Manal A. Alsaif1*, Moshtag Abdulbaqi1, Khalid Al Noaim2, Mustafa Aghbari1, Muneera Alabdulqader2 and Joan L. Robinson3.
1 Department of Pediatrics, King, Abdulaziz Hospital, King Abdullah International Medical Research Center, Al-Ahsa, Saudi Arabia.
2 Department of Pediatrics, King Faisal University, College of Medicine, Al-Ahsa, Saudi Arabia.
3 Department of Pediatrics, University of Alberta, Edmonton, Canada.
Correspondence to: Manal
A. Alsaif, MD, Department of Pediatrics, King Abdulaziz Hospital, King
Abdullah, International Medical Research Center, P.O. BOX 2477, Pin
Code 31982. Tel: +966 13 533 9999 Ext 33389, Fax: +966 13 533 9999 Ext
33844. E-mail:
saifma@ngha.med.sa
Published: January 1, 2021
Received: July 22, 2020
Accepted: December 7, 2020
Mediterr J Hematol Infect Dis 2021, 13(1): e2021002 DOI
10.4084/MJHID.2021.002
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Objective:
The main aim was to report the prevalence and severity of serious
bacterial infections (SBI) in children with sickle cell disease at King
Abdulaziz Hospital (KAH), Al Ahsa, Saudi Arabia, to aid in determining
whether outpatient management of such cases is appropriate.
Methods:
We conducted a retrospective chart review of febrile children less than
14 years of age admitted with sickle cell disease 2005 through 2015.
Results:
During 320 admissions, 25 children had SBIs (8%) including pneumonia
(n=11), osteomyelitis (n=8), bacteremia (n=3, all with Salmonella
species) and UTI (n=3). All recovered uneventfully.
Conclusion:
It appears that in the current era, less than 10% of febrile children
with sickle cell disease in our center are diagnosed with an SBI. Over
11 years, there were no sequelae or deaths from SBI. Given these
excellent outcomes, outpatient ceftriaxone should be considered for
febrile well-appearing children with sickle cell disease if they have
no apparent source and parents are judged to be reliable.
|
Introduction
Sickle-cell
disease (SCD) is one of the most common monogenic disorders worldwide,
characterized by wide variation in the associated disease's clinical
manifestations and severity. SCD is most prevalent in Africa, the
Middle East, the Indian subcontinent, and some Mediterranean
countries.[1]
In Saudi Arabia, SCD was first reported in Eastern
Province in the early 1960s. The prevalence varies significantly in
different parts of the country but is highest in Eastern, followed by
the southern provinces.[2]
Patients with SCD have an increased risk of invasive bacterial infections, particularly with encapsulated organisms including Streptococcus pneumoniae, Haemophilus influenzae, Neisseria meningitidis, Salmonella spp. and Escherichia coli.
The increased susceptibility to infections is related to many factors,
primarily functional hyposplenism, and impaired opsonization. Other
factors include genetic predisposition, mechanical risk factors, and
abnormalities in the defense mechanisms, including an abnormality in
the alternative pathway of complement activities, and defective
neutrophil function.[3,4]
An increased incidence of bacteremia in
children with SCD has been well documented in the literature.[5,6]
Fortunately, the incidence appears to have decreased following the
introduction of routine childhood H. influenzae
type B (Hib) and pneumococcal conjugate vaccines along with widespread
use of prophylactic oral penicillin for young children with SCD[7-11]
since it was proven to be effective in the late 1980s.[12] In one
study, the introduction of pneumococcal conjugate vaccines resulted in
an impressive reduction in invasive pneumococcal disease incidence by
90.8% in infants and 93.4% in children less than five years of age
living with SCD.[13-16] Another study reported that the infection rate
declined from 1.7 infections per 100 persons/year in 1995 to 2000 to
just 0.5 infections per 100 persons/ year in the following two years in
children ≤ 10 years of age.[17] However, other serious bacterial
infections (SBIs), including pneumonia and acute osteomyelitis,
continued to threaten SCD patients.
There are few studies of SBI
among febrile children with SCD. A study from the United States
reported that most had pneumonia.[18] The only previous study from
Saudi Arabia was done before introducing HIB and conjugated
pneumococcal vaccines.[19]
In terms of vaccines for encapsulated
organisms in Saudi Arabia, the HIB vaccine was introduced nationally in
2002. Seven valent pneumococcal conjugate vaccines (PCV7) was
introduced first in the Ministry of National Guard community only for
high-risk children (aged <2 years) in 2006 and then for all children
in that community in their first year of life starting January 2008.
The program was expanded to include all Saudi children in January 2009.
Thirteen valent pneumococcal conjugate vaccine (PCV13) was introduced
in the national immunization schedule in January 2011. The current
schedule includes four doses of HIB given at age 2,4,6 and 18 months,
four doses of PCV13 given at the age of 2,4,6 and 12 months, and three
doses of quadrivalent meningococcal vaccine (MCV4) given at nine and 12
months and 18 years of age. https://www.moh.gov.sa/en/HealthAwareness/EducationalContent/HealthTips/Documents/Immunization-Schedule.pdf
The
healthcare system is free of charge for all Saudis. There are two
hematological centers under the Ministry of Health's charge located in
two cities in Eastern Province (Al-Ahsa and Qatif) where sickle cell
disease and thalassemia are prevalent.[20] However, patients can access
any emergency room in any hospital in the province.
The KAH is the
second-largest tertiary hospital in the Al-Ahsa area and was
commissioned in late 2002 to provide healthcare for Saudi National
Guard employees and their dependents. It is accredited by the Joint
Commission of International Accreditation for Hospitals and has a
35-bed pediatric ward. Al-Ahsa is an oil and gas producing area located
approximately 60 km inland from Arabian Gulf. It has 543,000 km2 with a population of more than 1,220,655 (the year 2020).
Children
with sickle cell disease are cared for in KAH by a hematologist in an
outpatient clinic, and they have access to the emergency room for acute
management. Our current guideline states that all children with sickle
cell disease presenting with fever should be admitted to the hospital
and follow a standard protocol for management. Parents are educated to
pay meticulous attention to hygiene measures to reduce Salmonella
infection risk for preventive measures. Parents are encouraged to
monitor their children closely at home and seek advice if they have a
fever or respiratory symptoms. The importance of compliance with
vaccination and penicillin prophylaxis is reinforced during outpatient
visits and inpatient admission. All children with sickle cell disease
receive penicillin prophylaxis from diagnosis until the age of 5 years.
This study's main objective was to determine the current
incidence rate and outcome of SBI in febrile Saudi children with SCD.
If the incidence rate is relatively low and sequelae are rare, it may
be safe to manage well-appearing febrile children with SCD as
outpatients.
Methods
This
study was based on a retrospective chart review of all patients younger
than 14 years with SCD admitted to KAH 2005 through 2015 with a history
of fever at home or a documented fever in the E.D.
Exclusion
criteria were a) fever was not documented with a thermometer either at
home or in hospital b) the patient had incomplete medical records. If a
patient was discharged and then readmitted, this was recorded as
multiple admissions.
A Febrile illness was defined as
temperature ≥38°C measured by any method at any body site. Serious
bacterial infections (SBI) were defined as bacteremia, meningitis,
urinary tract infection, osteomyelitis, pneumonia, or bacteria's
isolation from a normally sterile site. Urinary tract infections (UTI)
has diagnosed if i) urine cultures grew more than 50 000 colony-forming
units per milliliter of a single organism from a catheterized urine
specimen or midstream urine and ii) pyuria was present (>5 WBC/HPF).
Bacteremia was diagnosed if a common pathogen was recovered from one or
more blood cultures or if an organism that is typical skin flora was
recovered in two or more blood cultures. Meningitis was diagnosed if i)
a true pathogen was recovered from the spinal fluid or ii) clinical
examination in conjunction with CSF indices was suggestive of bacterial
meningitis, but CSF was sterile as it was obtained after antibiotics
had been administered. For children with suspected pneumonia, chest
radiographs were interpreted by a radiologist blinded to the suspected
diagnosis. The diagnosis of pneumonia was then made by determining
which of the following 3 categories best described the case: 1) Viral
pneumonia: a) nontoxic child; b) proceeding upper airway symptoms (e.g.
rhinorrhea, congestion; c) diffuse and bilateral auscultatory findings;
d) bilateral diffuse interstitial infiltrate e) detection of a virus
from the respiratory tract, 2) Bacterial pneumonia: a) ill or toxic
appearing child; b) moderate or severe respiratory distress; d) focal
or few auscultatory findings; d) imaging study showed any of the
followings: lobar; segmental; or rounded consolidation; pneumatocele,
cavitation, large pleural effusion, or necrotizing process; e)
detection of bacteria that typically cause pneumonia from blood or
another sterile site or 3) Atypical pneumonia: a) presence of
constitutional findings including malaise, myalgia, headache,
photophobia or sore throat; b) gradual and worsening nonproductive
cough; d) diffuse crackles or wheezing on lung auscultation; d)
presence of dermatological or extrapulmonary findings; e) diffuse or
bronchopulmonary infiltrates. Acute chest syndrome (ACS) was defined
as a new pulmonary infiltrate on chest radiograph, hypoxia (low blood
oxygen concentration) accompanied by one or more of the following
symptoms: fever, cough, dyspnea, or tachypnea. However, as there is an
overlap with bacterial pneumonia, any patient who met both bacterial
pneumonia and the ACS definition was considered to have either
bacterial pneumonia or ACS. Osteomyelitis was diagnosed from reports of
imaging studies in correlation with clinical findings. The diagnosis
was considered to be confirmed if there was histopathologic evidence of
inflammation in a surgical specimen of bone or identification of a
pathogen by culture or gram stain in an aspirate of bone. The diagnosis
was considered to be probable in a child with compatible clinical,
laboratory, and/or radiologic findings in whom a pathogen was isolated
from blood, periosteal collection, or joint fluid. The diagnosis was
considered possible in a child with compatible clinical, laboratory,
and radiologic findings with negative cultures (or not cultures
obtained) and a response to empiric antimicrobial therapy.
The
following data were collected from patient charts for each admission:
age in months, gender, immunization status, presence of splenectomy and
central venous line, previous hemoglobin electrophoresis results, use
of hydroxyurea and penicillin prophylaxis before admission, compliance
with penicillin prophylaxis (if applicable), reported temperature at
home, E.D. triage vital signs, results of relevant cultures and
radiographs and patient outcome. The data was recorded and coded in
statistical software, SPSS 21 version.
Results
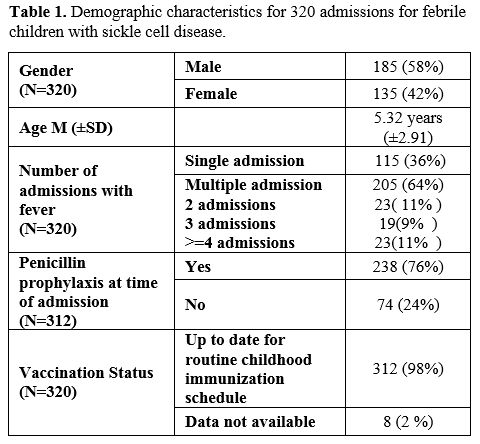
Three hundred twenty admissions met the eligibility criteria. Of them, 185 (58%) were male children (Table 1).
The mean age at admission was 5±3 years. Fever was documented in the
hospital for 106 admissions (33%) and only at home for 214 admissions
(67%).
 |
Table
1. Demographic characteristics for 320 admissions for febrile children with sickle cell disease.
|
Of the 320 admissions, 115 children (36%) had a single admission for fever, while the others had multiple admissions (Table 1).
All patients had homozygous sickle cell anemia except for 14 (4%) with
sickle cell beta-thalassemia (SCD-Thalassemia); all were SB0 type.
Completed immunizations for age were documented for 312 admissions
(98%) and were not documented for the remaining eight patients.
SBI was documented in 25 of the 320 admissions (8%; 95% CI 5.2-11.2%) (Table 2).
No child was admitted more than once with an SBI. None of the patients
with SBI had a CVL, one had a previous splenectomy, and 17 (68%) were
male (Table 3). All patients
with SBI had homozygous SCD and were fully immunized except for one
child with SCD-Thalassemia and no immunization record available. The
most common SBI was pneumonia (N=11; 4% of all admissions with 95%
confidence interval ((CI) 2.0-6.2%) of which 4 cases were presumed to
be viral, and seven were bacterial versus ACS. Eight children (3% of
all admissions; 95% CI 1.3-5.0%) had osteomyelitis (one confirmed, and
seven possible cases). Blood cultures were obtained for 283 of the 320
patients during their admission (89%), of which 8 had positive
cultures, but only 3 (1% of all admissions; 95% CI 0.3-2.8%) were
thought to be true pathogens (all Salmonella species). None of the 37
patients without a blood culture obtained were diagnosed with an SBI.
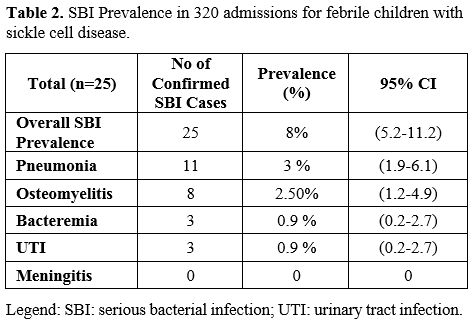 |
Table 2.SBI Prevalence in 320 admissions for febrile children with sickle cell disease. |
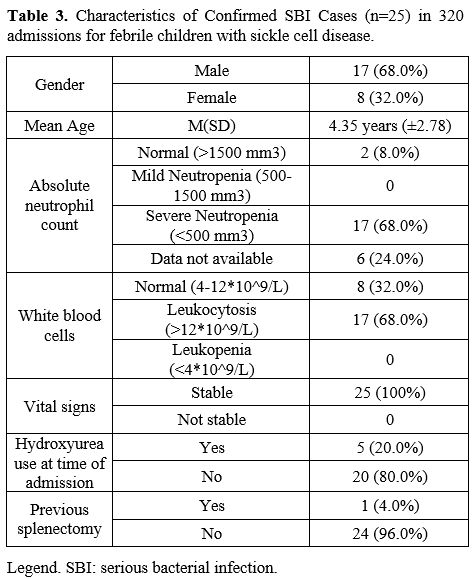 |
Table 3. Characteristics of Confirmed SBI Cases (n=25) in 320 admissions for febrile children with sickle cell disease. |
All
were well appearing except for the seven patients with bacterial
pneumonia versus ACS; all were ill-looking and required admission to
the intensive care unit; of them, one required mechanical ventilation.
Seventeen children (66%) presented with severe neutropenia and
leukocytosis (Table 3). All children survived to discharge.
For
the 320 admissions, the child was on penicillin prophylaxis with
suspected good compliance for 238 (74%), was not on penicillin, or
compliance was thought to be low for 74 (23%), while data were not
recorded for 8 (3%). SBI was diagnosed in 19 children on penicillin
prophylaxis (8%) versus ten, not on penicillin (14%; p=0.15) (Table 4).
Of the 19 children with SBI despite penicillin prophylaxis, three were
vaccinated with 7 valent pneumococcal vaccine, and the remaining 16
were vaccinated with PCV13. Seven children out of 10 who were not on
penicillin prophylaxis were vaccinated with PCV13, and the remaining
three children had incomplete records.
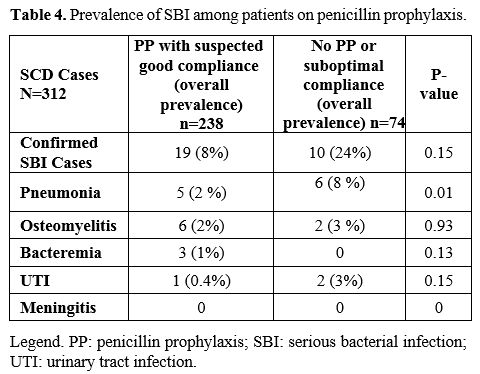 |
Table 4. Prevalence of SBI among patients on penicillin prophylaxis.
|
Discussion
The
overall prevalence of SBI in SCD patients admitted with fever was 8%,
with 68% of SBI cases occurring in males. The most common manifestation
was pneumonia, accounting for 3% of admissions.
A study from
Qatif central hospital in the Eastern region of Saudi Arabia was
conducted prior to introducing HIB and conjugated pneumococcal
vaccines.[19] Of 450 admitted febrile and afebrile children, 39 (8.6%)
had bacterial infections; Salmonella species predominated, and three
children died (fatality rate 7.6%)[19] (Versus none in the current
study). A recent study conducted in the Makkah region of Saudi Arabia
reported that infection (but not necessarily SBI) was the second most
common complication leading to admission in children with SCD,
accounting for 9% of admissions but was dwarfed by the veno-occlusive
disease, which accounted for 56% of admissions.[21]
Comparing to
studies conducted in other countries, a 2013 study from the United
States showed that the incidence of SBI in febrile children with SCD
presenting to an E.D. was 16% (30 of 188) with 26 having pneumonia.[18]
In a study conducted in Cameron of children with SCD hospitalized with
suspicion of bacterial infection, the rate of SBI was 9.7%; as in our
study, males predominated, accounting for 60% of cases.[22]
Pneumonia
appeared to be the most common SBI in the current study, but the low
incidence is presumably due to HIB and pneumococcal
immunizations.[9,14,23] In children with SCD, the diagnosis of acute
chest syndrome (ACS) is difficult to distinguish from pneumonia as both
present with fever, cough, and pulmonary infiltrates on CXR, and it
remains possible that some children diagnosed with pneumonia in the
current study had ACS. Unless the blood cultures are positive,
assigning an etiology to pediatric pneumonia is fraught with error, so
etiologies were not analyzed in the current study. Chlamydia pneumoniae
and Mycoplasma pneumoniae were the most common causes of pneumonia in
the Multicenter National Acute Chest Syndrome Study (NACSS), followed
by the respiratory syncytial virus (RSV), Staphylococcus aureus, and Streptococcus pneumoniae.[24]
As in previous reports, osteomyelitis was uncommon and typically accounted for less than 5% of SBI with SCD.[22,25,26]
Bacteremia
was rare in the current study, accounting for only 3 SBIs in 320
admissions for fever; none of the patients with pneumonia or
osteomyelitis were bacteremic. This datum differs markedly from studies
in Africa, where 14%[5] and 28%[27] of febrile children with SCD were
bacteremic. Although immunizations and penicillin prophylaxis may
account for some improvement in the current study, it is noteworthy
that many children in the African studies had pathogens such as Klebsiella pneumoniae[5] and S. aureus[27] that would not be impacted by these strategies.
The
consensus is that children with SCD should receive penicillin
prophylaxis until at least five years of age and potentially throughout
childhood to prevent pneumococcal sepsis.[28] Not unexpectedly, many
children in our study had SBIs despite penicillin prophylaxis, but it
is striking that not a single child had pneumococcal sepsis (although
some of the 11 pneumonia cases could have been pneumococcal). As the
number of pneumococcal serotypes in conjugated vaccines increases,
penicillin prophylaxis will become less useful, but it seems logical to
continue it for now.
Although bacteremia was rare, all cases were due to Salmonella
spp. A study from Cameroon also reported Salmonella spp to be the most
common pathogens in bacteremic children with SCD.[22] A study in the
Saudi population also revealed that Salmonella species were the leading
cause of SBIs in SCD patients.[19] Unfortunately, available Salmonella vaccines are designed to cover only S. typhi, which likely accounts for a minority of Salmonella bacteremia cases in SCD.
Children
with SCD are more inclined to develop UTIs than those without SCD. This
tendency could be caused by altered blood flow in the renal
vasculature, which causes papillary necrosis and loss of urinary
concentration and acidification of the nephrons, resulting in dilute
and alkaline urine favoring bacterial infection.[25] The children may
develop compromised renal function due to recurrent UTI and repeated
vaso-occlusive episodes;[23,29] Only three were admitted with UTI in
the current study, but this may be because most children with UTIs are
treated as outpatients even if febrile.
There were no cases of
bacterial meningitis in the present study. In previously published
studies from Saudi Arabia in the early 1990's the prevalence was 0.8%
and 5.5%,[19,30] respectively. Organisms implicated were S .pneumoniae, H. influenzae, N. meningitides, and Salmonella spp.[19,30] A low prevalence of meningitis has also been reported in recent studies from Cameroon and Brazil.[22,31]
It
is striking that there were only 25 cases of SBI diagnosed in the 11
years of this study, resulting in no deaths or apparent sequelae.
Therefore, it would seem reasonable that well appearing febrile
children in Saudi Arabia with reliable parents could be cultured, given
antibiotics promptly, observed for at least a few hours in the
emergency department, and discharged home with follow-up at 24 hours.
Discharge following one dose of ceftriaxone has been studied in other
countries. In the 1990s, this strategy was successful in 86[32] and
107[33] febrile episodes in children over six months of age in the U.S.
The same regimen was also successful in 60 children in West Africa who
had been febrile for < 36 hours.[34] In a more recent study from the
U.S., about half of 390 cases were successfully managed as
outpatients.[35] Although three patients managed as outpatients proved
to be bacteremic in another U.S. study, all did well.[36] Given the low
incidence of SBI in recent studies, perhaps antibiotics are not
indicated in all children with fever and SCD. However, there is a need
for further study of risk factors for and predictors of SBI before one
could recommend withholding antibiotics.
This study's main
limitation is that children could have had unrecognized SBIs that
improved with empiric antibiotics. Our methodology would not have
captured children who died of SBI before hospital admission. The
retrospective analysis of data barred us from obtaining all the
information necessary and having a control group to investigate factors
driving the occurrence of SBIs. Data were collected from only one
hospital.
Conclusion
The
current prevalence of SBI in children with SCD appears to be much lower
than previously reported, presumably due to penicillin prophylaxis and
immunizations. It appears safe to consider empiric outpatient
ceftriaxone therapy for well febrile children with SCD if they have a
UTI or no apparent source and a reliable family.
Ethical consideration
This
study was initiated after taking the ethical approval from the IRB of
King Abdullah International Medical Research Center, Saudi Arabia. The
identification of patients was kept anonymous, and data confidentiality
was also ensured.
Acknowledgment
I
wish to thank my colleagues from the imaging departments, Dr. Ahmed
Eid, head of the radiology department, and Dr. Ammar Ashraf, for their
contribution to CXR interpretation. Special thanks also to Dr. Mohammed
Aldarwish (hematologist) who works at Qatif central hospital, for his
contribution to H.B. electrophoresis interpretation.
References
- Piel FB, Patil AP, Howes RE, Nyangiri OA, Gething
PW, Williams TN, et al. Global distribution of the sickle cell gene and
geographical confirmation of the malaria hypothesis. Nature
communications. 2010;1(1):1-7. https://doi.org/10.1038/ncomms1104
PMid:21045822 PMCid:PMC3060623
- Al-Qurashi MM, El-Mouzan MI,
Al-Herbish AS, Al-Salloum AA, Al-Omar AA. The prevalence of sickle cell
disease in Saudi children and adolescents. Saudi Med J.
2008;29(10):1480-3. https://doi.org/10.4103/0256-4947.55163
PMid:19700891 PMCid:PMC2860399
- Di Nuzzo D, Fonseca SF. Sickle cell disease and infection. J Pediatr (Rio J). 2004;80(5):347-54. https://doi.org/10.2223/1218
- Elbashier
AM, Al-Salem AH, Aliama A. Salmonella as a causative organism of
various infections in patients with sickle cell disease. Annals of
Saudi Medicine. 2003. https://doi.org/10.5144/0256-4947.2003.358
PMid:16868368
- Brown B, Dada-Adegbola H, Trippe C, Olopade O.
Prevalence and etiology of bacteremia in febrile children with sickle
cell disease at a Nigeria tertiary hospital. Mediterranean journal of
hematology and infectious diseases. 2017;9(1).
https://doi.org/10.4084/mjhid.2017.039 PMid:28698782 PMCid:PMC5499496
- Williams
TN, Uyoga S, Macharia A, Ndila C, McAuley CF etal. Bacteraemia in
Kenyan children with sickle-cell anaemia: a retrospective cohort and
case-control study. Lancet. 2009 Oct 17;374(9698):1364-70
https://doi.org/10.1016/S0140-6736(09)61374-X
- Hernigou P, Daltro
G, Flouzat-Lachaniette C-H, Roussignol X, Poignard A. Septic arthritis
in adults with sickle cell disease often is associated with
osteomyelitis or osteonecrosis. Clinical Orthopaedics and Related
Research®. 2010;468(6):1676-81.
https://doi.org/10.1007/s11999-009-1149-3 PMid:19885711 PMCid:PMC2865595
- Chambers
JB, Forsythe DA, Bertrand SL, Iwinski HJ, Steflik DE. Retrospective
review of osteoarticular infections in a pediatric sickle cell age
group. Journal of pediatric orthopaedics. 2000;20(5):682-5.
https://doi.org/10.1097/01241398-200009000-00025 PMid:11008753
- Chang
TP, Kriengsoontorkij W, Chan LS, Wang VJ. Clinical factors and
incidence of acute chest syndrome or pneumonia among children with
sickle cell disease presenting with a fever: a 17-year review.
Pediatric emergency care. 2013;29(7):781-6.
https://doi.org/10.1097/PEC.0b013e31829829f7 PMid:23823253
- Gorham
M, Smith C, Smith S, Wong L, Kreze O. Vaccinations in sickle cell
disease: An audit of vaccination uptake in sickle cell patients
attending Newham University Hospital. Vaccine. 2015;33(38):5005-11.
https://doi.org/10.1016/j.vaccine.2015.06.028 PMid:26151544
- Piel
FB, Steinberg MH, Rees DC. Sickle cell disease. New England Journal of
Medicine. 2017;376(16):1561-73. https://doi.org/10.1056/NEJMra1510865
PMid:28423290
- Gaston MH, Verter JI, Woods G, Pegelow C, Kelleher
J, Presbury G, et al. Prophylaxis with oral penicillin in children with
sickle cell anemia. New England Journal of Medicine.
1986;314(25):1593-9. https://doi.org/10.1056/NEJM198606193142501
PMid:3086721
- Memish ZA, El-Saed A, Al-Otaibi B, Al Shaalan M, Al
Alola S, Thaqafi AO. Epidemiology of invasive pneumococcal infection in
children aged five years and under in Saudi Arabia: a five-year
retrospective surveillance study. International Journal of Infectious
Diseases. 2010;14(8):e708-e12.
https://doi.org/10.1016/j.ijid.2010.02.2242 PMid:20627645
- Halasa
NB, Shankar SM, Talbot TR, Arbogast PG, Mitchel EF, Wang WC, et al.
incidence of invasive pneumococcal disease among individuals with
sickle cell disease before and after the introduction of the
pneumococcal conjugate vaccine. Clinical Infectious Diseases.
2007;44(11):1428-33. https://doi.org/10.1086/516781 PMid:17479937
- Adamkiewicz
TV, Sarnaik S, Buchanan GR, Iyer RV, Miller ST, Pegelow CH, et al.
Invasive pneumococcal infections in children with sickle cell disease
in the era of penicillin prophylaxis, antibiotic resistance, and
23-valent pneumococcal polysaccharide vaccination. The Journal of
pediatrics. 2003;143(4):438-44.
https://doi.org/10.1067/S0022-3476(03)00331-7
- Knight-Madden J,
Serjeant GR. Invasive pneumococcal disease in homozygous sickle cell
disease: Jamaican experience 1973-1997. The Journal of pediatrics.
2001;138(1):65-70. https://doi.org/10.1067/mpd.2001.109709 PMid:11148514
- Adamkiewicz
TV, Silk BJ, Howgate J, Baughman W, Strayhorn G, Sullivan K, et al.
Effectiveness of the 7-valent pneumococcal conjugate vaccine in
children with sickle cell disease in the first decade of life.
Pediatrics. 2008;121(3):562-9. https://doi.org/10.1542/peds.2007-0018
PMid:18310206
- Bansil NH. Incidence of Serious Bacterial Infections
in Febrile Children With Sickle Cell Disease. clinical pediatrics. May
2013. https://doi.org/10.1177/0009922813488645 PMid:23661790
- Abu-srair.
incidence of major infection in sickle cell pediatric patients at qatif
Central Hospita. Annals of Saudi Medicine. 1991:267-70.
https://doi.org/10.5144/0256-4947.1991.267 PMid:17588101
- El Mouzan
MI, Al Awamy BH, Al Torki MT. Clinical features of sickle cell disease
in eastern Saudi Arab children. Journal of Pediatric
Hematology/Oncology. 1990;12(1):51-5.
https://doi.org/10.1097/00043426-199021000-00009 PMid:1689968
- Alkot
M, Almaghrabi W, Al-Najdi N, Al-Otaibi M, Shatla M. Prevalence of
complications of sickle cell disease at Makkah Al-Mukaramah, Saudi
Arabia, 2017. Ann Clin Lab Res. 2018;6(1):226.
https://doi.org/10.21767/2386-5180.1000226
- Yanda ANA, Nansseu
JRN, Awa HDM, Tatah SA, Seungue J, Eposse C, et al. Burden and spectrum
of bacterial infections among sickle cell disease children living in
Cameroon. BMC infectious diseases. 2017;17(1):211.
https://doi.org/10.1186/s12879-017-2317-9 PMid:28298206 PMCid:PMC5353947
- Saganuwan
SA. The Pattern of Sickle Cell Disease in Sickle Cell Patients from
Northwestern Nigeria. Clinical Medicine Insights: Therapeutics.
2016;8:CMT. S38164. https://doi.org/10.4137/CMT.S38164
- Vichinsky
EP, et al. Causes and outcomes of the acute chest syndrome in sickle
cell disease. National Acute Chest Syndrome Study Group. N Engl J Med
2000; 342:1855-1865. https://doi.org/10.1056/NEJM200006223422502
PMid:10861320
- Shinde S, Bakshi AP, Shrikhande A. Infections in sickle cell disease. IAIM Int ArchIntegr Med IAIM. 2015;2:34-26.
- Fontalis
A, Hughes K, Nguyen M, Williamson M, Yeo A, Lui D, et al. The challenge
of differentiating vaso-occlusive crises from osteomyelitis in children
with sickle cell disease and bone pain: A 15-year retrospective review.
Journal of children's orthopaedics. 2019;13(1):33-9.
https://doi.org/10.1302/1863-2548.12.180094 PMid:30838073
PMCid:PMC6376437
- Kizito M, Mworozi E, Ndugwa C, Serjeant GR.
Bacteraemia in homozygous sickle cell disease in Africa: is
pneumococcal prophylaxis justified? Archives of disease in childhood.
2007;92(1):21-3. https://doi.org/10.1136/adc.2005.088807 PMid:16531454
PMCid:PMC2083172
- AAP. Health Supervision for Children with Sickle
Cell Disease. Pediatrics 2002;109;526
https://doi.org/10.1542/peds.109.3.526 PMid:11875155
- Saborio P, Scheinman JI. Sickle cell nephropathy. Journal of the American Society of Nephrology. 1999;10(1):187-92.
- Hawasawi
ZM, Nabi G, Al Magamci M, Awad KS. Sickle cell disease in childhood in
Madina. Annals of Saudi medicine. 1998;18(4):293-5.
https://doi.org/10.5144/0256-4947.1998.293 PMid:17344675
- Chenou F,
Azevedo J, Leal HF, de Souza Gonçalves M, Reis JN. Bacterial meningitis
in patients with sickle cell anemia in Salvador, Bahia, Brazil: a
report on ten cases. Hematology, Transfusion and Cell Therapy. 2019.
https://doi.org/10.1016/j.htct.2019.06.006 PMid:31806417
PMCid:PMC7248505
- Wilimas JA, Flynn PM, Harris S, Day SW, Smith R,
Chesney PJ, Rodman JH, Eguiguren JM, Fairclough DL, Wang WC. A
randomized study of outpatient treatment with ceftriaxone for selected
febrile children with sickle cell disease. N Engl J Med. 1993 Aug
12;329(7):472-6 https://doi.org/10.1056/NEJM199308123290705 PMid:8332152
- LL
Williams , J A Wilimas, S C Harris, S W Day, R M Dancy, W C Wang.
Outpatient Therapy With Ceftriaxone and Oral Cefixime for Selected
Febrile Children With Sickle Cell Disease. J Pediatr Hematol Oncol .
1996 Aug;18(3):257-61 https://doi.org/10.1097/00043426-199608000-00004
PMid:8689337
- M C Rahimy 1, A Gangbo, G Ahouignan, S Anagonou, V
Boco, E Alihonou. Outpatient Management of Fever in Children With
Sickle Cell Disease (SCD) in an African Setting. Am J Hematol 1999
Sep;62(1):1-6
https://doi.org/10.1002/(SICI)1096-8652(199909)62:1<1::AID-AJH1>3.0.CO;2-C
- Sokol E, Obringer E, Palama B, Hageman J, Peddinti R. Outpatient
Management of Febrile Children With Sickle Cell Disease. Clin Pediatr
(Phila). 2016 Mar;55(3):268-71 https://doi.org/10.1177/0009922815594345
PMid:26149843
- Baskin MN, Goh XL, Heeney MM, Harper MB. Bacteremia
risk and outpatient management of febrile patients with sickle cell
disease. Pediatrics. 2013 Jun;131(6):1035-41
https://doi.org/10.1542/peds.2012-2139 PMid:2366952
[TOP]



