Nicola Sgherza1, Lidia Dalfino2, Antonio Palma1, Angelantonio Vitucci1, Daniela Campanale1, Salvatore Grasso3 and Pellegrino Musto4.
1 Unit of Hematology and Stem Cell Transplantation, Azienda Ospedaliero-Universitaria Consorziale Policlinico, Bari, Italy.
2
Anaesthesia and Intensive Care Unit II, General surgery, Gynecology and
Anaesthesia Department, Azienda Ospedaliero-Universitaria Consorziale
Policlinico, Bari, Italy.
3 University of Bari “Aldo Moro”, Emergency and Organ Transplantation department, Bari, Italy.
4
Chair of Hematology and Unit of Hematology and Stem Cell
Transplantation, "Aldo Moro" University School of Medicine, Azienda
Ospedaliero-Universitaria Consorziale Policlinico, Bari, Italy.
Corresponding
author: Nicola Sgherza, MD, PhD. Unit of Hematology and Stem Cell
Transplantation, Azienda Ospedaliero-Universitaria Consorziale
Policlinico, Piazza G. Cesare 11, 70124 Bari, Italy. Tel:
+390805594001; fax: +390805428978. E-mail:
nicolasgherza@libero.it
Published: November 1, 2020
Received: August 18, 2020
Accepted: October 9, 2020
Mediterr J Hematol Infect Dis 2020, 12(1): e2020076 DOI
10.4084/MJHID.2020.076
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
To the editor,
In
March 2020, "Coronavirus Disease 2019" (COVID-19) infection outbreak
has been declared a pandemic by the World Health Organization, and
until now, there are no proven drugs for the treatment of "Severe Acute
Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)" infection; therefore
affected symptomatic patients are treated with drugs already used for
other infections[1] such as hydroxychloroquine (HCQ)
or chloroquine, administered alone or in combination with other
medications. Hence the growing attention about G6PD
(Glucose-6-Phosphate Dehydrogenase)-deficiency since that chloroquine
or HCQ could trigger severe hemolysis in subjects with this inherited
abnormality.[2] From a literature review, four case
reports have been recently published about the use of HCQ in patients
with COVID-19 infection and G6PD-deficiency, developing acute hemolytic
anemia (AHA);[3-6] we are reporting the case of a
patient with SARS-CoV-2 infection and G6PD-deficiency treated with HCQ
without laboratory evidence of hemolysis. The patient, a 61-year-old
Caucasian man, was admitted to the Intensive Care Unit of "A.O.U.
Consorziale, Policlinico di Bari-Italy" for severe acute respiratory
failure, requiring invasive mechanical ventilation, and fever. Chest
X-ray showed multiple bilateral lung opacities. The suspected COVID-19
infection was confirmed by a real-time-PCR assay on a nasopharyngeal
swab. According to the institutional protocol, treatment with HCQ (200
mg, thrice a day), darunavir (800 mg, once a day), and azithromycin
(500 mg, once a day) was carried on for seven days. The patient's
medical history included hypertension controlled with nebivolol and
aspirin, along with some relatives having a suspected condition of
"favism," but there was no personal history of drug-induced hemolysis.
Since recent indications[7] for G6PD testing include a
family history of G6PD-deficiency, enzymatic activity was analyzed; a
diagnosis of G6PD-deficiency was made with a dosage of 5.6 UI/g Hb
(normal range: 6.9-9.0; reticulocytes: 1.63%; residual enzyme activity:
79%) and in consideration of ongoing treatment, close monitoring of
complete blood count and hemolysis parameters was carried on, without
stopping treatment. A decreased value of Hgb was reported, without
laboratory evidence of hemolysis during and after treatment (Figure 1);
no transfusion support with red blood cells was needed. Molecular
analysis was not performed, but considering that the incidence of class
III (WHO classification) G6PD deficiency in our region is high,
according to our previous paper,[8] the patient may be
a carrier of the Seattle variant, usually associated with a low risk of
AHA. G6PD deficiency is a global health problem representing a
paradigmatic example of a highly specific interaction between an
inherited abnormality and exogenous agents that trigger hemolysis;[7] it frequently occurs in Africa, Asia, and the Mediterranean region[9]
and remains the most common human enzymatic disorder of red blood cells
worldwide. G6PD, whose gene maps to the long arm of the X chromosome
(band Xq28), is expressed in all tissue cells where it catalyzes the
first step in the pentose phosphate pathway. In the red blood cell,
this is the unique pathway for NADPH production, which is required to
maintain glutathione in the reduced state. Failure of this process
impairs the red cell's ability to deal with oxidative stress, which may
lead to hemolytic episodes and anemia that can be severe and, in some
cases, fatal. Often G6PD deficiency is an asymptomatic condition that
remains undetected until subjects are exposed to an exogenous hemolytic
trigger such as fava beans ingestion, taking drugs with intracellular
oxidizing action, exposure to substances with intracellular oxidizing
action, bacterial and viral infections. We identify some interesting
issues concerning the management of the case report described. First,
"G6PD deficiency" does not necessarily mean "hemolysis" depending on
the severity of the deficiency and residual enzymatic activity.
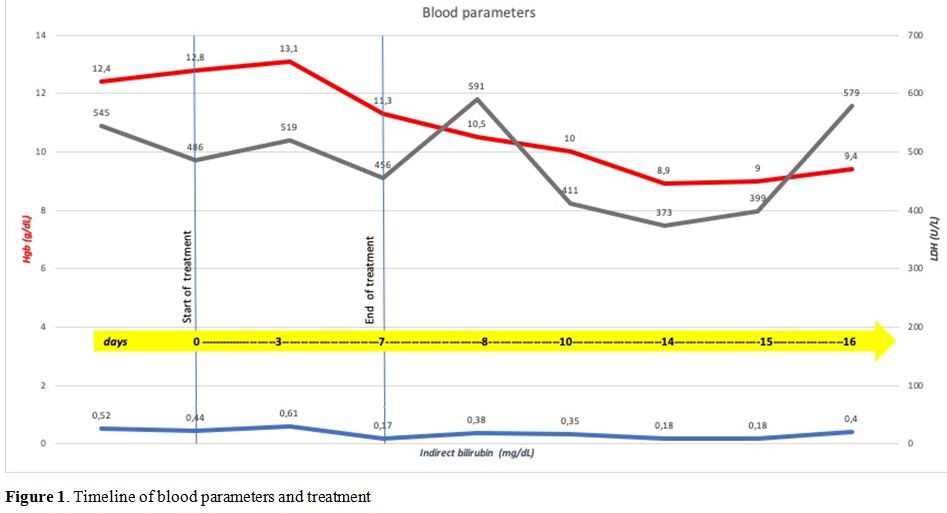 |
Figure 1. Timeline of blood parameters and treatment. |
Second,
the enzymatic activity deficiency is not a predictive parameter of the
severity of the clinical condition, as we reported in 2001.[8]
Although it is not yet clearly understood whether genetic or
extragenetic, other mechanisms must exist that offer protection from
the oxidative stresses that play a role in the clinical expression of
G6PD deficiency. Third, since G6PD-deficiency could be a susceptibility
factor to SARS-CoV2 infection[10] and that this
inherited abnormality is not so rare, it should be sought in case of
unexplained hemolysis or before administering drugs, a potential
trigger of hemolysis. According to literature data, we recommend
caution with using HCQ in all subjects with G6PD-deficiency, including
those with COVID-19 infection; at the same time, we believe that the
use of this drug in patients with G6PD deficiency should be
investigated with further studies, elucidating the role of residual
enzyme activity and genotype, factors that may influence AHA risk.
Moreover, although G6PD deficiency remains a contraindication to HCQ
use, our report suggests that several variables may influence AHA risk,
and these aspects should be investigated in further studies to
discriminate patients who may have some benefits from this specific
drug.
Acknowledgments
The authors thank Mark Mirizio for the language revision of the manuscript.
References
- Costanzo M, De Giglio MAR, Roviello GN. SARS-CoV-2:
Recent Reports on Antiviral Therapies Based on Lopinavir/Ritonavir,
Darunavir/Umifenovir, Hydroxychloroquine, Remdesivir, Favipiravir and
other Drugs for the Treatment of the New Coronavirus. Curr Med Chem.
2020;27(27):4536-4541. https://doi.org/10.2174/0929867327666200416131117 PMid:32297571
- ISS
COVID-19 Rare Diseases Working Group. Interim guidance for the
appropriate support of people with enzymopenia G6PD (favism) in the
current SARS-CoV-2 emergency scenario. Version April 14, 2020. Roma:
Istituto Superiore di Sanità; 2020. (Rapporto ISS COVID-19, n. 14/2020
- English version).
- Beauverd Y, Adam Y,
Assouline B, Samii K. COVID-19 infection and treatment with
hydroxychloroquine cause severe haemolysis crisis in a patient with
glucose-6-phosphate dehydrogenase deficiency [published online ahead of
print, 2020 Apr 23]. Eur J Haematol. 2020;10.1111/ejh.13432. https://doi.org/10.1111/ejh.13432 PMid:32324284 PMCid:PMC7264743
- Maillart
E, Leemans S, Van Noten H, et al. A case report of serious haemolysis
in a glucose-6-phosphate dehydrogenase-deficient COVID-19 patient
receiving hydroxychloroquine. Infect Dis (Lond). 2020;52(9):659-661. https://doi.org/10.1080/23744235.2020.1774644 PMid:32496938 PMCid:PMC7284136
- Sasi
S, Yassin MA, Nair AP, Al Maslamani MS. A Case of COVID-19 in a Patient
with Asymptomatic Hemoglobin D Thalassemia and Glucose-6-Phosphate
Dehydrogenase Deficiency. Am J Case Rep. 2020;21:e925788. Published
2020 Jul 22. https://doi.org/10.12659/AJCR.925788 PMid:32697769 PMCid:PMC7394553
- De
Franceschi L, Costa E, Dima F, Morandi M, Olivieri O. Acute hemolysis
by hydroxycloroquine was observed in G6PD-deficient patient with severe
COVD-19 related lung injury. Eur J Intern Med. 2020;77:136-137. https://doi.org/10.1016/j.ejim.2020.04.020 PMid:32381323 PMCid:PMC7167571
- Roper
D, Layton M, Rees D, et al. Laboratory diagnosis of G6PD deficiency. A
British Society for Haematology Guideline. Br J Haematol.
2020;189(1):24-38. https://doi.org/10.1111/bjh.16366 PMid:31991476
- Pietrapertosa
A, Palma A, Campanale D, Delios G, Vitucci A, Tannoia N. Genotype and
phenotype correlation in glucose-6-phosphate dehydrogenase deficiency.
Haematologica. 2001;86(1):30-35.
- Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008;371(9606):64-74. https://doi.org/10.1016/S0140-6736(08)60073-2
- Kassi
EN, Papavassiliou KA, Papavassiliou AG. G6PD and chloroquine: Selecting
the treatment against SARS-CoV-2?. J Cell Mol Med.
2020;24(9):4913-4914. https://doi.org/10.1111/jcmm.15312 PMid:32281268 PMCid:PMC7205832
[TOP]
