tumour lysis
syndrome have been updated to avoid allopurinol concomitant
administration.[104] Moreover, nearly half of 234
patients evaluated in a retrospective analysis developed at least one
infection, one third being severe.[105]
Data
coming from GALLIUM trial and presented at the ASH meeting in 2016,
showed an unexpectedly higher rate of deaths in patients treated with
bendamustine in association with rituximab or obinutuzumab.[106]
Moreover,
very few data recorded in literature are long-term toxicities of this
agent. Recently, Martin et al. reported long-term outcomes of 149
subjects treated with bendamustine in 3 clinical trials. With a median
follow-up of 8·9 years, 23/149 patients developed 25 cancers, including
8 patients with myelodysplastic syndrome/acute myeloid leukaemia.[107]
Rituximab
combinations with purine analogues are effective, leading to high rate
of responses with a median PFS exceeding 50 months.[86–89,101]
Nevertheless, as previously mentioned, myelosuppression as well as high
rate of long term toxicities are major concerns with these agents
confining the use to selected cases with high tumor burden and limited
therapeutic options. Moreover, a retrospective study focused on the
long term outcomes of patients treated with FCR or BR were reported in
2015.[109] Interestingly, although FCR showed a
higher number of toxicities during treatment course, discontinuation
rate was similar between the 2 regimens. Response rate and quality were
also comparable nevertheless, PFS was significantly superior with FCR
treatment although it did not diverge when considering only responding
patients. Event free survival did not differ between FCR and BR when
considering either the whole population or only responding patients.
Notably, a significant higher proportion of patients in the FCR group
developed a solid tumor or MDS/AML.
Results coming from DRC combination in the setting of previously treated patients were reported by Paludo et al in 2017.[110]
Overall response rate was 87% with 68% MR. Median PFS and time-to
next-therapy were 32 and 50 (95% CI: 35–60) months, respectively being
comparable to survival results obtained in the setting of treatment
naive patients. Notably, response achievement and outcomes were
independent of MYD88 mutation status.
Bortezomib remains a valid
option in previously treated patients allowing to obtain response rate
of 81% with a median time to IgM lowering of approximately 1 month.
Table 4 summarizes main regimens employed in previously treated patients.
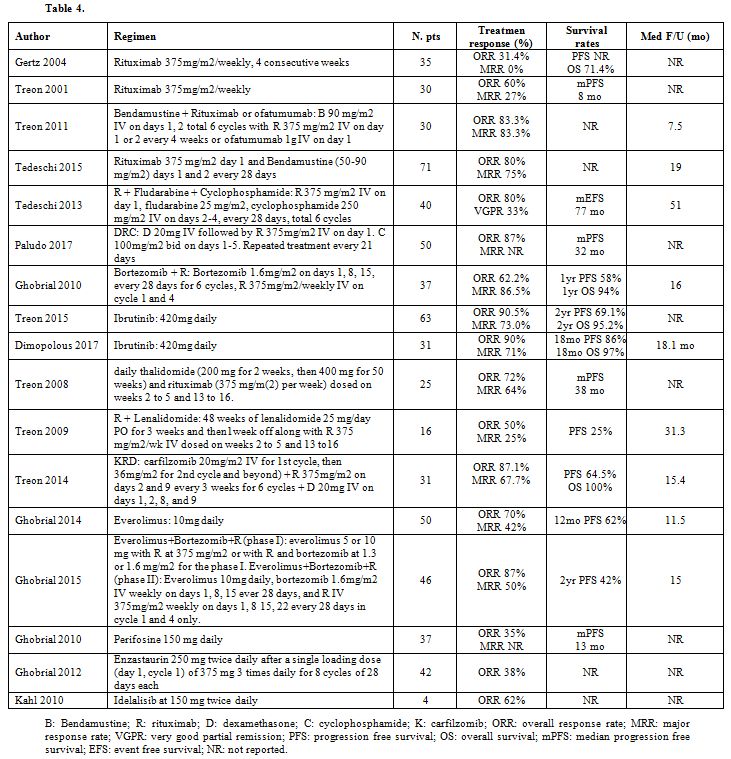 |
Table 4 |
Despite
considerable activity, it is noteworthy that response duration is short
as in previously treated patients, median time to progression and time
to next treatment were 16 and 17 months, respectively.[111]
Ibrutinib
Up to now ibrutinib is the only agent that has been specifically approved by FDA and EMA for the treatment of WM.[105,106]
In
preclinical studies ibrutinib demonstrated its efficacy in the
inhibition of IkB-alpha phosphorylation resulting in NF-kb signaling
block. Notably, as BTK is a downstream target of MYD88 L265P signaling,
ibrutinib exerts its action at higher levels in MYD88 L265P-expressing
cells rather in wild-type cells.[114]
In a single-arm phase II trial, ibrutinib was administered to 63 previously treated patients with WM.[115]
Overall response rate was 91% with 73% MR in a median time to response
of 4 weeks. Neutropenia, thrombocytopenia, anemia, atrial fibrillation
and infection were the most commonly reported grade 3-4 adverse events.
Based on these results, in January 2015 ibrutinib obtained FDA approval
as a breakthrough therapy for WM.[112] Notably,
response rate and quality significantly associated with genomic profile
as all MYD88 L265P mutated/CXCR4 WT cases obtained a response with
ibrutinib compared to 86% ORR among patients with MYD88 and CXCR4
mutations; furthermore, MR of patients carrying MYD88 but no CXCR4
mutations was 92% versus 62% in those who showed both mutations.
Overall, only 71% of patients with WT MYD88 status achieved a response
that was minor in all the cases. At 37 months follow up, 10 among the
initial 63 patients progressed and neither PFS nor OS was reached. The
prognostic impact of MYD88 and CXCR4 mutational status was confirmed at
longer observation. Moreover, compared to the initial report, no
significant difference in terms of side effects was reported.[116]
Finally,
INNOVATE is a single arm, multicenter, open-label, phase III study
exploring ibrutinib efficacy and tolerability in 31
rituximab-refractory WM.[117] Median number of prior
therapies was four and 42% of patients were classified as high risk per
the IPSSWM. Response rate and quality in such a high risk population
was superimposable to that previously reported by Treon et al. (90% ORR
with 71% MR). Once again, responses were rapid and durable. With a
median follow up of 18 months, estimated PFS and OS were 86% and 97%,
respectively. Common grade 3-4 adverse events included neutropenia,
hypertension, anemia, thrombocytopenia and diarrhea. Twenty six among
the 31 patients were continuing with ibrutinib at the time of the
report.
Despite much evidence of ibrutinib activity in Waldenstrom
Macroglobulinemia, clinical progression occurs while on therapy. It has
been demonstrated that WHIM-like CXCR4 S338X somatic mutation promotes
resistance to ibrutinib through the activation of AKT and ERK
signaling.[118] Moreover, similarly to chronic lymphocytic leukemia, also in WM activating mutations in BTKcys 481, PLCγ2 and CARD11 were detected and were frequently identified in CXCR4WHIM-like
cases. Interestingly, some patients carrying subclonal BTK mutations,
subsequently progressed while on treatment course. None of the
mutations was found at baseline confirming that acquisition of
mutations is probably linked to ibrutinib selective pressure on the
leukemic clone.[119]
Novel agents
Thalidomide and lenalidomide are immunomodulatory agents currently approved for the treatment of multiple myeloma.Rituximab combined with thalidomide or lenalidomide produced 72% and 50% response rate, respectively.[113,114]
Nevertheless, late responses (median after 9 to 12 cycles) and
development of acute anemia in more than 80% of patients, make this
drug unsuitable for WM. On the other hand, thalidomide demonstrated an
activity in WM only when administered at significantly
higher dosages than in multiple myeloma and this fact, together with
the well- known subclinical neuropathy that exists in patients with WM
predisposes them to enhanced thalidomide-related neurotoxicity.Pomalidomide
could be a promising alternative to the other immunomodulatory agents
and is currently under investigation in phase I clinical trials.Carfilzomib,
a second-generation selective proteasome inhibitor, showed a favourable
toxicity profile in the myeloma setting and has also been explored in
WM. Carfilzomib, rituximab and dexamethasone (CaRD) was administered as
front line therapy in 28 patients and led to 87% ORR (36% VGPR or CR)
and 64.5% PFS at 15.4 months. Responses were not affected by MYD88L265P
or CXCR4WHIM mutation status. No severe neurological toxicity was
reported.[122]The
phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt)/mammalian
target of rapamycin (mTOR)-signaling pathway showed to play a pivotal
role on the initiation and progression of B cell malignancies,
enhancing cell survival by stimulating cell proliferation, and
inhibiting apoptosis.[123] Following in-vitro data, the efficacy of mTOR inhibitor everolimus has been explored in phase I-II trials.[117,118]
When used as single agent, everolimus led to about 70% ORR.
Combinations of everolimus with bortezomib and/or rituximab allowed to
achieve 74% ORR with 5% CR.[126] However, a
considerable number of patients experienced grade >3 hematologic and
non-hematologic adverse events including pulmonary toxicity. Taking
into account such a safety profile, the use of this drug should be
considered only in selected patients in the context of clinical trials.[74]Perifosine is an Akt Inhibitor leading to 35% ORR in 37 previously treated WM.[127]
Median PFS was 12.4 months. Grade 1-2 gastrointestinal symptoms were
reported in most of the patients; hematologic toxicity was also
reported.Enzastaurin
is a serine/threonine kinase inhibitor that showed antiangiogenic,
antiproliferative, and proapoptotic properties in vitro and antitumor
activity in vivo in a xenograft WM model. Its efficacy was evaluated in
a phase II study on 42 patients who received previous treatment for
Waldenstrom disease. Almost 40% of patients obtained a response being
major in 2 cases. Grade 3 leukopenia occurred in one case while 1
patient died due to a septic shock.[128]PI3kδ inhibitor idelalisib was evaluated in 4 WM in the context of a phase I trial addressed to relapsed/refractory NHL.[129]
Overall, 62% of patients obtained a response. Nevertheless, a phase II
trial with this agent was prematurely interrupted due to the recurrence
of liver toxicity even when idelalisib was administered at lower dose
level.[130]Venetoclax,
a B-cell CLL/lymphoma 2 (BCL2) antagonist, was tested in-vitro on WM
cells and was found to be effective in cell lines with CXCR4WHIM. This
BCL2 inhibitor, combined with ibrutinib and idelalisib, enhanced
apoptosis in cell lines derived from WM patients presenting CXCR4WHIM
mutation.[131] M12-175 trial, a phase I study,
tested venetoclax for the first time in patients with relapsed and
refractory CLL and NHL. The BCL2 inhibitor demonstrated to be effective
and well tolerated in all lymphoma subtypes including 4 patients with
WM.[132]The
increasing knowledge of disease biology allowed to recognise new
potential therapeutic targets, such as CD38 that is expressed on the
surface of almost half of WM malignant cells.[133]
Daratumumab, a monoclonal antibody against CD38, approved for the
treatment of multiple myeloma, is a promising agent for the treatment
of WM.Considering the increased expression of CXCR4 on WM cells, agents active against this molecule are actually on study.[134]
Ulocuplumab, a fully human monoclonal antibody that targets CXCR4, was
recently tested in vitro and in vivo studies on xenograft models: as
monotherapy it showed antitumor activity against leukaemia, lymphoma
and myeloma.[135] Therefore, strategies targeting
CXCR4 may constitute an effective therapeutic approach for WM
potentially providing benefit even in ibrutinib resistant cases.Second
generation BTK inhibitors include acalabrutinib, BGB-3111, CC292 and
ONO-4059. These agents showed greater selectivity compared to ibrutinib
and phase I/II trials addressed to patients with WM are ongoing. A
phase III trial comparing BGB-3111 to ibrutinib in WM relapsed and
refractory patients is also currently enrolling patients.The
role of maintenance in WM is under investigation: MAINTAIN trial is
testing the efficacy on maintenance with rituximab after an induction
therapy with bendamustine and rituximab.
Conclusions
The
therapeutic landscape is expanding for Waldenstrom Macroglobulinemia.
Treatment choice in first, as well in subsequent lines of therapy,
should be driven by clinical features (age, comorbidities, concomitant
medications, eligibility for transplant procedures), disease-specific
characteristics at the time of progression and genetic profile.The
therapeutic objective should also be clear before starting treatment,
as some agents leading to deeper responses and, in this way, to a
prolonged survival, are often linked to a worse safety profile.One
of the main problems in treatment management of WM is that most of
currently administered regimens, are extrapolated from studies
involving indolent lymphoma while there is a lack of randomized
prospective trials specifically addressed to WM.Nevertheless,
what clearly emerged from clinical trials is that first line treatment
should always include rituximab. Patients receiving rituximab-based
regimens compared to those who didn’t in fact, showed significantly
better OS without differences in hospitalizations or plasmapheresis.
Moreover, survival appears not to differ when rituximab is administered
alone or in combination with chemotherapy with similar outcomes when
single agent rituximab is compared with either purine analogues or
alkylating agents administered as monotherapies.[136]It is important to take into account disease-specific characteristics, at the time of treatment decision.Rituximab
alone or in combination, is a valid option for neuropathy although the
occurrence of IgM flare could even worsen the neurologic clinical
picture. In case of bulky adenopathies, the addition of bendamustine to
rituximab is an effective regimen. Chemoimmunotherapy with DRC should
be considered instead in elderly patients, in the presence of cytopenia
taking into account the reduced myelotoxicity of this regimen.
Bortezomib and carlfizomib-based combinations are also effective in
this setting and guarantee a rapid reduction of IgM levels together
with improvement of cytopenia.Ibrutinib
is currently the only therapeutic agent approved for
relapsed/refractory WM. Nevertheless, data on ibrutinib are very
limited and ibrutinib resistance is getting more frequent while follow
up is extending. Moreover, patients with unmutated MYD88 status are
more likely to do worse with ibrutinib than with chemoimmunotherapeutic
or proteasome inhibitor based combinations.Considering
the emergence of mutations, ibrutinib combinations with other biologic
agents, acting on alternative ways of BCR signaling pathway, could be a
reasonable option in order to avoid the occurrence of resistance.
Besides MYD88, targeting of CXCR4-CXCL12 axis through CXCR4-antagonists
may offer a complementary mode of action by affecting CXCR4-expressing
tumor cells.Finally,
despite the rarity of the disease, large prospective international
trials are warranted to better understand the most appropriate clinical
use and long term side effects of new agents in Waldenstrom patients.
.
References
- Owen RG, Treon SP, Al-Katib A, Fonseca R, Greipp
PR, McMaster ML, Morra E, Pangalis GA, San Miguel JF, Branagan AR,
Dimopoulos MA. Clinicopathological definition of Waldenstrom's
macroglobulinemia: consensus panel recommendations from the Second
International Workshop on Waldenstrom's macroglobulinemia. Semin Oncol.
2003;30:110-5. https://doi.org/10.1053/sonc.2003.50082 PMid:12720118

- Swerdlow
SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R,
Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the
World Health Organization classification of lymphoid neoplasms. Blood.
2016;127:2375-90. https://doi.org/10.1182/blood-2016-01-643569 PMid:26980727 PMCid:PMC4874220

- Sekhar
J, Sanfilippo K, Zhang Q, Trinkaus K, Vij R, Morgensztern D.
Waldenstrom macroglobulinemia: a Surveillance, Epidemiology, and End
Results database review from 1988 to 2005. Leuk Lymphoma.
2012;53:1625-26. https://doi.org/10.3109/10428194.2012.656103 PMid:22239669

- Dimopoulos
MA, Panayiotidis P, Moulopoulos LA, Sfikakis P, Dalakas M.
Waldenström's macroglobulinemia: clinical features, complications, and
management. J Clin Oncol. 2000;18:214-26. https://doi.org/10.1200/JCO.2000.18.1.214 PMid:10623712

- Leblond
V, Kastritis E, Advani R, Ansell SM, Buske C, Castillo JJ, García-Sanz
R, Gertz M, Kimby E, Kyriakou C, Merlini G, Minnema MC, Morel P, Morra
E, Rummel M, Wechalekar A, Patterson CJ, Treon S, Dimopoulos MA.
Treatment recommendations from the Eighth International Workshop on
Waldenström's Macroglobulinemia. Blood. 2016;128:1321-8. https://doi.org/10.1182/blood-2016-04-711234 PMid:27432877

- Treon
SP, Xu L, Yang G, Zhou Y, Liu X, Cao Y, Sheehy P, Manning RJ, Patterson
CJ, Tripsas C, Arcaini L, Pinkus GS, Rodig SJ, Sohani AR, Harris NL,
Laramie JM, Skifter DA, Lincoln SE, Hunter ZR. MYD88 L265P somatic
mutation in Waldenstrom's macroglobulinemia. N Engl J Med.
2012;367:826-33. https://doi.org/10.1056/NEJMoa1200710 PMid:22931316

- Hunter
ZR, Xu L, Yang G, Zhou Y, Liu X, Cao Y, Manning RJ, Tripsas C,
Patterson CJ, Sheehy P, Treon SP. The genomic landscape of Waldenstrom
macroglobulinemia is characterized by highly recurring MYD88 and
WHIM-like CXCR4 mutations, and small somatic deletions associated with
B-cell lymphomagenesis. Blood. 2014;123:1637-46. https://doi.org/10.1182/blood-2013-09-525808 PMid:24366360

- Dimopoulos
MA, Kyle RA, Anagnostopoulos A, Treon SP. Diagnosis and management of
Waldenstrom's macroglobulinemia. J Clin Oncol. 2005;23:1564-77. https://doi.org/10.1200/JCO.2005.03.144 PMid:15735132

- Ciccarelli
BT, Patterson CJ, Hunter ZR, Hanzis C, Ioakimidis L, Manning R, Yang G,
Xu L, Zhou Y, Sun J, Liu X, Tseng H, Cao Y, Sheehy P, Rodig SJ, Treon
SP. Hepcidin is produced by lymphoplasmacytic cells and is associated
with anemia in Waldenström's macroglobulinemia. Clin Lymphoma Myeloma
Leuk. 2011;11:160-3. https://doi.org/10.3816/CLML.2011.n.038 PMid:21454222

- Treon
SP, Tripsas CK, Ciccarelli BT, Manning RJ, Patterson CJ, Sheehy P,
Hunter ZR. Patients with Waldenström macroglobulinemia commonly present
with iron deficiency and those with severely depressed transferrin
saturation levels show response to parenteral iron administration. Clin
Lymphoma Myeloma Leuk. 2013;13:241-3. https://doi.org/10.1016/j.clml.2013.02.016 PMid:23523274

- Kapoor
P, Paludo J, Vallumsetla N, Greipp PR. Waldenström macroglobulinemia:
What a hematologist needs to know. Blood Rev. 2015;29:301-19. https://doi.org/10.1016/j.blre.2015.03.001 PMid:25882617

- García-Sanz
R, Montoto S, Torrequebrada A, de Coca AG, Petit J, Sureda A,
Rodríguez-García JA, Massó P, Pérez-Aliaga A, Monteagudo MD, Navarro I,
Moreno G, Toledo C, Alonso A, Besses C, Besalduch J, Jarque I, Salama
P, Rivas JA, Navarro B, Bladé J, Miguel JF. Waldenstrom
macroglobulinaemia: presenting features and outcome in a series with
217 cases. Br J Haematol. 2001;115:575-82. https://doi.org/10.1046/j.1365-2141.2001.03144.x PMid:11736938

- Mackenzie
MR, Babcock J. Studies of the hyperviscosity syndrome. II.
Macroglobulinemia. J Lab Clin Med. 1975;85:227-34.
PMid:803540

- Fahey JL, Barth WF, Solomon A. Serum hyperviscosity syndrome. JAMA. 1965;192:120-3. https://doi.org/10.1001/jama.1965.03080190030008

- Stone MJ, Bogen SA. Evidence-based focused review of management of hyperviscosity syndrome. Blood 2012;119:2205-8. https://doi.org/10.1182/blood-2011-04-347690 PMid:22147890

- Mehta J, Singhal S. Hyperviscosity syndrome in plasma cell dyscrasias. Semin Thromb Hemost. 2003;29:467-71. https://doi.org/10.1055/s-2003-44554 PMid:14631546

- Gustine
JN, Meid K, Dubeau T, Hunter ZR, Xu L, Yang G, Ghobrial IM, Treon SP,
Castillo JJ. Serum IgM level as predictor of symptomatic hyperviscosity
in patients with Waldenström macroglobulinaemia. Br J Haematol.
2017;177:717-25. https://doi.org/10.1111/bjh.14743 PMid:28485115

- Menke
MN, Feke GT, McMeel JW, Branagan A, Hunter Z, Treon SP.
Hyperviscosity-related retinopathy in Waldenström's macroglobulinemia.
Arch Ophthalmol. 2006;124:1601-6. https://doi.org/10.1001/archopht.124.11.1601 PMid:17102008

- Ghobrial IM. Are you sure this is Waldenstrom macroglobulinemia? Hematology Am Soc Hematol Educ Program 2012;2012:586-94.

- Kyle
RA. Neuropathy associated with the monoclonal gammopathies. In:
Noseworthy JH, ed. Neurologic therapeautics: principles and practice.
London, New York. Martin Dunitz. 2003;2126-36.
- D'Sa
S, Kersten MJ, Castillo JJ, Dimopoulos M, Kastritis E, Laane E, Leblond
V, Merlini G, Treon SP, Vos JM, Lunn MP. Investigation and management
of IgM and Waldenström-associated peripheral neuropathies:
recommendations from the IWWM-8 consensus panel. Br J Haematol.
2017;176:728-42. https://doi.org/10.1111/bjh.14492 PMid:28198999

- Nobile-Orazio
E, Meucci N, Baldini L, Di Troia A, Scarlato G. Long-term prognosis of
neuropathy associated with anti-MAG IgM M-proteins and its relationship
to immune therapies. Brain. 2000;123:710-17. https://doi.org/10.1093/brain/123.4.710 PMid:10734002

- Treon
SP, Hanzis CA, Ioakimidis LI, Patterson CJ, Hunter ZR, Brodsky PS,
Sheehy PS, Manning RJ. Clinical characteristics and treatment outcome
of disease-related peripheral neuropathy in Waldenstrom's
macroglobulinemia (WM). J Clin Oncol. 2010;28:15s. https://doi.org/10.1200/jco.2010.28.15 _suppl.8114

- Willison
HJ, O'Leary CP, Veitch J, Blumhardt LD, Busby M, Donaghy M, Fuhr P,
Ford H, Hahn A, Renaud S, Katifi HA, Ponsford S, Reuber M, Steck A,
Sutton I, Schady W, Thomas PK, Thompson AJ, Vallat JM, Winer J. The
clinical and laboratory features of chronic sensory ataxic neuropathy
with anti-disialosyl IgM antibodies. Brain. 2001;124: 1968-77. https://doi.org/10.1093/brain/124.10.1968 PMid:11571215

- Rajkumar
SV, Gertz MA, Kyle RA. Prognosis of patients with primary systemic
amyloidosis who present with dominant neuropathy. Am J of Med.
1988;104:232-7. https://doi.org/10.1016/S0002-9343(98)00037-0

- Themistocleous
AC, Ramirez JD, Serra J, Bennett DL. The clinical approach to small
fibre neuropathy and painful channelopathy. Prac Neur. 2014;14:368-79. https://doi.org/10.1136/practneurol-2013-000758 PMid:24778270 PMCid:PMC4251302

- Palladini
G, Russo P, Bosoni T, Sarais G, Lavatelli F, Foli A, Bragotti LZ,
Perfetti V, Obici L, Bergesio F, Albertini R, Moratti R, Merlini G. AL
amyloidosis associated with IgM monoclonal protein: a distinct clinical
entity. Clin Lymph Myel. 2009;9:80-3. https://doi.org/10.3816/CLM.2009.n.021 PMid:19362981

- Minnema
MC, Kimby E, D'Sa S, Fornecker LM, Poulain S, Snijders TJ, Kastritis E,
Kremer S, Fitsiori A, Simon L, Davi F, Lunn M, Castillo JJ, Patterson
CJ, Le Garff-Tavernier M, Costopoulos M, Leblond V, Kersten MJ,
Dimopoulos MA, Treon SP. Guideline for the diagnosis, treatment and
response criteria for Bing-Neel syndrome. Haematologica.
2017;102:43-51. https://doi.org/10.3324/haematol.2016.147728 PMid:27758817 PMCid:PMC5210231

- Lipsker
D, Veran Y, Grunenberger F, Cribier B, Heid E, Grosshans E. The
Schnitzler syndrome: four new cases and review of the literature.
Medicine. 2001;80:37- 44. https://doi.org/10.1097/00005792-200101000-00004 PMid:11204501

- Ansell
SM, Kyle RA, Reeder CB, Fonseca R, Mikhael JR, Morice WG, Bergsagel PL,
Buadi FK, Colgan JP, Dingli D, Dispenzieri A, Greipp PR, Habermann TM,
Hayman SR, Inwards DJ, Johnston PB, Kumar SK, Lacy MQ, Lust JA,
Markovic SN, Micallef IN, Nowakowski GS, Porrata LF, Roy V, Russell SJ,
Short KE, Stewart AK, Thompson CA, Witzig TE, Zeldenrust SR, Dalton RJ,
Rajkumar SV, Gertz MA. Diagnosis and management of Waldenstrom
macroglobulinemia: mayo stratification of macroglobulinemia and
risk-adapted therapy (mSMART) guidelines. Mayo Clin Proc.
2010;85:824–33. https://doi.org/10.4065/mcp.2010.0304 PMid:20702770 PMCid:PMC2931618

- Bassarova
A, Trøen G, Spetalen S, Micci F, Tierens A, Delabie J.
Lymphoplasmacytic lymphoma and marginal zone lymphoma in the bone
marrow: paratrabecular involvement as an important distinguishing
feature. Am J Clin Pathol. 2015 Jun;143(6):797-806. https://doi.org/10.1309/AJCP6ZODWV1CIDME PMid:25972321

- Feiner
HD, Rizk CC, Finfer MD, Bannan M, Gottesman SR, Chuba JV, Amorosi E.
IgM monoclonal gammopathy/Waldenstrom's macroglobulinemia: a
morphological and immunophenotypic study of the bone marrow. Mod
Pathol. 1990;3:348–56. PMid:2114024

- San
Miguel JF, Vidriales MB, Ocio E, Mateo G, Sánchez-Guijo F, Sánchez ML,
Escribano L, Bárez A, Moro MJ, Hernández J, Aguilera C, Cuello R,
García-Frade J, López R, Portero J, Orfao A. Immunophenotypic analysis
of Waldenstrom's macroglobulinemia. Semin Oncol. 2003;30:187–95. https://doi.org/10.1053/sonc.2003.50074 PMid:12720134

- Hunter
ZR, Branagan AR, Manning R, Patterson CJ, Santos DD, Tournilhac O,
Dorfman DM, Treon SP. CD5, CD10, CD23 expression in Waldenstrom's
macroglobulinemia. Clin Lymph. 2005;5:246–9. https://doi.org/10.3816/CLM.2005.n.008

- Treon
SP, Hunter ZR, Aggarwal A, Ewen EP, Masota S, Lee C, Santos DD,
Hatjiharissi E, Xu L, Leleu X, Tournilhac O, Patterson CJ, Manning R,
Branagan AR, Morton CC. Characterization of familial Waldenstrom's
macroglobulinemia. Ann. Oncol. 2006;17:488–94 https://doi.org/10.1093/annonc/mdj111 PMid:16357024

- Nguyen-Khac
F, Lambert J, Chapiro E, Grelier A, Mould S, Barin C, Daudignon A,
Gachard N, Struski S, Henry C, Penther D, Mossafa H, Andrieux J,
Eclache V, Bilhou-Nabera C, Luquet I, Terre C, Baranger L, Mugneret F,
Chiesa J, Mozziconacci MJ, Callet-Bauchu E, Veronese L, Blons H, Owen
R, Lejeune J, Chevret S, Merle-Beral H, Leblondon V; Groupe Français
d'Etude de la Leucémie Lymphoïde Chronique et Maladie de Waldenström
(GFCLL/MW); Groupe Ouest-Est d'étude des Leucémie Aiguës et Autres
Maladies du Sang (GOELAMS); Groupe d'Etude des Lymphomes de l'Adulte
(GELA). Chromosomal aberrations and their prognostic value in a series
of 174 untreated patients with Waldenström's macroglobulinemia.
Haematologica. 2013;98:649-54 https://doi.org/10.3324/haematol.2012.07045 PMid:23065509 PMCid:PMC3659998

- Avet-Loiseau
H, Garand R, Lodé L, Harousseau JL, Bataille R; Intergroupe Francophone
du Myélome. Translocation t(11;14)(q13;q32) is the hallmark of IgM,
IgE, and nonsecretory multiple myeloma variants. Blood.
2003;101(4):1570-71. https://doi.org/10.1182/blood-2002-08-2436 PMid:12393502

- Nguyen-Khac
F, Lejeune J, Chapiro E, Mould S, Barin C, Daudignon A. Cytogenetic
abnormalities in a cohort of 171 patients with Waldenström
macroglobulinemia before treatment: clinical and biological
correlations. Blood. 2010;116:abstract 801

- Chang
H, Qi C, Trieu Y, Jiang A, Young KH, Chesney A, Jani P, Wang C, Reece
D, Chen C. Prognostic relevance of 6q deletion in Waldenstrom's
macroglobulinemia: a multicenter study. Clin Lymph Myel. 2009;9:36–8 https://doi.org/10.3816/CLM.2009.n.008 PMid:19362968

- Treon
SP, Xu L, Zhou Y, Liu X, Yang G, Cao Y, Hanzis C, Sheehy P, Manning R,
Patterson CJ, Laramie JM, Skifter DA, Lincoln SE, Hunter Z. Whole
genome sequencing reveals a widely expressed mutation (MYD88 L265P)
with oncogenic activity in Waldenstrom's macroglobulinemia. Blood.
2011;118:abstract 300.

- Xu
L, Hunter ZR, Yang G, Zhou Y, Cao Y, Liu X, Morra E, Trojani A, Greco
A, Arcaini L, Varettoni M, Brown JR, Tai YT, Anderson KC, Munshi NC,
Patterson CJ, Manning RJ, Tripsas CK, Lindeman NI, Treon SP. MYD88
L265P in Waldenstrom macroglobulinemia, immunoglobulin M monoclonal
gammopathy, and other B-cell lymphoproliferative disorders using
conventional and quantitative allele-specific polymerase chain
reaction. Blood. 2013;121:2051–8. https://doi.org/10.1182/blood-2012-09-454355 PMid:23321251 PMCid:PMC3596964

- Hunter
ZR, Xu L, Yang G, Zhou Y, Liu X, Cao Y, Manning RJ, Tripsas C,
Patterson CJ, Sheehy P, Treon SP. The genomic landscape of Waldenstrom
macroglobulinemia is characterized by highly recurring MYD88 and
WHIM-like CXCR4 mutations, and small somatic deletions associated with
B-cell lymphomagenesis. Blood. 2014;123:1637-46. https://doi.org/10.1182/blood-2013-09-525808 PMid:24366360

- Yang
G1, Zhou Y, Liu X, Xu L, Cao Y, Manning RJ, Patterson CJ, Buhrlage SJ,
Gray N, Tai YT, Anderson KC, Hunter ZR, Treon SP. A mutation in MYD88
(L265P) supports the survival of lymphoplasmacytic cells by activation
of Bruton tyrosine kinase in Waldenström macroglobulinemia. Blood
2013;122:1222-32. https://doi.org/10.1182/blood-2012-12-475111 PMid:23836557

- Treon
SP, Xu L, Yang G, Zhou Y, Liu X, Cao Y, Sheehy P, Manning RJ, Patterson
CJ, Tripsas C, Arcaini L, Pinkus GS, Rodig SJ, Sohani AR, Harris NL,
Laramie JM, Skifter DA, Lincoln SE, Hunter ZR. MYD88 L265P somatic
mutation in Waldenstrom's macroglobulinemia. N Engl J Med.
2012;367:826-33. https://doi.org/10.1056/NEJMoa1200710 PMid:22931316

- Xu
L, Hunter ZR, Yang G, Cao Y, Liu X, Manning R, Tripsas C, Chen J,
Patterson CJ, Kluk M, Kanan S, Castillo J, Lindeman N, Treon SP.
Detection of MYD88 L265P in peripheral blood of patients with
Waldenstrom's Macroglobulinemia and IgM monoclonal gammopathy of
undetermined significance. Leukemia. 2014;28:1698-704. https://doi.org/10.1038/leu.2014.65 PMid:24509637

- Roccaro
AM, Sacco A, Jimenez C, Maiso P, Moschetta M, Mishima Y, Aljawai Y,
Sahin I, Kuhne M, Cardarelli P, Cohen L, San Miguel JF, Garcia-Sanz R,
Ghobrial IM. C1013G/CXCR4 acts as a driver mutation of tumor
progression and modulator of drug resistance in lymphoplasmacytic
lymphoma. Blood. 2014;123:4120-31. https://doi.org/10.1182/blood-2014-03-564583 PMid:24711662

- Chakraborty
R, Novak AJ, Ansell SM, Muchtar E, Kapoor P, Hayman SR, Dispenzieri A,
Buadi FK, Lacy MQ, King RL, Gertz MA. First report of MYD88 L265P
somatic mutation in IgM-associated light-chain amyloidosis. Blood.
2016;127:2936-8. https://doi.org/10.1182/blood-2016-02-702035 PMid:27034430

- Gachard
N, Parrens M, Soubeyran I, Petit B, Marfak A, Rizzo D, Devesa M,
Delage-Corre M, Coste V, Laforêt MP, de Mascarel A, Merlio JP,
Bouabdhalla K, Milpied N, Soubeyran P, Schmitt A, Bordessoule D, Cogné
M, Feuillard J. IGHV gene features and MYD88 L265P mutation separate
the three marginal zone lymphoma entities and Waldenstrom
macroglobulinemia/ lymphoplasmacytic lymphomas. Leukemia.
2013;27:183-9. https://doi.org/10.1038/leu.2012.257 PMid:22944768

- Landgren O, Staudt L. MYD88 L265P somatic mutation in IgM MGUS. N Engl J Med. 2012;367:2255–6. https://doi.org/10.1056/NEJMc1211959 PMid:23215570

- Treon
SP, Cao Y, Xu L, Yang G, Liu X, Hunter ZR. Somatic mutations in MYD88
and CXCR4 are determinants of clinical presentation and overall
survival in Waldenstrom macroglobulinemia. Blood. 2014;123:2791-6. https://doi.org/10.1182/blood-2014-01-550905 PMid:24553177

- Hunter
ZR, Xu L, Yang G, Zhou Y, Liu X, Cao Y, Manning RJ, Tripsas C,
Patterson CJ, Sheehy P, Treon SP. The genomic landscape of Waldenstom's
macroglobulinemia is characterized by highly recurring MYD88 and
WHIM-like CXCR4 mutations, and small somatic deletions associated with
B-cell lymphomagenesis. Blood. 2014;123:1637–46. https://doi.org/10.1182/blood-2013-09-525808 PMid:24366360

- Dotta
L, Tassone L, Badolato R. Clinical and genetic features of Warts,
Hypogammaglobulinemia, Infections and Myelokathexis (WHIM) syndrome.
Curr Mol Med. 2011;11:317–25. https://doi.org/10.2174/156652411795677963 PMid:21506920

- Braggio
E, Keats JJ, Leleu X, Van Wier S, Jimenez-Zepeda VH, Valdez R, Schop
RF, Price-Troska T, Henderson K, Sacco A, Azab F, Greipp P, Gertz M,
Hayman S, Rajkumar SV, Carpten J, Chesi M, Barrett M, Stewart AK, Dogan
A, Bergsagel PL, Ghobrial IM, Fonseca R. Identification of copy number
abnormalities and inactivating mutations in two negative regulators of
nuclear factor-kappaB signaling pathways in Waldenstrom's
macroglobulinemia. Cancer Res. 2009;69:3579–88. https://doi.org/10.1158/0008-5472.CAN-08-3701 PMid:19351844 PMCid:PMC2782932

- Poulain
S, Roumier C, Galiègue-Zouitina S, Daudignon A, Herbaux C, Aiijou R,
Lainelle A, Broucqsault N, Bertrand E, Manier S, Renneville A, Soenen
V, Tricot S, Roche-Lestienne C, Duthilleul P, Preudhomme C, Quesnel B,
Morel P, Leleu X. Genome Wide SNP Array identified multiple mechanisms
of genetic changes in Waldenstrom macroglobulinemia. Am J Hematol.
2013;88:948–54. https://doi.org/10.1002/ajh.23545 PMid:23861223

- Facon
T, Brouillard M, Duhamel A, Morel P, Simon M, Jouet JP, Bauters F,
Fenaux P. Prognostic factors in Waldenstrom's macroglobulinemia: a
report of 167 cases. J Clin Oncol. 1993;11:1553-8. https://doi.org/10.1200/JCO.1993.11.8.1553 PMid:8336194

- Morel
P, Monconduit M, Jacomy D, Lenain P, Grosbois B, Bateli C, Facon T,
Dervite I, Bauters F, Najman A, De Gramont A, Wattel E. Prognostic
factors in Waldenstrom's macroglobulinemia: a report on 232 patients
with the description of a new scoring system and its validation on 253
other patients. Blood. 2000;96:852-8 PMid:10910896

- Merlini
G, Baldini L, Broglia C, Comelli M, Goldaniga M, Palladini G, Deliliers
GL, Gobbi PG. Prognostic factors in symptomatic Waldenstrom's
macroglobulinemia. Semin Oncol. 2003;30:211-5. https://doi.org/10.1053/sonc.2003.50064 PMid:12720138

- García-Sanz
R, Montoto S, Torrequebrada A, de Coca AG, Petit J, Sureda A,
Rodríguez-García JA, Massó P, Pérez-Aliaga A, Monteagudo MD, Navarro I,
Moreno G, Toledo C, Alonso A, Besses C, Besalduch J, Jarque I, Salama
P, Rivas JA, Navarro B, Bladé J, Miguel JF; Spanish Group for the Study
of Waldenström Macroglobulinaemia and PETHEMA (Programme for the Study
and Treatment of Haematological Malignancies). Waldenstrom
macroglobulinaemia: presenting features and outcome in a series with
217 cases. Br J Haematol. 2001;115:575-82. https://doi.org/10.1046/j.1365-2141.2001.03144.x PMid:11736938

- Owen
RG, Barrans SL, Richards SJ, O'Connor SJ, Child JA, Parapia LA, Morgan
GJ, Jack AS. Waldenstrom macroglobulinemia: development of diagnostic
criteria and identification of prognostic factors. Am J Clin Pathol.
2001;116:420-8. https://doi.org/10.1309/4LCN-JMPG-5U71-UWQB PMid:11554171

- Dimopoulos
MA, Hamilos G, Zervas K, Symeonidis A, Kouvatseas G, Roussou P, Gika D,
Karmiris T, Bourantas K, Zomas A, Mitsouli C, Xilouri I, Vervessou E,
Matsis K, Anagnostopoulos N, Economopoulos T; Greek Myeloma Study
Group. Survival and prognostic factors after initiation of treatment in
Waldenstrom's macroglobulinemia. Ann Oncol. 2003;14:1299-305. https://doi.org/10.1093/annonc/mdg334

- Ghobrial
IM, Fonseca R, Gertz MA, Plevak MF, Larson DR, Therneau TM, Wolf RC,
Hoffmann RJ, Lust JA, Witzig TE, Lacy MQ, Dispenzieri A, Vincent
Rajkumar S, Zeldenrust SR, Greipp PR, Kyle RA. Prognostic model for
disease-specific and overall mortality in newly diagnosed symptomatic
patients with Waldenstrom macroglobulinaemia. Br J Haematol.
2006;133:158-64. https://doi.org/10.1111/j.1365-2141.2006.06003.x PMid:16611306

- Kastritis
E1, Zervas K, Repoussis P, Michali E, Katodrytou E, Zomas A, Simeonidis
A, Terpos E, Delimbassi S, Vassou A, Gika D, Dimopoulos MA; Greek
Myeloma Study Group. Prognostication in young and old patients with
Waldenström's macroglobulinemia: importance of the International
Prognostic Scoring System and of serum lactate dehydrogenase. Clin
Lymphoma Myeloma. 2009;9:50-2. https://doi.org/10.3816/CLM.2009.n.012 PMid:19362972

- Kristinsson
SY, Eloranta S, Dickman PW, Andersson TM, Turesson I, Landgren O,
Björkholm M. Patterns of survival in lymphoplasmacytic
lymphoma/Waldenström macroglobulinemia: a population-based study of
1,555 patients diagnosed in Sweden from 1980 to 2005. Am J Hematol.
2013;60-5. https://doi.org/10.1002/ajh.23351 PMid:23165980

- Castillo
JJ1, Olszewski AJ, Kanan S, Meid K, Hunter ZR, Treon SP. Overall
survival and competing risks of death in patients with Waldenström
macroglobulinaemia: an analysis of the Surveillance, Epidemiology and
End Results database. Br J Haematol. 2015;169:81-9. https://doi.org/10.1111/bjh.13264 PMid:25521528

- Morel
P, Duhamel A, Gobbi P, Dimopoulos MA, Dhodapkar MV, McCoy J, Crowley J,
Ocio EM, Garcia-Sanz R, Treon SP, Leblond V, Kyle RA, Barlogie B,
Merlini G. International prognostic scoring system for Waldenstrom
macroglobulinemia. Blood. 2009;113:4163-7. https://doi.org/10.1182/blood-2008-08-174961 PMid:19196866

- Poulain
S, Roumier C, Venet-Caillault A, Figeac M, Herbaux C, Marot G, Doye E,
Bertrand E, Geffroy S, Lepretre F, Nibourel O, Decambron A, Boyle EM,
Renneville A, Tricot S, Daudignon A, Quesnel B, Duthilleul P,
Preudhomme C, Leleu X. Genomic Landscape of CXCR4 Mutations in
Waldenström Macroglobulinemia. Clin Cancer Res. 2016;22:1480-8. https://doi.org/10.1158/1078-0432.CCR-15-0646 PMid:26490317

- Schmidt
J, Federmann B, Schindler N, Steinhilber J, Bonzheim I, Fend F,
Quintanilla-Martinez L. MYD88 L265P and CXCR4 mutations in
lymphoplasmacytic lymphoma identify cases with high disease activity.Br
J Haematol. 2015;169:795-803. https://doi.org/10.1111/bjh.13361 PMid:25819228

- Kristinsson
SY, Björkholm M, Goldin LR, McMaster ML, Turesson I, Landgren O. Risk
of lymphoproliferative disorders among first-degree relatives of
lymphoplasmacytic lymphoma/Waldenstrom's macroglobulinemia patients: a
population-based study in Sweden. Blood. 2008;112:3052–6. https://doi.org/10.1182/blood-2008-06-162768 PMid:18703425 PMCid:PMC2569164

- Hanzis
C, Ojha RP, Hunter Z, Manning R, Lewicki M, Brodsky P, Ioakimidis L,
Tripsas C, Patterson CJ, Sheehy P, Treon SP. Associated malignancies in
patients with Waldenstro¨m's macroglobulinemia and their kin. Clin
Lymph Myel Leuk. 2011;11:88–92. https://doi.org/10.3816/CLML.2011.n.016 PMid:21454200

- Steingrímsson
v, Lund SH, Turesson I, Goldin LR, Björkholm M, Landgren O, Kristinsson
SY. Population-based study on the impact of the familial form of
Waldenstrom macroglobulinemia on overall survival. Blood.
2015;125:2174–5. https://doi.org/10.1182/blood-2015-01-622068 PMid:25814489 PMCid:PMC4375112

- Treon
SP, Hunter ZR, Aggarwal A, Ewen EP, Masota S, Lee C, Santos DD,
Hatjiharissi E, Xu L, Leleu X, Tournilhac O, Patterson CJ, Manning R,
Branagan AR, Morton CC. Characterization of familial Waldenstrom's
macroglobulinemia. Ann Oncol. 2006;17:488–94. https://doi.org/10.1093/annonc/mdj111 PMid:16357024

- Kristinsson
SY, Goldin LR, Turesson I, Bjorkholm M, Landgren O. Familial
aggregation of lymphoplasmacytic lymphoma/Waldenstrom macroglobulinemia
with solid tumors and myeloid malignancies. Acta Haematol.
2012;127:173–7. https://doi.org/10.1159/000335618 PMid:22310551 PMCid:PMC3326274

- Treon
SP, Tripsas C, Hanzis C, Ioakimidis L, Patterson CJ, Manning RJ, Sheehy
P, Turnbull B, Hunter ZR. Familial disease predisposition impacts
treatment outcome in patients with Waldenstrom macroglobulinemia. Clin
Lymph Myel Leuk. 2012;12D6:433–7.

- Leblond
V, Kastritis E, Advani R, Ansell SM, Buske C, Castillo JJ, García-Sanz
R, Gertz M, Kimby E, Kyriakou C, Merlini G, Minnema MC, Morel P, Morra
E, Rummel M, Wechalekar A, Patterson CJ, Treon SP, Dimopoulos MA.
Treatment recommendations from the Eighth International Workshop on
Waldenström's Macroglobulinemia. Blood 2016;128:1321-8. https://doi.org/10.1182/blood-2016-04-711234 PMid:27432877

- Glennie
MJ, French RR, Cragg MS, Taylor RP. Mechanisms of killing by anti-CD20
monoclonal antibodies. Mol Immunol. 2007;44:3823–37. https://doi.org/10.1016/j.molimm.2007.06.151 PMid:17768100

- Dimopoulos
MA, Zervas C, Zomas A, Kiamouris C, Viniou NA, Grigoraki V, Karkantaris
C, Mitsouli C, Gika D, Christakis J, Anagnostopoulos N. Treatment of
Waldenstrom's macroglobulinemia with rituximab. J Clin Oncol.
2002;20:2327–33. https://doi.org/10.1200/JCO.2002.09.039 PMid:11981004

- Gertz
MA, Rue M, Blood E, Kaminer LS, Vesole DH, Greipp PR. Multicenter phase
2 trial of rituximab for Waldenstrom macroglobulinemia (WM): an Eastern
Cooperative Oncology Group Study (E3A98). Leuk Lymphoma.
2004;45:2047–55. https://doi.org/10.1080/10428190410001714043 PMid:15370249

- Dimopoulos
MA, Zervas C, Zomas A, Hamilos G, Gika D, Efstathiou E, Panayiotidis P,
Vervessou E, Anagnostopoulos N, Christakis J. Extended rituximab
therapy for previously untreated patients with Waldenstrom's
macroglobulinemia. Clin Lymphoma. 2002;3:163-6. https://doi.org/10.3816/CLM.2002.n.022 PMid:12521393

- Treon
SP1, Emmanouilides C, Kimby E, Kelliher A, Preffer F, Branagan AR,
Anderson KC, Frankel SR; Waldenström's Macroglobulinemia Clinical
Trials Group. Extended rituximab therapy in Waldenstro¨m's
macroglobulinemia. Ann Oncol. 2005;16:132–8. https://doi.org/10.1093/annonc/mdi022 PMid:15598950

- Kapoor
P, Ansell SM, Fonseca R, Chanan-Khan A, Kyle RA, Kumar SK, Mikhael JR,
Witzig TE, Mauermann M, Dispenzieri A, Ailawadhi S, Stewart AK, Lacy
MQ, Thompson CA, Buadi FK, Dingli D, Morice WG, Go RS, Jevremovic D,
Sher T, King RL, Braggio E, Novak A, Roy V, Ketterling RP, Greipp PT,
Grogan M, Micallef IN, Bergsagel PL, Colgan JP, Leung N, Gonsalves WI,
Lin Y, Inwards DJ, Hayman SR, Nowakowski GS, Johnston PB, Russell SJ,
Markovic SN, Zeldenrust SR, Hwa YL, Lust JA, Porrata LF, Habermann TM,
Rajkumar SV, Gertz MA, Reeder CB. Diagnosis and Management of
Waldenström Macroglobulinemia: Mayo Stratification of Macroglobulinemia
and Risk-Adapted Therapy (mSMART) Guidelines 2016. JAMA Oncol.
2017;3:1257-65. https://doi.org/10.1001/jamaoncol.2016.5763 PMid:28056114 PMCid:PMC5556979

- Campagnolo
M, Zambello R, Nobile-Orazio E, Benedetti L, Marfia GA, Riva N,
Castellani F, Bianco M, Salvalaggio A, Garnero M, Ruiz M, Mataluni G,
Fazio R, Ermani M, Briani C. IgM MGUS and Waldenstrom-associated
anti-MAG neuropathies display similar response to rituximab therapy. J
Neurol Neurosurg Psychiatry. 2017. Epub 2017 May 13. https://doi.org/10.1136/jnnp-2017-315736

- Teeling
JL, French RR, Cragg MS, van den Brakel J, Pluyter M, Huang H, Chan C,
Parren PW, Hack CE, Dechant M, Valerius T, van de Winkel JG, Glennie
MJ. Characterization of new human CD20 monoclonal antibodies with
potent cytolytic activity against non-Hodgkin lymphomas. Blood.
2004;104:1793-800. https://doi.org/10.1182/blood-2004-01-0039 PMid:15172969

- Furman
RR, Eradat H, DiRienzo CG, Hayman SR, Hofmeister CC, Avignon NA,
Leonard JP, Coleman M, Advani R, Switzky JC, Liao Q, Shah DN, Lisby S,
Lin TS. A phase II trial of ofatumumab in subjects with Waldenstrom's
macroglobulinemia. Blood. 2011;118:abstract 3701.

- Castillo
JJ, Kanan S, Meid K, Manning R, Hunter ZR, Treon SP. Rituximab
intolerance in patients with Waldenstrom macroglobulinaemia. Br J
Haematol. 2016; 174: 645-48. https://doi.org/10.1111/bjh.13794 PMid:26523929

- Kyle
RA, Greipp PR, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Therneau TM.
Waldenström's macroglobulinaemia: a prospective study comparing daily
with intermittent oral chlorambucil. Br J Haematol. 2000;108:737-42. https://doi.org/10.1046/j.1365-2141.2000.01918.x PMid:10792277

- Leblond
V, Johnson S, Chevret S, Copplestone A, Rule S, Tournilhac O, Seymour
JF, Patmore RD, Wright D, Morel P, Dilhuydy MS, Willoughby S, Dartigeas
C, Malphettes M, Royer B, Ewings M, Pratt G, Lejeune J, Nguyen-Khac F,
Choquet S, Owen RG. Results of a randomized trial of chlorambucil
versus fludarabine for patients with untreated Waldenström
macroglobulinemia, marginal zone lymphoma, or lymphoplasmacytic
lymphoma. J Clin Oncol. 2013;31:301-7. https://doi.org/10.1200/JCO.2012.44.7920 PMid:23233721

- Dimopoulos
MA, Anagnostopoulos A, Kyrtsonis MC, Zervas K, Tsatalas C, Kokkinis G,
Repoussis P, Symeonidis A, Delimpasi S, Katodritou E, Vervessou E,
Michali E, Pouli A, Gika D, Vassou A, Terpos E, Anagnostopoulos N,
Economopoulos T, Pangalis G. Primary treatment of Waldenstrom's
macroglobulinemia with dexamethasone, rituximab and cyclophosphamide. J
Clin Oncol. 2007;25:3344–9. https://doi.org/10.1200/JCO.2007.10.9926 PMid:17577016

- Kastritis
E, Gavriatopoulou M, Kyrtsonis MC, Roussou M, Hadjiharissi E,
Symeonidis A, Repoussis P, Michalis E, Delimpasi S, Tsatalas K,
Tsirigotis P, Vassou A, Vervessou E, Katodritou E, Gika D, Terpos E,
Dimopoulos MA. Dexamethasone, rituximab, and cyclophosphamide as
primary treatment of Waldenström macroglobulinemia: final analysis of a
phase 2 study. Blood. 2015;126:1392-4. https://doi.org/10.1182/blood-2015-05-647420 PMid:26359434

- Treon
SP, Branagan AR, Ioakimidis L, Soumerai JD, Patterson CJ, Turnbull B,
Wasi P, Emmanouilides C, Frankel SR, Lister A, Morel P, Matous J,
Gregory SA, Kimby E. Long-term outcomes to fludarabine and rituximab in
Waldenström macroglobulinemia. Blood. 2009;113:3673-8. https://doi.org/10.1182/blood-2008-09-177329 PMid:19015393 PMCid:PMC2670786

- Tedeschi
A, Benevolo G, Varettoni M, Battista ML, Zinzani PL, Visco C, Meneghini
V, Pioltelli P, Sacchi S, Ricci F, Nichelatti M, Zaja F, Lazzarino M,
Vitolo U, Morra E. Fludarabine plus cyclophosphamide and rituximab in
Waldenstrom macroglobulinemia: an effective but myelosuppressive
regimen to be offered to patients with advanced disease. Cancer.
2012;118:434-43. https://doi.org/10.1002/cncr.26303 PMid:21732338

- Souchet
L, Levy V, Ouzegdouh M, Tamburini J, Delmer A, Dupuis J, Le Gouill S,
Pégourié-Bandelier B, Tournilhac O, Boubaya M, Vargaftig J, Choquet S,
Leblond V; FILO (French Innovative Leukemia Organization). Efficacy and
long-term toxicity of the rituximab-fludarabine-cyclophosphamide
combination therapy in Waldenstrom's macroglobulinemia. Am J Hematol.
2016;91:782-6. https://doi.org/10.1002/ajh.24405 PMid:27135784

- Laszlo
D, Andreola G, Rigacci L, Fabbri A, Rabascio C, Pinto A, Negri M,
Martinelli G. Rituximab and subcutaneous 2-chloro-2'-deoxyadenosine as
therapy in untreated and relapsed Waldenström's macroglobulinemia. Clin
Lymp Myel Leuk. 2011;11:130-2. https://doi.org/10.3816/CLML.2011.n.029 PMid:21454213

- Rummel
MJ1, Niederle N, Maschmeyer G, Banat GA, von Grünhagen U, Losem C,
Kofahl-Krause D, Heil G, Welslau M, Balser C, Kaiser U, Weidmann E,
Dürk H, Ballo H, Stauch M, Roller F, Barth J, Hoelzer D, Hinke A,
Brugger W; Study group indolent Lymphomas (StiL). Bendamustine plus
rituximab versus CHOP plus rituximab as first-line treatment for
patients with indolent and mantle-cell lymphomas: an open-label,
multicentre, randomised, phase 3 non-inferiority trial. Lancet.
2013;381:1203–10. https://doi.org/10.1016/S0140-6736(12)61763-2

- Castillo
JJ, Olszewski AJ, Kanan S, Meid K, Hunter ZR, Treon SP. Survival trends
in patients with Waldenstrom's macroglobulinemia: an analysis of the
Surveillance, Epidemiology, and End Results database. Blood.
2014;123:3999–4000. https://doi.org/10.1182/blood-2014-05-574871 PMid:24948623

- Treon
SP, Ioakimidis L, Soumerai JD, Patterson CJ, Sheehy P, Nelson M, Willen
M, Matous J, Mattern J 2nd, Diener JG, Keogh GP, Myers TJ, Boral A,
Birner A, Esseltine DL, Ghobrial IM. Primary therapy of Waldenstrom's
macroglobulinemia with Bortezomib, Dexamethasone and Rituximab: results
of WMCTG clinical trial 05-180. J Clin Oncol. 2009;27:3830–5. https://doi.org/10.1200/JCO.2008.20.4677 PMid:19506160 PMCid:PMC2727288

- Treon
SP, Meid K, Gustine J, Patterson CJ, Matous JV, Ghobrial IM, Castillo
JJ. Long-term outcome of a prospective study of bortezomib,
dexamethasone and rituximab (BDR) in previously untreated, symptomatic
patients with Waldenstrom's macroglobulinemia. Blood. 2015; abstract
1833. PMid:26002963

- Ghobrial
IM, Xie W, Padmanabhan S, Badros A, Rourke M, Leduc R, Chuma S, Kunsman
J, Warren D, Poon T, Harris B, Sam A, Anderson KC, Richardson PG, Treon
SP, Weller E, Matous J. Phase II trial of weekly bortezomib in
combination with rituximab in untreated patients with Waldenstrom
macroglobulinemia. Am J Hematol. 2010;85:670–4. https://doi.org/10.1002/ajh.21788 PMid:20652865

- Dimopoulos
MA, García-Sanz R, Gavriatopoulou M, Morel P, Kyrtsonis MC, Michalis E,
Kartasis Z, Leleu X, Palladini G, Tedeschi A, Gika D, Merlini G,
Kastritis E, Sonneveld P. Primary therapy of Waldenstrom
macroglobulinemia (WM) with weekly bortezomib, low-dose dexamethasone,
and rituximab (BDR): long-term results of a phase 2 study of the
European Myeloma Network (EMN). Blood. 2013;122:3276–82. https://doi.org/10.1182/blood-2013-05-503862 PMid:24004667

- Gavriatopoulou
M, García-Sanz R, Kastritis E, Morel P, Kyrtsonis MC, Michalis E,
Kartasis Z, Leleu X, Palladini G, Tedeschi A, Gika D, Merlini G,
Sonneveld P, Dimopoulos MA. BDR in newly diagnosed patients with WM:
final analysis of a phase 2 study after a minimum follow-up of 6 years.
Blood 2017;129:456-9. https://doi.org/10.1182/blood-2016-09-742411 PMid:27872060

- Treon
SP, Hanzis C, Manning RJ, Ioakimidis L, Patterson CJ, Hunter ZR, Sheehy
P, Turnbull B. Maintenance rituximab is associated with improved
clinical outcome in rituximab nai¨ve patients with Waldenstrom's
macroglobulinemia who respond to a rituximab containing regimen. Br J
Haematol. 2011;154:357–62. https://doi.org/10.1111/j.1365-2141.2011.08750.x PMid:21615385

- Treon
SP, Agus TB, Link B, Rodrigues G, Molina A, Lacy MQ, Fisher DC,
Emmanouilides C, Richards AI, Clark B, Lucas MS, Schlossman R,
Schenkein D, Lin B, Kimby E, Anderson KC, Byrd JC. CD20-Directed
antibody-mediated immunotherapy induces responses and facilitates
hematologic recovery in patients with Waldenstrom's macroglobulinemia.
J Immunother. 2001;24:272–9. https://doi.org/10.1097/00002371-200105000-00012

- Treon
SP, Hanzis C, Tripsas C, Ioakimidis L, Patterson CJ, Manning RJ, Sheehy
P. Bendamustine therapy in patients with relapsed or refractory
Waldenström's macroglobulinemia. Clin Lymph Myel Leuk. 2011;11:133-5. https://doi.org/10.3816/CLML.2011.n.030 PMid:21454214

- Tedeschi
A, Picardi P, Ferrero S, Benevolo G, Margiotta Casaluci G, Varettoni M,
Baratè C, Motta M, Gini G, Goldaniga MC, Visco C, Zaja F, Belsito
Petrizi V, Ravelli E, Gentile M, Urbano MA, Franceschetti S, Ghione P,
Orsucci L, Frustaci AM, Gaidano G, Vitolo U, Morra E. Bendamustine and
rituximab combination is safe and effective as salvage regimen in
Waldenström macroglobulinemia. Leuk Lymphoma. 2015;56:2637-42. https://doi.org/10.3109/10428194.2015.101271 PMid:25651423

- Treanda (bendamustine hydrochloride) [prescribing information]. North Wales, PA: Teva Pharmaceuticals USA, Inc. November 2015.
- Gafter-Gvili
A, Ribakovsky E, Mizrahi N, Avigdor A, Aviv A, Vidal L, Ram R, Perry C,
Avivi I, Kedmi M, Nagler A, Raanani P, Gurion R. Infections associated
with bendamustine containing regimens in hematological patients: a
retrospective multi-center study. Leuk Lymphoma. 2016;57:63-9. https://doi.org/10.3109/10428194.2015.1046862 PMid:25944378

- Marcus
RE, Davies AJ, Ando K, Klapper W, Opat S, Owen CJ, Phillips EH, Sangha
R, Schlag R, Seymour JF, Townsend W, Trnený M, Wenger MK,
Fingerle-Rowson G, Rufibach K, Moore T, Herold M, Hiddemann W.
Obinutuzumab-Based Induction and Maintenance Prolongs Progression-Free
Survival (PFS) in Patients with Previously Untreated Follicular
Lymphoma: Primary Results of the Randomized Phase 3 GALLIUM Study.
Blood ASH Annual Meet. 2016; 128, 22.

- Martin
P, Chen Z, Cheson BD, Robinson KS, Williams M, Rajguru SA, Friedberg
JW, van der Jagt RH, LaCasce AS, Joyce R, Ganjoo KN, Bartlett NL,
Lemieux B, VanderWalde A, Herst J, Szer J, Bar MH, Cabanillas F, Dodds
AJ, Montgomery PG, Pressnail B, Ellis T, Smith MR, Leonard JP.
Long-term outcomes, secondary malignancies and stem cell collection
following bendamustine in patients with previously treated non-Hodgkin
lymphoma. Br J Haematol. 2017;178:250-256. https://doi.org/10.1111/bjh.14667 PMid:28419413

- Tedeschi
A, Ricci F, Goldaniga MC, Benevolo G, Varettoni M, Motta M, Pioltelli
P, Gini G, Barate' C, Luraschi A, Vismara E, Frustaci AM, Nichelatti M,
Vitolo U, Baldini L, Morra E. Fludarabine, cyclophosphamide, and
rituximab in salvage therapy of Waldenström's macroglobulinemia. Clin
Lymph Myel Leuk. 2013;13:231-4. https://doi.org/10.1016/j.clml.2013.02.011 PMid:23490992

- Tedeschi
A, Picardi P, Goldaniga MC, Margiotta Casaluci G, Benevolo G, Ferrero
S, Varettoni M, Baratè C, Gini G, Visco C, Motta M, Belsito Petrizzi V,
Zaja F, Ravelli E, Gentile M, Frustaci AM, Orsucci L, Morra E, Gaidano
G, Cairoli R. Long Term Toxicity and Follow-up of Waldenstrom’s
Macroglobulinemia Patients after Salvage Treatment with Fludarabine
Cyclophosphamide Rituximab or Bendamustine and Rituximab. Blood ASH
Annual Meet. 2015;623:abstract 3958.

- Paludo
J, Abeykoon JP, Kumar S, Shreders A, Ailawadhi S, Gertz MA, Kourelis T,
King RL, Reeder CB, Leung N, Kyle RA, Buadi FK, Habermann TM, Dingli D,
Witzig TE, Dispenzieri A, Lacy MQ, Go RS, Lin Y, Gonsalves WI, Warsame
R, Lust JA, Rajkumar SV, Ansell SM, Kapoor P. Dexamethasone, rituximab
and cyclophosphamide for relapsed and/or refractory and treatment-naïve
patients with Waldenstrom macroglobulinemia. Br J Haematol.
2017;179:98-105. https://doi.org/10.1111/bjh.14826 PMid:28786474

- Ghobrial
IM, Hong F, Padmanabhan S, Badros A, Rourke M, Leduc R, Chuma S,
Kunsman J, Warren D, Harris B, Sam A, Anderson KC, Richardson PG, Treon
SP, Weller E, Matous J. Phase II trial of weekly bortezomib in
combination with rituximab in relapsed or relapsed and refractory
Waldenstrom macroglobulinemia. J Clin Oncol 2010;28:1422-8. https://doi.org/10.1200/JCO.2009.25.3237 PMid:20142586 PMCid:PMC2834499

- IMBRUVICA™ (ibrutinib). US Prescribing Information. Pharmacyclics, Inc; 2015 Jan
- IMBRUVICA™ (ibrutinib). European Medical Agency. EMEA/H/C/003791. Pharmacyclics, Inc; 2015 Jul
- Treon SP, Hunter ZR, Castillo JJ, Merlini G. Waldenstrom Macroglobulinemia. Hematol Oncol Clin North Am. 2014;28:945-7. https://doi.org/10.1016/j.hoc.2014.06.003 PMid:25212891

- Treon
SP, Tripsas CK, Meid K, Warren D, Varma G, Green R, Argyropoulos KV,
Yang G, Cao Y, Xu L, Patterson CJ, Rodig S, Zehnder JL, Aster JC,
Harris NL, Kanan S, Ghobrial I, Castillo JJ, Laubach JP, Hunter ZR,
Salman Z, Li J, Cheng M, Clow F, Graef T, Palomba ML, Advani RH.
Ibrutinib in previously treated Waldenström's macroglobulinemia. N Engl
J Med. 2015;372:1430–40. https://doi.org/10.1056/NEJMoa1501548 PMid:25853747

- Palomba
ML, Bantilan K, Meid K, Tripsas CK, Argyropoulos KV, Yang G, Cao Y, Xu
L, Patterson CJ, Ghobrial I, Castillo JJ, Laubach JP, Hunter ZR, Advani
RH and Treon SP. Long-term follow-up of a pivotal phase II trial of
ibrutinib for relapsed Waldenström's Macroglobulinemia. IWWM-9 Session
9, October 7, 2016.

- Dimopoulos
MA, Trotman J, Tedeschi A, Matous JV, Macdonald D, Tam C, Tournilhac O,
Ma S, Oriol A, Heffner LT, Shustik C, García-Sanz R, Cornell RF, de
Larrea CF, Castillo JJ, Granell M, Kyrtsonis MC, Leblond V, Symeonidis
A, Kastritis E, Singh P, Li J, Graef T, Bilotti E, Treon S, Buske C;
iNNOVATE Study Group and the European Consortium for Waldenström's
Macroglobulinemia. Ibrutinib for patients with rituximab-refractory
Waldenström's macroglobulinaemia (iNNOVATE): an open-label substudy of
an international, multicentre, phase 3 trial. Lancet Oncol.
2017;18:241-50. https://doi.org/10.1016/S1470-2045(16)30632-5

- Cao
Y, Hunter ZR, Liu X, Xu L, Yang G, Chen J, Patterson CJ, Tsakmaklis N,
Kanan S, Rodig S, Castillo JJ, Treon SP. The WHIM-like CXCR4(S338X)
somatic mutation activates AKT and ERK, and promotes resistance to
ibrutinib and other agents used in the treatment of Waldenstrom's
Macroglobulinemia. Leukemia. 2015;29:169-76. https://doi.org/10.1038/leu.2014.187 PMid:24912431

- Xu
L, Tsakmaklis N, Yang G, Chen JG, Liu X, Demos M, Kofides A, Patterson
CJ, Meid K, Gustine J, Dubeau T, Palomba ML, Advani R, Castillo JJ,
Furman RR, Hunter ZR, Treon SP. Acquired mutations associated with
ibrutinib resistance in Waldenström macroglobulinemia. Blood.
2017;129:2519-25. https://doi.org/10.1182/blood-2017-01-761726 PMid:28235842

- Treon
SP, Soumerai JD, Branagan AR, Hunter ZR, Patterson CJ, Ioakimidis L,
Briccetti FM, Pasmantier M, Zimbler H, Cooper RB, Moore M, Hill J 2nd,
Rauch A, Garbo L, Chu L, Chua C, Nantel SH, Lovett DR, Boedeker H,
Sonneborn H, Howard J, Musto P, Ciccarelli BT, Hatjiharissi E, Anderson
KC. Thalidomide and rituximab in Waldenstrom's macroglobulinemia.
Blood. 2008;112:4452–7. https://doi.org/10.1182/blood-2008-04-150854 PMid:18713945 PMCid:PMC2597120

- Treon
SP, Soumerai JD, Branagan AR, Hunter ZR, Patterson CJ, Ioakimidis L,
Chu L, Musto P, Baron AD, Nunnink JC, Kash JJ, Terjanian TO, Hyman PM,
Nawfel EL, Sharon DJ, Munshi NC, Anderson KC. Lenalidomide and
rituximab in Waldenstrom's macroglobulinemia. Clin Cancer Res.
2008;15:355–60. https://doi.org/10.1158/1078-0432.CCR-08-0862 PMid:19118065

- Treon
SP, Tripsas CK, Meid K, Kanan S, Sheehy P, Chuma S, Xu L, Cao Y, Yang
G, Liu X, Patterson CJ, Warren D, Hunter ZR, Turnbull B, Ghobrial IM,
Castillo JJ. Carfilzomib, rituximab and dexamethasone (CaRD) is active
and offers a neuropathy-sparing approach for proteasomeinhibitor based
therapy in Waldenstrom's macroglobulinemia. Blood. 2014;124:503–10. https://doi.org/10.1182/blood-2014-03-566273 PMid:24859363

- Leleu
X, Jia X, Runnels J, Ngo HT, Moreau AS, Farag M, Spencer JA,
Pitsillides CM, Hatjiharissi E, Roccaro A, O'Sullivan G, McMillin DW,
Moreno D, Kiziltepe T, Carrasco R, Treon SP, Hideshima T, Anderson KC,
Lin CP, Ghobrial IM. The Akt pathway regulates survival and homing in
Waldenstrom macroglobulinemia. Blood. 2007;110:4417–26. https://doi.org/10.1182/blood-2007-05-092098 PMid:17761832 PMCid:PMC2234792

- Ghobrial
IM, Gertz M, Laplant B, Camoriano J, Hayman S, Lacy M, Chuma S, Harris
B, Leduc R, Rourke M, Ansell SM, Deangelo D, Dispenzieri A, Bergsagel
L, Reeder C, Anderson KC, Richardson PG, Treon SP, Witzig TE. Phase II
Trial of the Oral Mammalian Target of Rapamycin Inhibitor Everolimus in
Relapsed or Refractory Waldenstrom Macroglobulinemia. J Clin Oncol.
2010;28:1408–14. https://doi.org/10.1200/JCO.2009.24.0994 PMid:20142598 PMCid:PMC2834498

- Ghobrial
IM, Witzig TE, Gertz M, LaPlant B, Hayman S, Camoriano J, Lacy M,
Bergsagel PL, Chuma S, DeAngelo D, Treon SP. Long-term results of the
phase II trial of the oral mTOR inhibitor everolimus (RAD001) in
relapsed or refractory Waldenstrom macroglobulinemia. Am J Hematol.
2014;89:237–42. https://doi.org/10.1002/ajh.23620 PMid:24716234

- Ghobrial
IM, Redd R, Armand P, Banwait R, Boswell E, Chuma S, Huynh D, Sacco A,
Roccaro AM, Perilla-Glen A, Noonan K, MacNabb M, Leblebjian H, Warren
D, Henrick P, Castillo JJ, Richardson PG, Matous J, Weller E, Treon SP.
Phase I/II trial of everolimus in combination with bortezomib and
rituximab (RVR) in relapsed/refractory Waldenstrom macroglobulinemia.
Leukemia. 2015;29:2338- 46. https://doi.org/10.1038/leu.2015.164 PMid:26139427

- Ghobrial
IM, Roccaro A, Hong F, Weller E, Rubin N, Leduc R, Rourke M, Chuma S,
Sacco A, Jia X, Azab F, Azab AK, Rodig S, Warren D, Harris B,
Varticovski L, Sportelli P, Leleu X, Anderson KC, Richardson PG.
Clinical and translational studies of a phase II trial of the novel
oral Akt inhibitor perifosine in relapsed or relapsed/refractory
Waldenstrom's macroglobulinemia. Clin Cancer Res. 2010;16:1033-41. https://doi.org/10.1158/1078-0432.CCR-09-1837 PMid:20103671 PMCid:PMC2885252

- Ghobrial
IM, Moreau P, Harris B, Poon T, Jourdan E, Maisonneuve H, Benhadji KA,
Hossain AM, Nguyen TS, Wooldridge JE, Leblond V. A multicenter phase II
study of single-agent enzastaurin in previously treated Waldenstrom
macroglobulinemia. Clin Cancer Res. 2012;18:5043-50. https://doi.org/10.1158/1078-0432.CCR-12-0181 PMid:22879385

- Kahl
BS, Byrd J, Flinn IW. Clinical Safety and Activity in a Phase 1 Study
of CAL-101, An Isoform-Selective Inhibitor of Phosphatidylinositol
3-Kinase P110d, In Patients with Relapsed or Refractory Non-Hodgkin
Lymphoma. Blood ASH Annual Meet. 2010;116:abstract 1777.

- Castillo
JJ, Gustine JN, Meid K, Dubeau T, Yang G, Xu L, Hunter ZR, Treon SP.
Idelalisib in Waldenstrom macroglobulinemia: high incidence of
hepatotoxicity. Leuk Lymphoma. 2017;58:1002–4. https://doi.org/10.1080/10428194.2016.1222380 PMid:27562445

- Cao
Y, Yang G, Hunter ZR, Liu X, Xu L, Chen J, Tsakmaklis N, Hatjiharissi
E, Kanan S, Davids MS, Castillo JJ, Treon SP. The BCL2 antagonist
ABT-199 triggers apoptosis, and augments ibrutinib and idelalisib
mediated cytotoxicity in CXCR4 Wild-type and CXCR4 WHIM mutated
Waldenstrom macroglobulinaemia cells. Br J Haematol 2015; 170: 134-8. https://doi.org/10.1111/bjh.13278 PMid:25582069

- Davids
MS, Roberts AW, Seymour JF, Pagel JM, Kahl BS, Wierda WG, Puvvada S,
Kipps TJ, Anderson MA, Salem AH, Dunbar M, Zhu M, Peale F, Ross JA,
Gressick L, Desai M, Kim SY, Verdugo M, Humerickhouse RA, Gordon GB,
Gerecitano JF. Phase I First-in-Human Study of Venetoclax in Patients
With Relapsed or Refractory Non-Hodgkin Lymphoma. J Clin Oncol 2017;
35: 826-833. https://doi.org/10.1200/JCO.2016.70.4320 PMid:28095146

- Konoplev
S, Medeiros LJ, Bueso-Ramos CE, Jorgensen JL, Lin P. Immunophenotypic
profile of lymphoplasmacytic lymphoma/Waldenstrom macroglobulinemia. Am
J Clin Pathol 2005;124:414-20 https://doi.org/10.1309/3G1XDX0DVHBNVKB4 PMid:16191510

- Ngo
HT, Leleu X, Lee J, Jia X, Melhem M, Runnels J, et al. SDF-1/CXCR4 and
VLA-4 interaction regulates homing in Waldenstrom macroglobulinemia.
Blood 2008;112:150-8. https://doi.org/10.1182/blood-2007-12-12939 PMid:18448868 PMCid:PMC2435685

- Peng
SB, Zhang X, Paul D, Kays LM, Ye M, Vaillancourt P, et al. Inhibition
of CXCR4 by LY2624587, a fully humanized antiCXCR4 antibody induces
apoptosis of hematologic malignancies. PLoS One 2016;11:e0150585 https://doi.org/10.1371/journal.pone.0150585 PMid:26954567 PMCid:PMC4782998

- Olszewski
AJ, Chen C, Gutman R, Treon SP, Castillo JJ. Comparative outcomes of
immunochemotherapy regimens in Waldenström macroglobulinaemia. Br J
Haematol. 2017;179:106-15. https://doi.org/10.1111/bjh.14828 PMid:28677830

[TOP]




