Margherita Migone De Amicis1, Alessandro Rimondi2,3, Luca Elli2,3 and Irene Motta1,4
1 General Medicine Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy.
2 Gastroenterology and Endoscopy Unit, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan, Italy.
3 Department of Pathophysiology and Transplantation, University of Milan, Milan, Italy.
4 Department of Clinical Sciences and Community Health, University of Milan, Milan, Italy.
Correspondence to:
Irene Motta, MD. Department of Clinical Sciences and Community
Health, Università degli Studi di Milano,General Medicine Unit, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico. Tel: +390255033493. E-mail:
irene.motta@unimi.it
Published: May 1, 2021
Received: October 22, 2020
Accepted: April 6, 2021
Mediterr J Hematol Infect Dis 2021, 13(1): e2021028 DOI
10.4084/MJHID.2021.028
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Anemia
is a global health problem affecting one-third of the world population,
and half of the cases are due to iron deficiency (ID). Iron deficiency
anemia (IDA) is the leading cause of disability in several countries.
Although multiple mechanisms may coexist, ID and IDA causes can be
classified as i) insufficient iron intake for the body requirement, ii)
reduced absorption, and iii) blood losses. Oral iron represents the
mainstay of IDA treatment. IDA is defined as "refractory" when the
hematologic response after 4 to 6 weeks of treatment with oral iron (an
increase of >=1 g/dL of Hb) is absent. The cause of iron-refractory
anemia is usually acquired and frequently related to gastrointestinal
pathologies, although a rare genetic form called iron-refractory iron
deficiency anemia (IRIDA) exists. In some pathological circumstances,
either genetic or acquired, hepcidin increases, limiting the absorption
in the gut, remobilization, and recycling of iron, thereby reducing
iron plasma levels. Indeed, conditions with high hepcidin levels are
often under-recognized as iron refractory, leading to inappropriate and
unsuccessful treatments. This review provides an overview of the iron
refractory anemia underlying conditions, from gastrointestinal
pathologies to hepcidin dysregulation and iatrogenic or provoked
conditions, and the specific diagnostic and treatment approach.
|
Introduction
Anemia
is a global health problem affecting one-third of the world population,
with half of the cases due to iron deficiency (ID).[1]
The prevalence of iron deficiency without anemia remains elusive,
although it has been estimated that it is at least double that of iron
deficiency anemia (IDA). IDA itself is the leading cause of disability
in several countries, with children and females of childbearing age
being the most affected.[2] Moreover, ID is the cause of anemia in a significant proportion of elderly patients admitted to medical units.[3]
IDA
is usually microcytic, defined as a decrease in mean red cell volume
(MCV) due to reduced hemoglobin (Hb) production. However, in some
circumstances, IDA can be normocytic if folate or vitamin B12
deficiencies coexist.
ID and IDA are the presenting signs and, in
some cases, the sole of different medical conditions. ID and IDA causes
can be classified as: i) insufficient iron intake for the body
requirement, ii) reduced absorption, and iii) blood losses, although
multiple mechanisms may coexist. Age, sex, clinical history, and
symptoms are essential in guiding the diagnostic work-up for the
identification of the underlying cause.[4] Oral iron
represents the mainstay of IDA treatment. IDA is defined as
"refractory" when the hematologic response after 4 to 6 weeks of
treatment with oral iron (an increase of >= 1 g/dL of Hb)[5]
is absent. The underlying cause of iron-refractory anemia is usually
acquired and frequently related to gastrointestinal (GI) pathologies,
although a rare genetic form called iron-refractory iron deficiency
anemia (IRIDA) exists (See definitions reported in table 1).
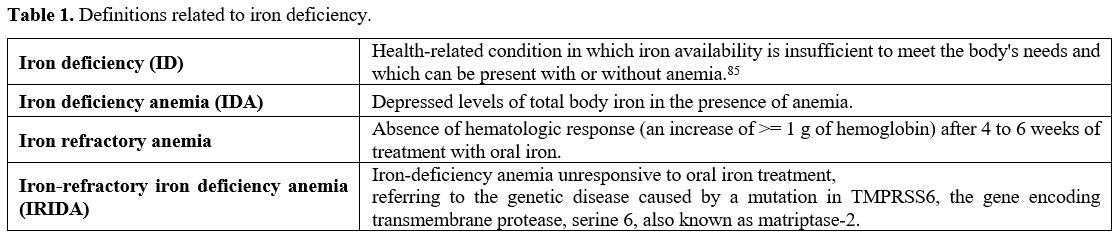 |
Table 1. Definitions related to iron deficiency.
|
Iron
absorption is limited to 1 to 2 mg daily, while most of the iron needed
is provided through recycling by macrophages that phagocytize senescent
erythrocytes. The two latter mechanisms are controlled by the hormone
hepcidin, the master regulator of iron homeostasis.[6] Hepcidin is a peptide primarily produced by the liver,[7] which regulates the systemic flux of iron by modulating ferroportin levels through its degradation.[8]
Ferroportin is highly expressed in duodenal enterocytes to transport
the iron absorbed with the diet, in hepatocytes to transport stored
iron, and macrophages to transport recycled iron. Hepcidin expression
is regulated by several mechanisms, including iron status,
erythropoietic stimuli, and inflammation. In ID, hepcidin synthesis is
suppressed to facilitate the absorption of iron.
However, in some
pathological circumstances, either genetic or acquired, hepcidin
increases, reducing surface ferroportin, limiting the absorption,
remobilization, and recycling of iron, thereby reducing iron plasma
levels.[8] Indeed, conditions with high hepcidin
levels are often under-recognized as iron refractory, leading to
inappropriate and unsuccessful treatments. This review provides an
overview of the iron-refractory anemia underlying conditions (Figure 1), from GI pathologies to hepcidin dysregulation and iatrogenic or provoked conditions.
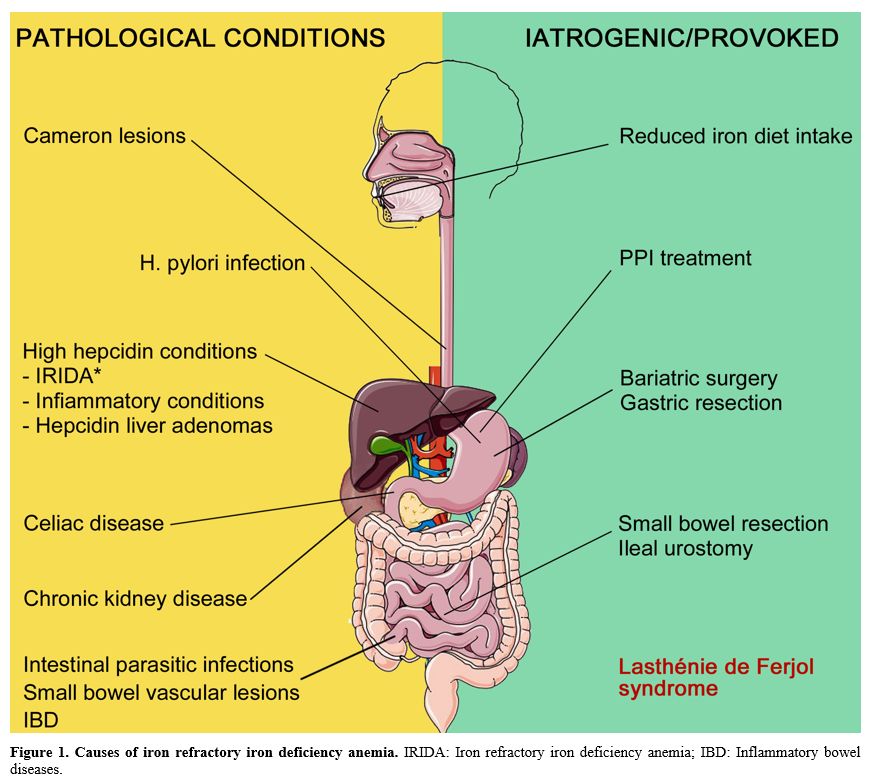 |
Figure 1. Causes of iron
refractory iron deficiency anemia. IRIDA: Iron refractory iron
deficiency anemia; IBD: Inflammatory bowel diseases.
|
Autoimmune Atrophic Gastritis
Chronic
atrophic gastritis (CAG) is defined as a loss of gastric glands
replaced by pseudo-pyloric or intestinal metaplasia or fibrosis,
causing impaired gastric acid secretion (hypochlorhydria/achlorhydria)
and intrinsic factor deficiency. Historically, two main types of CAG
have been documented: type A and type B chronic atrophic gastritis.
Type A is associated with autoimmune etiology, anti-parietal cell
antibodies (APCA), and corpus involvement with the antrum sparing. On
the other hand, type B is usually associated with environmental factors
such as Helicobacter Pylori (HP) infection and generally affects antral
mucosa.[9]However,
more recent consensus and guidelines have revised the terminology,
regrouping CAG by considering morphology, topography, distribution of
gastritis, and the possible coexistence of multiple etiologies. Asides
from classical dichotomous classification, it is now assumed that
histological sampling can hint at unclassified damage to the gastric
mucosa, and multiple patterns of gastritis can be found in the same
patient. Moreover, an overlap between different types of gastritis
(e.g., H. Pylori corpus predominant pattern) has been described in some
patients.[10,11] Chronic atrophic gastritis
prevalence changes extensively among epidemiological studies as
different diagnosis and definition criteria are adopted. However, a
systematic review esteems a prevalence of CAG as high as 23.9% in the
general population and up to 27% in selected groups when serological
criteria, such as pepsinogen I or pepsinogen I/pepsinogen II ratio, are
adopted.[12]Patients
with CAG are usually affected by a certain degree of anemia. Vice
versa, some studies show that up to 20-27% with refractory IDA are
diagnosed with autoimmune gastritis.[13,14] Nonetheless, the burden and clinical effects of CAG are relatively underestimated by GI specialists.[15] As
HP infection is examined separately in this review, henceforth, we will
discuss autoimmune chronic atrophic gastritis and its relationship with
IDA. According to the literature, roughly 2% of the general population
is affected by autoimmune atrophic gastritis (AAG). This illness is
usually found in females over 60 years old with related autoimmune
diseases.[16] Once established, AAG can be
responsible for many pathologic alterations involving multiple
apparatuses (e.g., gastrointestinal, hematological, neurological). As
AAG affects oxyntic gland production of hydrochloric acid and intrinsic
factor, both B12 and iron deficiency anemia are reported in these
patients. An acidic environment is essential for the reduction,
solubilization, and absorption of iron and intrinsic factor, a
well-known element for the assimilation of cobalamin. In this subset of
patients, current evidence shows that IDA usually occurs before B12
deficiency. It has also been observed that IDA can be the only sign of
AAG at clinical presentation in up to 53% of cases. However, subtle
alterations, such as anisocytosis, could hint at an early stage of the
disease and should always be considered when evaluating a patient. Patients
with AAG-associated IDA are usually younger (up to 20 years) and
female. This occurs probably because the reserves of Vitamin B12 can
last for a fair amount of time before macrocytic anemia shows up.
Overlapping menstrual losses that worsen iron deficiency could explain
why premenopausal women account for a more significant percentage of
AAG-associated IDA. On the contrary, older male patients with AAG are
often diagnosed with B12 deficiency anemia, probably due to a
long-lasting unidentified disease. Some studies report dimorphic anemia
as a consequence of both iron and B12 deficiency affecting erythrocyte
development.Several
studies showed that AAG could be the only cause for iron-refractory
anemia. Thus, when examining a patient with IDA who does not respond
well to oral iron supplementation, a thorough interrogation on upper
gastrointestinal disturbances is mandatory. These patients are often
affected by several unspecific symptoms such as abdominal bloating,
pain, and nausea. Sometimes symptoms such as early satiety,
postprandial fullness, and epigastric pain are also reported, giving
rise to differential diagnosis between other common gastrointestinal
diseases (e.g., functional disorders, celiac disease). Nonetheless, patients without enteric symptoms should also be suspected of gastrointestinal involvement. Besides,
attention should be given to those patients with IDA affected by other
autoimmune diseases classically related to AAG (e.g., diabetes mellitus
type 1, autoimmune thyroiditis).[13,14,16,17]To
our current knowledge, the mainstays for AAG diagnosis are endoscopic
gastric evaluation and mucosal biopsies taken following the Sydney
Houston protocol.This
protocol allows a correct gastric evaluation and detects 90% of
intestinal metaplasia when attained. APCA and anti-intrinsic factor
antibodies (AIFA) were once considered a mainstay of the diagnosis.
However, current guidelines report a relatively low sensitivity for
AIFA, low specificity for APCA, and an overall low diagnostic accuracy
when serological markers are considered alone. The best practice
imposes accurate histological examination, whereas the serological test
may help in corroborating the diagnosis.[13,17,18]
Recent laboratory-based scores taking into account gastrin, hemoglobin,
and mean cell volume levels have been proposed for identifying subjects
more likely to be affected by AAG. Notably, when proton-pump inhibitor
(PPI) use and Zollinger-Ellison syndrome have been excluded, gastrin
alone stands out as a good indicator of AAG in the atrophic stage, and
markedly elevated levels are associated with type 1 neuroendocrine
tumor.[19,20]
Helicobacter Pylori
The
relationship between Helicobacter pylori and IDA has always been
debated. Chronic gastritis resulting in hypochlorhydria overlapping
AAG, occult blood loss due to chronic erosive gastritis and peptic
ulcers, reduced ascorbic acid, subsequent intestinal iron absorption,
and iron sequestration and utilization by the pathogen itself are the
supposed mechanisms for IDA in HP infection.[21] The
2017 Maastricht V/Florence Consensus Report declares that HP
association with IDA has been conclusively proven in the adult and
pediatric population, thus recommending HP eradication in patients with
moderate to severe anemia with negative bidirectional endoscopy.[22]
However, the authors stated that the overall level of evidence is very
low, and the recommendation grade is weak. Notably, this statement is
mainly based on relatively old meta-analyses and multicenter studies.
Similarly, the American College of Gastroenterology 2017 Guidelines
suggest that patients with unexplained IDA despite an appropriate
evaluation should be tested for HP infection. Treatment should be given
to those who tested positive. However, there are multiple concerns over
HP testing (low positive predicting value in low prevalence scenarios)
and extensive treatment.[22,23] Furthermore, thanks
to the broader availability of capsule endoscopy, it is no longer
generally recommended to stop endoscopic examinations after a negative
bidirectional endoscopy and encourage to look for other bleeding
sources throughout the small GI.[24]Moreover,
a recent retrospective single cohort study based on many consecutive
patients undergoing esophagogastroduodenoscopy and standard testing for
HP infection excluded an association with IDA in univariate and
multiple logistic regression analyses.[25] When
approaching a patient with IDA at the current state of the art, HP
should be regarded as a possible cause, but it should not prevent
further examinations. We recommend ruling out more frequent causes of
IDA while planning patient follow-up and dismissal.
Celiac Disease
Celiac
disease (CeD) is an autoimmune enteropathy triggered by gluten in
genetically predisposed subjects carrying HLA DQ2 or DQ8 genes. The
duodenum and the proximal small bowel (jejunum) are the
gastrointestinal tracts mostly affected by mucosal inflammation.[26]
Eventually, mucosal inflammation ends in mucosal atrophy and villa
blunting when left untreated, causing nutrients malabsorption. Anemia
and CeD are strictly related since the absorption of these nutrients is
necessary for red cell production. Elemental iron and heme compounds
are absorbed exactly in the gastrointestinal mucosal area involved in
CeD.[17,27] Several
studies and a meta-analysis showed that anemia affects up to 69% of
patients diagnosed with CeD, especially adult females. Moreover, up to
4% of patients affected by anemia are diagnosed with CeD if an active
serological screening strategy is pursued.[28,29]Notably,
since iron is absorbed in the duodenum, IDA is the most common
extra-intestinal sign of CeD. Also, IDA could be the main and only
manifestation in relatively asymptomatic celiac patients.[30]Recent
studies showed that there is indeed a relationship between the degree
of villous atrophy and iron serological markers. Consequently, severe
anemia cases are associated with severe histological patterns of CeD
(Marsh-Oberhuber 3b and 3c). Besides, it has been demonstrated that
iron regulatory proteins expressed by the enterocyte (e.g., divalent
metal transporter 1 DMT1, hephaestin, ferroportin 1 FP1, Transferrin
receptor 1 TfR1, and Duodenal cytochrome B Dcytb) are regularly present
in CeD, thus denying a possible impaired iron mechanism disorder in
this subset of patients.[31,32] Currently,
malabsorption is believed to be the primary pathogenic mechanism for
IDA in CeD; other genetic factors (e.g., polymorphisms or mutations in
TMPRSS6) could worsen the clinical scenario.[33] For
the reasons mentioned above, CeD should always be considered when
evaluating a patient with IDA, especially if the patient is young and
complains of gastrointestinal symptoms (dyspepsia, bloating, diarrhea,
constipation). Current guidelines suggest this active case-finding policy in patients affected with unexplained IDA or iron refractory IDA.[34-36]Life-long
Gluten-Free Diet (GFD) is the mandatory and most effective treatment
for this specific type of IDA. However, in the persistence of IDA or
slow repletion of iron storage during GFD, iron supplementation could
be needed. This clinical scenario is typically related to higher iron
demands in premenopausal women or slow normalization of villous
atrophy. In adult patients, this last process could last longer than
three years.[37]
Surgical Procedures
Several
surgical procedures may lead to ID due to malabsorption. ID is well
documented in bariatric surgery, but other surgical procedures can be
involved. With the growing prevalence of obesity worldwide, bariatric
surgery will be more frequent, given its more significant and sustained
improvement in weight loss than non-surgical treatments. Good results
on weight also have their side effects, including the risk of
nutritional deficiency, with ID as one of the most relevant. According
to recent data,[38] postoperatively, iron deficiency
prevalence varies between 18 and 53 % after Roux-en-Y gastric bypass
and between 1 and 54% after sleeve gastrectomy. The prevalence of ID is
also proportional to the follow-up length, and it is higher in
premenopausal women.[39,40] Different
factors contribute to iron deficiency development after surgery: the
pre-existing predisposition related to obesity itself, since
obesity-induced systemic inflammation may increase hepcidin levels and
contribute to ID; the iron intake becomes inadequate to major
requirement due to a higher blood volume.[41,42]
Several
studies proved a reduced iron intake after bariatric surgery, primarily
due to increased satiety and reduced appetite with a reduction of
micronutrient intake,[43] together with a lower tolerance for red meat38 and poor adherence to dietary indications and recommended supplementations.[44]
The digestion and absorption of iron are also affected by the induced
alteration in gastrointestinal anatomy and physiology, leading to the
reduction of the area involved in iron absorption and reduced
hydrochloric acid secretion (essential for the reduction of the ferric
ion into its ferrous absorbable form).Other
types of surgery, with various indications, may lead to IDA. These
include gastric resection, procedures involving the first tract of the
small intestine, and proctocolectomy. A frequent complication in
patients undergoing surgery for ulcerative colitis is pouchitis, which
is associated with IDA (6-21% of patients with functional pouches) due
to mucosal bleeding and impaired iron absorption.[45]Considering
urological surgery, procedures that lead to excluding a bowel segment
from the GI may cause metabolic and nutritional disturbances. Folic
acid and vitamin B12 deficiency have been described, while the
importance of ID is debated: Bambach and Hill described a "frequently
found ID after major resection of the small intestine (100±330 cm)",[46]
while Salomon et al. did not found any evidence of metabolic disorders,
including iron deficiency, after an extended follow-up after Camey type
I ileal enterocystoplasty, considering this result possibly related to
the short ileal length (35 cm) used in the Camey type I operation.[47]
A more recent study, in a population of children and adolescents that
underwent ileal urostomy, revealed a reduced level of serum iron and
increased total iron-binding capacity (TIBC) in the long term follow up
in a small proportion of patients, suggesting as "prudent" to
investigate iron parameters in these patients.[48]
Occult Blood Losses
Diaphragmatic hernia and esophagitis.
Gastric bleeding from Cameron lesions (linear gastric ulcers or
erosions on the mucosal folds at the diaphragmatic impression) is a
documented cause of IDA in large diaphragmatic or hiatus hernia;[49,50] however, also axial and para-esophageal hernia, in the absence of Cameron lesions, have been related to IDA.[51] IDA reported incidence for all types of hernia varies from 8% to 42% in different cohorts, with an average of 20%.[52] The presence of hiatal hernia itself, independently of esophagitis, has been shown to increase IDA risk.[53]
The hypothesis for these forms of IDA has been related to mechanical
trauma plus esophagitis, and erosions, or gastroesophageal acid reflux.
Of note, the absence of endoscopically detectable erosions does not
exclude their causal role in most patients with hernia-related IDA.
Considering the treatment of hernia and esophagitis, surgery, in
combination with PPI, did not show better results compared to PPI
therapy alone in treating and preventing the recurrence of IDA, even in
the case of larger diaphragmatic hernia.[51,52]Small bowel vascular lesions. Small bowel bleedings account for approximately 5% of all cases of GI bleeding[54]
and consist of the majority of obscure gastrointestinal bleeding
(OGIB), defined as GI bleeding from an unidentified origin that
persists despite a comprehensive upper and lower GI evaluation.[97]
Age of onset and detailed medical history, including information on
comorbidities, is essential for determining small bowel bleeding
origin. The
American College of Gastroenterology guidelines in 2015 included
inflammatory bowel disease, Dieulafoy's lesion, neoplasia, Meckel's
diverticulum, and polyposis syndromes among the most common causes in
subjects under the age of 40, while angioectasia, Dieulafoy's lesion,
neoplasia, and non-steroidal anti-inflammatory drugs ulcers in those
over the age of 40.[55] Also, rare conditions can cause small bowel bleedings.Capsule
endoscopy (CE) is the first-line diagnostic tool for patients with IDA
and suspected obscure midgut bleeding, while device-assisted
enteroscopy should be performed as the second-line intervention and in
case of operative enteroscopy or bioptic sampling.[30]Intestinal parasitic infections. Several studies have shown parasitic infections, especially T. trichiura
and hookworm infections, to be strongly associated with IDA. Hookworm
infections are associated with mucosal damage and endogenous loss of
iron, while T. trichiura and E. histolytica
cause bleeding and dysentery by invading the mucosa of the large
intestine. Given these mechanisms, parasitic infections need to be
considered in the presence of IDA, especially if iron -refractory.[56-58]
Drug-Related Iron Refractory Anemia
Hypo-
or achlorhydria induced by proton pump inhibitors increase the risk of
ID and IDA. A recent population-based case-control study in the United
Kingdom showed that continuous use of PPI for one year or longer
increased the risk of ID compared to the non-exposed subjects or
patients treated for less than one year.[59] Suboptimal response to oral iron treatment in patients with ID receiving a PPI has been documented.[60]
Several studies demonstrate that PPIs are overprescribed; thus, more
attention is required to evaluate benefit and risk balance.
Self-Induced Bleeding
Lasthénie
de Ferjol syndrome (LF) is a rare psychiatric disease, affecting women
mainly. It is characterized by severe recurrent IDA caused by repeated
episodes of self-induced blood-letting. The microcytic anemia observed
in a patient with LF syndrome is non-specific, reflecting only chronic
blood loss. The work-up is negative for gastrointestinal or
gynecological bleeding and nutritional deficiencies.Anemia
improves with therapy, but patients almost inevitably interrupt the
treatment with the reappraisal of blood-letting sessions. Despite
severe debilitation, most LF syndrome patients continue to fulfill
their professional and social duties; they appear highly cooperative
and willingly submit to even the most invasive diagnostic procedures to
discover the actual cause of their symptoms. The diagnosis is based on
the findings of anemia, together with a unique psychological history.
Early diagnosis is usually difficult, but it may prevent repeated
hospitalizations and the risks associated with invasive diagnostic
procedures; management is usually challenging but should be based on
long-term psychotherapy.[61,62]
Iron Refractory Anemia and Hepcidin Dysregulation
High
hepcidin levels induce ferroportin degradation, limiting the absorption
of iron in the gut and the recycling from the liver and macrophages in
the spleen.Iron-refractory iron deficiency anemia (IRIDA).
Iron refractory iron deficiency anemia (IRIDA) is a rare hereditary
recessive form of microcytic hypochromic anemia. It is caused by
mutations in the transmembrane protease serine 6 (TMPRSS6)
gene, encoding for matriptase-2, which lead to inappropriately high
hepcidin expression for the low iron status and restricted intestinal
iron absorption.[63] Patients present with low
transferrin saturation and variable values of serum ferritin. Diagnosis
is made through molecular analysis, usually during childhood. The
disease is refractory to oral iron treatment but shows a slow response
to intravenous iron injections with partial correction of the anemia.[64]Anemia of chronic disease.
Elevated hepcidin levels are among the hallmarks of anemia of
inflammation (AI), also known as anemia of chronic disease (ACD). It is
estimated that up to 40% of all the anemia cases can be considered AI
or with significant AI contribution, accounting for 1 billion affected
individuals.[2] Under conditions of chronic
inflammation, increased expression of interleukin-6 (IL 6) results in
the activation of transcription 3 (STAT3) mediated hepcidin expression
and, consequently, limited dietary iron absorption and availability for
erythropoiesis.[65,66] AI is usually mild to moderate
normochromic and normocytic. Characteristically, AI has reduced
circulating iron concentrations and transferrin saturation (TSAT) and
reduced reticulocyte count. Serum ferritin can be normal or increased,
depending on the underlying condition.[67]Inflammatory bowel diseases.
Anemia is the most common extra-intestinal complication in inflammatory
bowel diseases (IBD), with a reported prevalence higher than 70%.[68] The etiology of anemia in IBD is multifactorial, mostly involving ID and chronic inflammation.[68]
IDA, the main cause of anemia in these patients, is due to chronic
blood loss in the gastrointestinal tract, reduced iron intake, and
malabsorption. AI is mediated by inflammatory cytokines, including
tumor necrosis factor-α, interleukin-1, IL-6, interleukin-10, and
interferon-γ.[69,70] Indeed, hepcidin has been demonstrated to be high in IBD patients.[71-73]
Hepcidin levels may vary according to the disease activity and the
opposite contribution of inflammatory activity versus iron deficiency.Chronic kidney disease.
Hepcidin is cleared by the kidney, where it is mainly reabsorbed and
degraded by the proximal tubular mechanism that metabolizes other
peptides. In chronic kidney disease (CKD), iron metabolism is disrupted
because of high hepcidin concentrations.[74] Several
mechanisms contribute to high hepcidin levels and include the
inflammatory pathogenesis of kidney disorder, decreased clearance, and
hemodialysis or peritoneal dialysis-related complications.[75,76]
In these patients, ID is a consequence of decreased iron absorption and
increased iron losses, mostly from gastrointestinal bleeding[77]
but also from iatrogenic effects. Indeed, hemodialysis causes blood
loss due to residual blood in the dialysis equipment, anticoagulation,
and frequent laboratory examinations.[74]Hepcidin adenomas.
A form of microcytic hypochromic iron deficiency anemia, refractory to
oral treatment, was described in a cohort of patients affected by type
1a glycogen storage disease (GSD1a), a rare recessive disorder due to
deficiency of glucose-6 phosphatase, which catalyzes a reaction
involved in both glycogenolysis and gluconeogenesis. This condition is
characterized by hypoglycemia, and current treatment leads to prolonged
survival of affected subjects up to adulthood, with the development of
several complications, including liver adenomas. The observation of IDA
correction after adenomas resection led to further studies that showed
inappropriately high expression of hepcidin mRNA in adenoma tissue
(while hepcidin suppression was documented in the normal liver tissue).
The aberrant high-level hepcidin mRNA expression is supposed to play a
direct role in anemia pathogenesis, which presents the same
characteristics of IRIDA.[78,79]
Diagnostic Approach
Considering
the refractory IDA definition, we will focus on specific requirements
after the IDA first-line approach has been performed (Figure 2).
It can be complicated and even experienced, and trained physicians may
be misled by low compliance and factitious anemia. C-reactive protein
and other acute-phase markers need to be assessed, together with kidney
function; coexisting cobalamin or other vitamin deficiency can help
address the diagnosis.
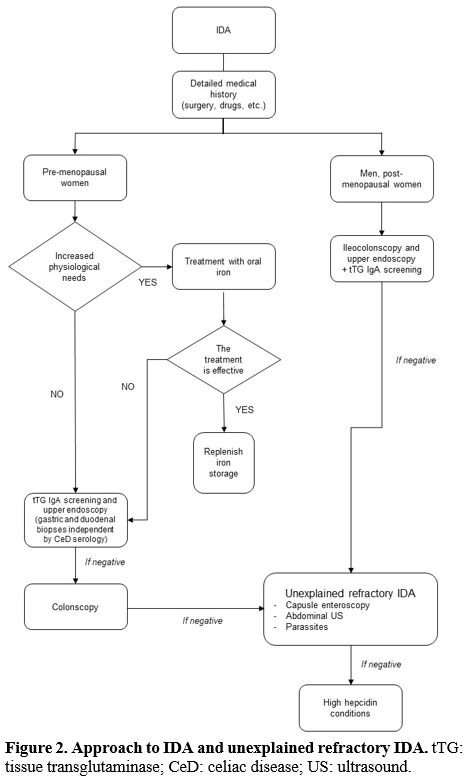 |
Figure 2. Characteristics, indications, and side effects of oral and IV iron formulation.
|
Medical
and pharmacological history needs to be re-evaluated, focusing on GI
symptoms and the use of PPI or other drugs that can impair gastric
acidity; occult blood losses can be induced by drugs or by hereditary
bleeding disorders. History of travel or other risk factors for
parasitic infection has to be considered. The
probability of HP infection is higher in men and postmenopausal women,
while refractory IDA stating during childhood can be found in IRIDA
patients. A specific approach for each condition is reported in Table 3.
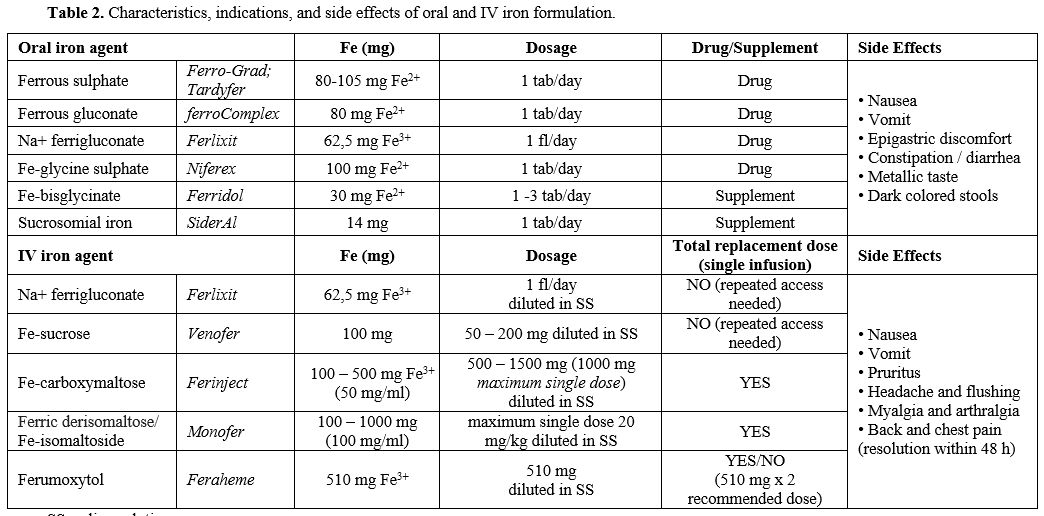 |
Table 2. Characteristics, indications, and side effects of oral and IV iron formulation. |
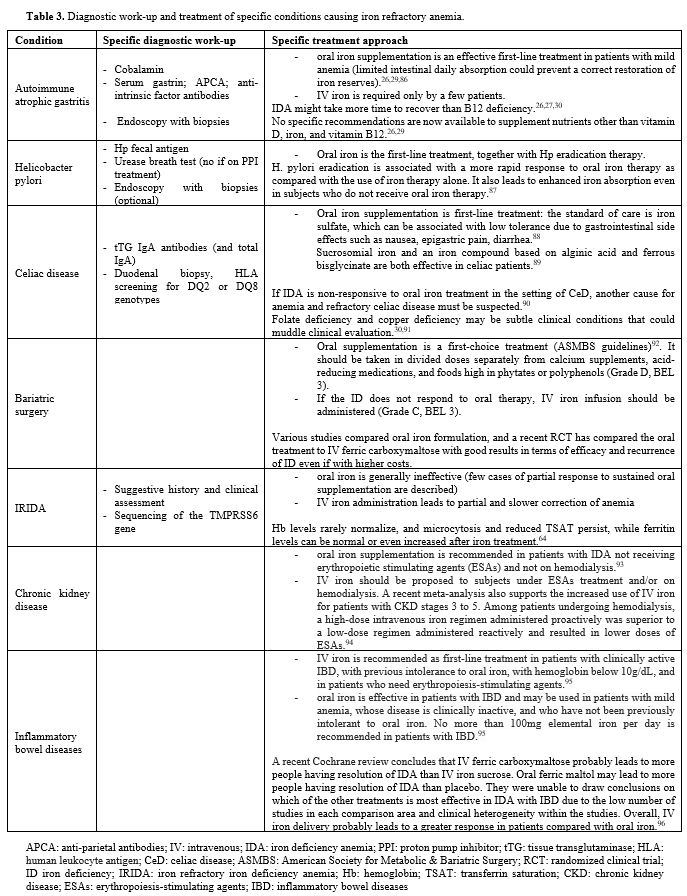 |
Table 3. Diagnostic work-up and treatment of specific conditions causing iron refractory anemia. |
Treatment
The
basis of IDA treatment is the administration of iron to restore the
deposits and induce Hb production, together with the removal of the
causes, whenever possible. The mainstay is oral iron administration,
which should be prolonged for at least three months to replete iron
stores and normalize ferritin levels.
Oral iron is cheap and does not require hospital access. However, it is
associated with frequent side effects, especially GI symptoms that
often lead to treatment interruption.[80] It has been
recently shown that oral alternate-day administration can have fewer
gastrointestinal side effects and reduced hepcidin levels than
conventional daily dosage, ameliorating iron absorption and improving
patient tolerability.[81,82] Thus, in oral treatment failure, low adherence should be suspected, but iron refractoriness causes should also be considered. Iron
can also be administered intravenously (IV), with more rapid and
effective correction of ID and IDA bypassing iron absorption. It has
higher costs, but with more recent formulation, such as ferric
carboximaltose, fewer/single-dose administration is often sufficient,
with shorter time for anemia correction and reduced hospital accesses.
New IV iron formulations, compared to those available in the past, are
safe and exhibit a low immunogenic potential.[83] The cost-benefit ratio favors IV supplementation, as indicated by the European Medicines Agency (EMEA: http://www.emea.europe.eudocument WC500150771, 2013).Characteristics, indications, and side effects of oral and IV formulation are reported in Table 2.
The choice between different formulations is mainly based on Hb values,
IDA etiology, comorbidities, and oral formulation tolerance. The specific treatment approach for each condition is presented in Table 3.
Conclusions
IDA
is a highly prevalent disorder affecting all ages. However, it remains
under-recognized, and unique definitions of ID, anemia, and IDA are
necessary to reach a proper diagnosis.[84] Once
diagnosed, IDA requires appropriate diagnostic work-up to identify the
underlying cause and start the proper treatment. Oral iron refractory
conditions require specialist management and specific treatment to
avoid the worsening of IDA and consequences on the patient's quality of
life.
References
- Disease GBD, Injury I, Prevalence C. Global,
regional, and national incidence, prevalence, and years lived with
disability for 310 diseases and injuries, 1990-2015: a systematic
analysis for the Global Burden of Disease Study 2015. Lancet.
2016;388(10053):1545-1602. https://doi.org/10.1016/S0140-6736(16)31678-6
- Kassebaum
NJ, Jasrasaria R, Naghavi M, Wulf SK, Johns N, Lozano R, Regan M,
Weatherall D, Chou DP, Eisele TP, Flaxman SR, Pullan RL, Brooker SJ,
Murray CJ. A systematic analysis of global anemia burden from 1990 to
2010. Blood. 2014;123(5):615-624. https://doi.org/10.1182/blood-2013-06-508325 PMid:24297872 PMCid:PMC3907750
- Migone
De Amicis M, Poggiali E, Motta I, Minonzio F, Fabio G, Hu C, Cappellini
MD. Anemia in elderly hospitalized patients: prevalence and clinical
impact. Intern Emerg Med. 2015;10(5):581-586. https://doi.org/10.1007/s11739-015-1197-5 PMid:25633233
- Camaschella C. Iron deficiency. Blood. 2019;133(1):30-39. https://doi.org/10.1182/blood-2018-05-815944 PMid:30401704
- Hershko C, Camaschella C. How I treat unexplained refractory iron deficiency anemia. Blood. 2014;123(3):326-333. https://doi.org/10.1182/blood-2013-10-512624 PMid:24215034
- Nicolas
G, Bennoun M, Devaux I, Beaumont C, Grandchamp B, Kahn A, Vaulont S.
Lack of hepcidin gene expression and severe tissue iron overload in
upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci
U S A. 2001;98(15):8780-8785. https://doi.org/10.1073/pnas.151179498 PMid:11447267 PMCid:PMC37512
- Park
CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial
peptide synthesized in the liver. J Biol Chem. 2001;276(11):7806-7810. https://doi.org/10.1074/jbc.M008922200 PMid:11113131
- Nemeth
E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan
J. Hepcidin regulates cellular iron efflux by binding to ferroportin
and inducing its internalization. Science. 2004;306(5704):2090-2093. https://doi.org/10.1126/science.1104742 PMid:15514116
- Strickland
RG, Mackay IR. A reappraisal of the nature and significance of chronic
atrophic gastritis. Am J Dig Dis. 1973;18(5):426-440. https://doi.org/10.1007/BF01071995 PMid:4573514
- Dixon
MF, Genta RM, Yardley JH, Correa P. Classification and grading of
gastritis. The updated Sydney System. International Workshop on the
Histopathology of Gastritis, Houston 1994. Am J Surg Pathol.
1996;20(10):1161-1181. https://doi.org/10.1097/00000478-199610000-00001 PMid:8827022
- Sugano
K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, Haruma K, Asaka
M, Uemura N, Malfertheiner P, faculty members of Kyoto Global Consensus
C. Kyoto global consensus report on Helicobacter pylori gastritis. Gut.
2015;64(9):1353-1367. https://doi.org/10.1136/gutjnl-2015-309252 PMid:26187502 PMCid:PMC4552923
- Marques-Silva
L, Areia M, Elvas L, Dinis-Ribeiro M. Prevalence of gastric
precancerous conditions: a systematic review and meta-analysis. Eur J
Gastroenterol Hepatol. 2014;26(4):378-387. https://doi.org/10.1097/MEG.0000000000000065 PMid:24569821
- Lahner
E, Zagari RM, Zullo A, Di Sabatino A, Meggio A, Cesaro P, Lenti MV,
Annibale B, Corazza GR. Chronic atrophic gastritis: Natural history,
diagnosis and therapeutic management. A position paper by the Italian
Society of Hospital Gastroenterologists and Digestive Endoscopists
[AIGO], the Italian Society of Digestive Endoscopy [SIED], the Italian
Society of Gastroenterology [SIGE], and the Italian Society of Internal
Medicine [SIMI]. Dig Liver Dis. 2019;51(12):1621-1632. https://doi.org/10.1016/j.dld.2019.09.016 PMid:31635944
- Lenti
MV, Lahner E, Bergamaschi G, Miceli E, Conti L, Massironi S, Cococcia
S, Zilli A, Caprioli F, Vecchi M, Maiero S, Cannizzaro R, Corazza GR,
Annibale B, Di Sabatino A. Cell Blood Count Alterations and Patterns of
Anaemia in Autoimmune Atrophic Gastritis at Diagnosis: A Multicentre
Study. J Clin Med. 2019;8(11). https://doi.org/10.3390/jcm8111992 PMid:31731715 PMCid:PMC6912578
- Lenti
MV, Miceli E, Cococcia S, Klersy C, Staiani M, Guglielmi F, Giuffrida
P, Vanoli A, Luinetti O, De Grazia F, Di Stefano M, Corazza GR, Di
Sabatino A. Determinants of diagnostic delay in autoimmune atrophic
gastritis. Aliment Pharmacol Ther. 2019;50(2):167-175. https://doi.org/10.1111/apt.15317 PMid:31115910
- Zilli
A, Cavalcoli F, Ciafardini C, Massironi S. Deficiency of micronutrients
in patients affected by chronic atrophic autoimmune gastritis: A
single-institution observational study. Dig Liver Dis.
2019;51(4):505-509. https://doi.org/10.1016/j.dld.2018.08.028 PMid:30236765
- Bergamaschi
G, Di Sabatino A, Corazza GR. Pathogenesis, diagnosis and treatment of
anaemia in immune-mediated gastrointestinal disorders. Br J Haematol.
2018;182(3):319-329. https://doi.org/10.1111/bjh.15254 PMid:29732532
- Banks
M, Graham D, Jansen M, Gotoda T, Coda S, di Pietro M, Uedo N, Bhandari
P, Pritchard DM, Kuipers EJ, Rodriguez-Justo M, Novelli MR, Ragunath K,
Shepherd N, Dinis-Ribeiro M. British Society of Gastroenterology
guidelines on the diagnosis and management of patients at risk of
gastric adenocarcinoma. Gut. 2019;68(9):1545-1575. https://doi.org/10.1136/gutjnl-2018-318126 PMid:31278206 PMCid:PMC6709778
- Miceli
E, Padula D, Lenti MV, Gallia A, Albertini R, Di Stefano M, Klersy C,
Corazza GR. A laboratory score in the diagnosis of autoimmune atrophic
gastritis: a prospective study. J Clin Gastroenterol. 2015;49(1):e1-5. https://doi.org/10.1097/MCG.0000000000000101 PMid:24583750
- Lenti
MV, Rugge M, Lahner E, Miceli E, Toh BH, Genta RM, De Block C, Hershko
C, Di Sabatino A. Autoimmune gastritis. Nat Rev Dis Primers.
2020;6(1):56. https://doi.org/10.1038/s41572-020-0187-8 PMid:32647173
- DuBois
S, Kearney DJ. Iron-deficiency anemia and Helicobacter pylori
infection: a review of the evidence. Am J Gastroenterol.
2005;100(2):453-459. https://doi.org/10.1111/j.1572-0241.2005.30252.x PMid:15667507
- Malfertheiner
P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F,
Gasbarrini A, Atherton J, Graham DY, Hunt R, Moayyedi P, Rokkas T,
Rugge M, Selgrad M, Suerbaum S, Sugano K, El-Omar EM, European H,
Microbiota Study G, Consensus p. Management of Helicobacter pylori
infection-the Maastricht V/Florence Consensus Report. Gut.
2017;66(1):6-30. https://doi.org/10.1136/gutjnl-2016-312288 PMid:27707777
- Chey
WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline:
Treatment of Helicobacter pylori Infection. Am J Gastroenterol.
2017;112(2):212-239. https://doi.org/10.1038/ajg.2016.563 PMid:28071659
- Pennazio
M, Spada C, Eliakim R, Keuchel M, May A, Mulder CJ, Rondonotti E, Adler
SN, Albert J, Baltes P, Barbaro F, Cellier C, Charton JP, Delvaux M,
Despott EJ, Domagk D, Klein A, McAlindon M, Rosa B, Rowse G, Sanders
DS, Saurin JC, Sidhu R, Dumonceau JM, Hassan C, Gralnek IM. Small-bowel
capsule endoscopy and device-assisted enteroscopy for diagnosis and
treatment of small-bowel disorders: European Society of
Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy.
2015;47(4):352-376. https://doi.org/10.1055/s-0034-1391855 PMid:25826168
- John
S, Baltodano JD, Mehta N, Mark K, Murthy U. Unexplained iron deficiency
anemia: does Helicobacter pylori have a role to play? Gastroenterol Rep
(Oxf). 2018;6(3):215-220. https://doi.org/10.1093/gastro/goy001 PMid:30151206 PMCid:PMC6101634
- Oberhuber
G, Granditsch G, Vogelsang H. The histopathology of coeliac disease:
time for a standardized report scheme for pathologists. Eur J
Gastroenterol Hepatol. 1999;11(10):1185-1194. https://doi.org/10.1097/00042737-199910000-00019 PMid:10524652
- Leffler
DA, Green PH, Fasano A. Extraintestinal manifestations of coeliac
disease. Nat Rev Gastroenterol Hepatol. 2015;12(10):561-571. https://doi.org/10.1038/nrgastro.2015.131 PMid:26260366
- Mahadev
S, Laszkowska M, Sundstrom J, Bjorkholm M, Lebwohl B, Green PHR,
Ludvigsson JF. Prevalence of Celiac Disease in Patients With Iron
Deficiency Anemia-A Systematic Review With Meta-analysis.
Gastroenterology. 2018;155(2):374-382 e371. https://doi.org/10.1053/j.gastro.2018.04.016 PMid:29689265 PMCid:PMC7057414
- Corazza
GR, Valentini RA, Andreani ML, D'Anchino M, Leva MT, Ginaldi L, De
Feudis L, Quaglino D, Gasbarrini G. Subclinical coeliac disease is a
frequent cause of iron-deficiency anaemia. Scand J Gastroenterol.
1995;30(2):153-156. https://doi.org/10.3109/00365529509093254 PMid:7732338
- Elli
L, Norsa L, Zullo A, Carroccio A, Girelli C, Oliva S, Romano C, Leandro
G, Bellini M, Marmo R, Soncini M, Monica F, De Francesco V, Paulon E,
Cappellini MD, Motta I, Ferretti F, Orlando S, Mansueto P, Buscarini E,
Manfredi G, Agostoni C, Tomba C, Cannizzaro R. Diagnosis of chronic
anaemia in gastrointestinal disorders: A guideline by the Italian
Association of Hospital Gastroenterologists and Endoscopists (AIGO) and
the Italian Society of Paediatric Gastroenterology Hepatology and
Nutrition (SIGENP). Dig Liver Dis. 2019;51(4):471-483. https://doi.org/10.1016/j.dld.2019.01.022 PMid:30850345
- Barisani
D, Ceroni S, Del Bianco S, Meneveri R, Bardella MT. Hemochromatosis
gene mutations and iron metabolism in celiac disease. Haematologica.
2004;89(11):1299-1305.
- Barisani
D, Parafioriti A, Bardella MT, Zoller H, Conte D, Armiraglio E, Trovato
C, Koch RO, Weiss G. Adaptive changes of duodenal iron transport
proteins in celiac disease. Physiol Genomics. 2004;17(3):316-325. https://doi.org/10.1152/physiolgenomics.00211.2003 PMid:15054143
- Elli
L, Poggiali E, Tomba C, Andreozzi F, Nava I, Bardella MT, Campostrini
N, Girelli D, Conte D, Cappellini MD. Does TMPRSS6 RS855791
polymorphism contribute to iron deficiency in treated celiac disease?
Am J Gastroenterol. 2015;110(1):200-202. https://doi.org/10.1038/ajg.2014.354 PMid:25567183
- Elli
L, Ferretti F, Orlando S, Vecchi M, Monguzzi E, Roncoroni L, Schuppan
D. Management of celiac disease in daily clinical practice. Eur J
Intern Med. 2019;61:15-24. https://doi.org/10.1016/j.ejim.2018.11.012 PMid:30528262
- Ludvigsson
JF, Bai JC, Biagi F, Card TR, Ciacci C, Ciclitira PJ, Green PH,
Hadjivassiliou M, Holdoway A, van Heel DA, Kaukinen K, Leffler DA,
Leonard JN, Lundin KE, McGough N, Davidson M, Murray JA, Swift GL,
Walker MM, Zingone F, Sanders DS, Group BSGCDGD, British Society of G.
Diagnosis and management of adult coeliac disease: guidelines from the
British Society of Gastroenterology. Gut. 2014;63(8):1210-1228. https://doi.org/10.1136/gutjnl-2013-306578 PMid:24917550 PMCid:PMC4112432
- Rubio-Tapia
A, Hill ID, Kelly CP, Calderwood AH, Murray JA, American College of G.
ACG clinical guidelines: diagnosis and management of celiac disease. Am
J Gastroenterol. 2013;108(5):656-676; quiz 677. https://doi.org/10.1038/ajg.2013.79 PMid:23609613 PMCid:PMC3706994
- Elli
L, Zini E, Tomba C, Bardella MT, Bosari S, Conte D, Runza L, Roncoroni
L, Ferrero S. Histological evaluation of duodenal biopsies from coeliac
patients: the need for different grading criteria during follow-up. BMC
Gastroenterol. 2015;15:133. https://doi.org/10.1186/s12876-015-0361-8 PMid:26467310 PMCid:PMC4604755
- Steenackers
N, Van der Schueren B, Mertens A, Lannoo M, Grauwet T, Augustijns P,
Matthys C. Iron deficiency after bariatric surgery: what is the real
problem? Proc Nutr Soc. 2018;77(4):445-455. https://doi.org/10.1017/S0029665118000149 PMid:29619914
- Schijns
W, Ligthart MAP, Berends FJ, Janssen IMC, van Laarhoven C, Aarts EO, de
Boer H. Changes in Iron Absorption After Roux-en-Y Gastric Bypass. Obes
Surg. 2018;28(6):1738-1744. https://doi.org/10.1007/s11695-017-3088-5 PMid:29327182
- Ruz
M, Carrasco F, Rojas P, Codoceo J, Inostroza J, Rebolledo A, Basfi-fer
K, Csendes A, Papapietro K, Pizarro F, Olivares M, Sian L, Westcott JL,
Hambidge KM, Krebs NF. Iron absorption and iron status are reduced
after Roux-en-Y gastric bypass. Am J Clin Nutr. 2009;90(3):527-532. https://doi.org/10.3945/ajcn.2009.27699 PMid:19625680
- Ruivard
M, Laine F, Ganz T, Olbina G, Westerman M, Nemeth E, Rambeau M, Mazur
A, Gerbaud L, Tournilhac V, Abergel A, Philippe P, Deugnier Y, Coudray
C. Iron absorption in dysmetabolic iron overload syndrome is decreased
and correlates with increased plasma hepcidin. J Hepatol.
2009;50(6):1219-1225. https://doi.org/10.1016/j.jhep.2009.01.029 PMid:19398238
- Aigner E, Feldman A, Datz C. Obesity as an emerging risk factor for iron deficiency. Nutrients. 2014;6(9):3587-3600. https://doi.org/10.3390/nu6093587 PMid:25215659 PMCid:PMC4179177
- Moize
V, Andreu A, Flores L, Torres F, Ibarzabal A, Delgado S, Lacy A,
Rodriguez L, Vidal J. Long-term dietary intake and nutritional
deficiencies following sleeve gastrectomy or Roux-En-Y gastric bypass
in a mediterranean population. J Acad Nutr Diet. 2013;113(3):400-410. https://doi.org/10.1016/j.jand.2012.11.013 PMid:23438491
- Hood
MM, Corsica J, Bradley L, Wilson R, Chirinos DA, Vivo A. Managing
severe obesity: understanding and improving treatment adherence in
bariatric surgery. J Behav Med. 2016;39(6):1092-1103. https://doi.org/10.1007/s10865-016-9772-4 PMid:27444752
- M'Koma
AE, Wise PE, Schwartz DA, Muldoon RL, Herline AJ. Prevalence and
outcome of anemia after restorative proctocolectomy: a clinical
literature review. Dis Colon Rectum. 2009;52(4):726-739. https://doi.org/10.1007/DCR.0b013e31819ed571 PMid:19404082 PMCid:PMC4154485
- Bambach
CP, Hill GL. Long term nutritional effects of extensive resection of
the small intestine. Aust N Z J Surg. 1982;52(5):500-506. https://doi.org/10.1111/j.1445-2197.1982.tb06039.x PMid:6816204
- Salomon
L, Lugagne PM, Herve JM, Barre P, Lebret T, Botto H. No evidence of
metabolic disorders 10 to 22 years after Camey type I ileal
enterocystoplasty. J Urol. 1997;157(6):2104-2106. https://doi.org/10.1016/S0022-5347(01)64685-8
- Abd-el-Gawa
G, Abrahamsson K, Norlen L, Hjalmas K, Hanson E. Vitamin B12 and folate
after 5-12 years of continent ileal urostomy (Kock reservoir) in
children and adolescents. Eur Urol. 2002;41(2):199-205. https://doi.org/10.1016/S0302-2838(01)00032-X
- Cameron
AJ, Higgins JA. Linear gastric erosion. A lesion associated with large
diaphragmatic hernia and chronic blood loss anemia. Gastroenterology.
1986;91(2):338-342. https://doi.org/10.1016/0016-5085(86)90566-4
- Zullo
A, Manta R, De Francesco V, Fiorini G, Lahner E, Vaira D, Annibale B.
Cameron lesions: A still overlooked diagnosis. Case report and
systematic review of literature. Clin Res Hepatol Gastroenterol.
2018;42(6):604-609. https://doi.org/10.1016/j.clinre.2018.05.002 PMid:29910147
- Carrott
PW, Markar SR, Hong J, Kuppusamy MK, Koehler RP, Low DE.
Iron-deficiency anemia is a common presenting issue with giant
paraesophageal hernia and resolves following repair. J Gastrointest
Surg. 2013;17(5):858-862. https://doi.org/10.1007/s11605-013-2184-7 PMid:23515913
- Panzuto
F, Di Giulio E, Capurso G, Baccini F, D'Ambra G, Delle Fave G, Annibale
B. Large hiatal hernia in patients with iron deficiency anaemia: a
prospective study on prevalence and treatment. Aliment Pharmacol Ther.
2004;19(6):663-670. https://doi.org/10.1111/j.1365-2036.2004.01894.x PMid:15023168
- Crooks
CJ, West J, Card TR. Upper gastrointestinal haemorrhage and
deprivation: a nationwide cohort study of health inequality in hospital
admissions. Gut. 2012;61(4):514-520. https://doi.org/10.1136/gutjnl-2011-300186 PMid:21757448 PMCid:PMC3292712
- Longstreth
GF. Epidemiology and outcome of patients hospitalized with acute lower
gastrointestinal hemorrhage: a population-based study. Am J
Gastroenterol. 1997;92(3):419-424.
- Gerson
LB, Fidler JL, Cave DR, Leighton JA. ACG Clinical Guideline: Diagnosis
and Management of Small Bowel Bleeding. Am J Gastroenterol.
2015;110(9):1265-1287; quiz 1288. https://doi.org/10.1038/ajg.2015.246 PMid:26303132
- Hesham
MS, Edariah AB, Norhayati M. Intestinal parasitic infections and
micronutrient deficiency: a review. Med J Malaysia. 2004;59(2):284-293.
- Kassebaum NJ, Bertozzi-Villa A,
Coggeshall MS, Shackelford KA, Steiner C, Heuton KR, Gonzalez-Medina D,
Barber R, Huynh C, Dicker D, Templin T, Wolock TM, Ozgoren AA,
Abd-Allah F, Abera SF, Abubakar I, Achoki T, Adelekan A, Ademi Z, Adou
AK, Adsuar JC, Agardh EE, Akena D, Alasfoor D, Alemu ZA,
Alfonso-Cristancho R, Alhabib S, Ali R, Al Kahbouri MJ, Alla F, Allen
PJ, AlMazroa MA, Alsharif U, Alvarez E, Alvis-Guzman N, Amankwaa AA,
Amare AT, Amini H, Ammar W, Antonio CA, Anwari P, Arnlov J, Arsenijevic
VS, Artaman A, Asad MM, Asghar RJ, Assadi R, Atkins LS, Badawi A,
Balakrishnan K, Basu A, Basu S, Beardsley J, Bedi N, Bekele T, Bell ML,
Bernabe E, Beyene TJ, Bhutta Z, Bin Abdulhak A, Blore JD, Basara BB,
Bose D, Breitborde N, Cardenas R, Castaneda-Orjuela CA, Castro RE,
Catala-Lopez F, Cavlin A, Chang JC, Che X, Christophi CA, Chugh SS,
Cirillo M, Colquhoun SM, Cooper LT, Cooper C, da Costa Leite I, Dandona
L, Dandona R, Davis A, Dayama A, Degenhardt L, De Leo D, del Pozo-Cruz
B, Deribe K, Dessalegn M, deVeber GA, Dharmaratne SD, Dilmen U, Ding
EL, Dorrington RE, Driscoll TR, Ermakov SP, Esteghamati A, Faraon EJ,
Farzadfar F, Felicio MM, Fereshtehnejad SM, de Lima GM, Forouzanfar MH,
Franca EB, Gaffikin L, Gambashidze K, Gankpe FG, Garcia AC, Geleijnse
JM, Gibney KB, Giroud M, Glaser EL, Goginashvili K, Gona P,
Gonzalez-Castell D, Goto A, Gouda HN, Gugnani HC, Gupta R, Gupta R,
Hafezi-Nejad N, Hamadeh RR, Hammami M, Hankey GJ, Harb HL, Havmoeller
R, Hay SI, Pi IB, Hoek HW, Hosgood HD, Hoy DG, Husseini A, Idrisov BT,
Innos K, Inoue M, Jacobsen KH, Jahangir E, Jee SH, Jensen PN, Jha V,
Jiang G, Jonas JB, Juel K, Kabagambe EK, Kan H, Karam NE, Karch A,
Karema CK, Kaul A, Kawakami N, Kazanjan K, Kazi DS, Kemp AH, Kengne AP,
Kereselidze M, Khader YS, Khalifa SE, Khan EA, Khang YH, Knibbs L,
Kokubo Y, Kosen S, Defo BK, Kulkarni C, Kulkarni VS, Kumar GA, Kumar K,
Kumar RB, Kwan G, Lai T, Lalloo R, Lam H, Lansingh VC, Larsson A, Lee
JT, Leigh J, Leinsalu M, Leung R, Li X, Li Y, Li Y, Liang J, Liang X,
Lim SS, Lin HH, Lipshultz SE, Liu S, Liu Y, Lloyd BK, London SJ, Lotufo
PA, Ma J, Ma S, Machado VM, Mainoo NK, Majdan M, Mapoma CC, Marcenes W,
Marzan MB, Mason-Jones AJ, Mehndiratta MM, Mejia-Rodriguez F, Memish
ZA, Mendoza W, Miller TR, Mills EJ, Mokdad AH, Mola GL, Monasta L, de
la Cruz Monis J, Hernandez JC, Moore AR, Moradi-Lakeh M, Mori R,
Mueller UO, Mukaigawara M, Naheed A, Naidoo KS, Nand D, Nangia V, Nash
D, Nejjari C, Nelson RG, Neupane SP, Newton CR, Ng M, Nieuwenhuijsen
MJ, Nisar MI, Nolte S, Norheim OF, Nyakarahuka L, Oh IH, Ohkubo T,
Olusanya BO, Omer SB, Opio JN, Orisakwe OE, Pandian JD, Papachristou C,
Park JH, Caicedo AJ, Patten SB, Paul VK, Pavlin BI, Pearce N, Pereira
DM, Pesudovs K, Petzold M, Poenaru D, Polanczyk GV, Polinder S, Pope D,
Pourmalek F, Qato D, Quistberg DA, Rafay A, Rahimi K, Rahimi-Movaghar
V, ur Rahman S, Raju M, Rana SM, Refaat A, Ronfani L, Roy N, Pimienta
TG, Sahraian MA, Salomon JA, Sampson U, Santos IS, Sawhney M, Sayinzoga
F, Schneider IJ, Schumacher A, Schwebel DC, Seedat S, Sepanlou SG,
Servan-Mori EE, Shakh-Nazarova M, Sheikhbahaei S, Shibuya K, Shin HH,
Shiue I, Sigfusdottir ID, Silberberg DH, Silva AP, Singh JA, Skirbekk
V, Sliwa K, Soshnikov SS, Sposato LA, Sreeramareddy CT, Stroumpoulis K,
Sturua L, Sykes BL, Tabb KM, Talongwa RT, Tan F, Teixeira CM, Tenkorang
EY, Terkawi AS, Thorne-Lyman AL, Tirschwell DL, Towbin JA, Tran BX,
Tsilimbaris M, Uchendu US, Ukwaja KN, Undurraga EA, Uzun SB, Vallely
AJ, van Gool CH, Vasankari TJ, Vavilala MS, Venketasubramanian N,
Villalpando S, Violante FS, Vlassov VV, Vos T, Waller S, Wang H, Wang
L, Wang X, Wang Y, Weichenthal S, Weiderpass E, Weintraub RG, Westerman
R, Wilkinson JD, Woldeyohannes SM, Wong JQ, Wordofa MA, Xu G, Yang YC,
Yano Y, Yentur GK, Yip P, Yonemoto N, Yoon SJ, Younis MZ, Yu C, Jin KY,
El Sayed Zaki M, Zhao Y, Zheng Y, Zhou M, Zhu J, Zou XN, Lopez AD,
Naghavi M, Murray CJ, Lozano R. Global, regional, and national levels
and causes of maternal mortality during 1990-2013: a systematic
analysis for the Global Burden of Disease Study 013. Lancet.
2014;384(9947):980-1004.
- Crompton DW,
Nesheim MC. Nutritional impact of intestinal helminthiasis during the
human life cycle. Annu Rev Nutr. 2002;22:35-59. https://doi.org/10.1146/annurev.nutr.22.120501.134539 PMid:12055337
- Tran-Duy
A, Connell NJ, Vanmolkot FH, Souverein PC, de Wit NJ, Stehouwer CDA,
Hoes AW, de Vries F, de Boer A. Use of proton pump inhibitors and risk
of iron deficiency: a population-based case-control study. J Intern
Med. 2019;285(2):205-214. https://doi.org/10.1111/joim.12826 PMid:30141278
- Ajmera
AV, Shastri GS, Gajera MJ, Judge TA. Suboptimal response to ferrous
sulfate in iron-deficient patients taking omeprazole. Am J Ther.
2012;19(3):185-189. https://doi.org/10.1097/MJT.0b013e3181f9f6d2 PMid:21150767
- Piccillo
GA, Miele L, Mondati EG, Scuderi R, Nicolosi M, Forgione A, Gabrieli
ML, Cefalo C, Gasbarrini G, Grieco A. Eighteen needles to forget...an
unnamed past. J Forensic Leg Med. 2007;14(5):304-306. https://doi.org/10.1016/j.jcfm.2006.07.005 PMid:17055322
- Palmer SR, Thanarajasingam G, Wolanskyj AP. 39-year-old woman with an obscure case of anemia. Mayo Clin Proc. 2010;85(1):e1-4. https://doi.org/10.4065/mcp.2008.0721 PMid:20042553 PMCid:PMC2800282
- Finberg
KE, Heeney MM, Campagna DR, Aydinok Y, Pearson HA, Hartman KR, Mayo MM,
Samuel SM, Strouse JJ, Markianos K, Andrews NC, Fleming MD. Mutations
in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA). Nat
Genet. 2008;40(5):569-571. https://doi.org/10.1038/ng.130 PMid:18408718 PMCid:PMC3104019
- De
Falco L, Sanchez M, Silvestri L, Kannengiesser C, Muckenthaler MU,
Iolascon A, Gouya L, Camaschella C, Beaumont C. Iron refractory iron
deficiency anemia. Haematologica. 2013;98(6):845-853. https://doi.org/10.3324/haematol.2012.075515 PMid:23729726 PMCid:PMC3669438
- Gardenghi
S, Renaud TM, Meloni A, Casu C, Crielaard BJ, Bystrom LM,
Greenberg-Kushnir N, Sasu BJ, Cooke KS, Rivella S. Distinct roles for
hepcidin and interleukin-6 in the recovery from anemia in mice injected
with heat-killed Brucella abortus. Blood. 2014;123(8):1137-1145. https://doi.org/10.1182/blood-2013-08-521625 PMid:24357729 PMCid:PMC3931188
- Theurl
I, Aigner E, Theurl M, Nairz M, Seifert M, Schroll A, Sonnweber T,
Eberwein L, Witcher DR, Murphy AT, Wroblewski VJ, Wurz E, Datz C, Weiss
G. Regulation of iron homeostasis in anemia of chronic disease and iron
deficiency anemia: diagnostic and therapeutic implications. Blood.
2009;113(21):5277-5286. https://doi.org/10.1182/blood-2008-12-195651 PMid:19293425
- Weiss G, Ganz T, Goodnough LT. Anemia of inflammation. Blood. 2019;133(1):40-50. https://doi.org/10.1182/blood-2018-06-856500 PMid:30401705 PMCid:PMC6536698
- Kulnigg S, Gasche C. Systematic review: managing anaemia in Crohn's disease. Aliment Pharmacol Ther. 2006;24(11-12):1507-1523. https://doi.org/10.1111/j.1365-2036.2006.03146.x PMid:17206940
- Desreumaux
P, Ernst O, Geboes K, Gambiez L, Berrebi D, Muller-Alouf H, Hafraoui S,
Emilie D, Ectors N, Peuchmaur M, Cortot A, Capron M, Auwerx J, Colombel
JF. Inflammatory alterations in mesenteric adipose tissue in Crohn's
disease. Gastroenterology. 1999;117(1):73-81. https://doi.org/10.1016/S0016-5085(99)70552-4
- Strong
SA, Pizarro TT, Klein JS, Cominelli F, Fiocchi C. Proinflammatory
cytokines differentially modulate their own expression in human
intestinal mucosal mesenchymal cells. Gastroenterology.
1998;114(6):1244-1256. https://doi.org/10.1016/S0016-5085(98)70431-7
- Oustamanolakis
P, Koutroubakis IE, Messaritakis I, Malliaraki N, Sfiridaki A,
Kouroumalis EA. Serum hepcidin and prohepcidin concentrations in
inflammatory bowel disease. Eur J Gastroenterol Hepatol.
2011;23(3):262-268. https://doi.org/10.1097/MEG.0b013e328343b885 PMid:21285884
- Mecklenburg
I, Reznik D, Fasler-Kan E, Drewe J, Beglinger C, Hruz P, Swiss IBDCSG.
Serum hepcidin concentrations correlate with ferritin in patients with
inflammatory bowel disease. J Crohns Colitis. 2014;8(11):1392-1397. https://doi.org/10.1016/j.crohns.2014.04.008 PMid:24825446
- Martinelli
M, Strisciuglio C, Alessandrella A, Rossi F, Auricchio R, Campostrini
N, Girelli D, Nobili B, Staiano A, Perrotta S, Miele E. Serum Hepcidin
and Iron Absorption in Paediatric Inflammatory Bowel Disease. J Crohns
Colitis. 2016;10(5):566-574. https://doi.org/10.1093/ecco-jcc/jjv242 PMid:26733407 PMCid:PMC4957448
- Babitt JL, Lin HY. Mechanisms of anemia in CKD. J Am Soc Nephrol. 2012;23(10):1631-1634. https://doi.org/10.1681/ASN.2011111078 PMid:22935483 PMCid:PMC3458456
- Atkinson
MA, Kim JY, Roy CN, Warady BA, White CT, Furth SL. Hepcidin and risk of
anemia in CKD: a cross-sectional and longitudinal analysis in the CKiD
cohort. Pediatr Nephrol. 2015;30(4):635-643. https://doi.org/10.1007/s00467-014-2991-4 PMid:25380788 PMCid:PMC4336204
- Troutt
JS, Butterfield AM, Konrad RJ. Hepcidin-25 concentrations are markedly
increased in patients with chronic kidney disease and are inversely
correlated with estimated glomerular filtration rates. J Clin Lab Anal.
2013;27(6):504-510. https://doi.org/10.1002/jcla.21634 PMid:24218134 PMCid:PMC6807340
- Sohal
AS, Gangji AS, Crowther MA, Treleaven D. Uremic bleeding:
pathophysiology and clinical risk factors. Thromb Res.
2006;118(3):417-422. https://doi.org/10.1016/j.thromres.2005.03.032 PMid:15993929
- Weinstein
DA, Roy CN, Fleming MD, Loda MF, Wolfsdorf JI, Andrews NC.
Inappropriate expression of hepcidin is associated with iron refractory
anemia: implications for the anemia of chronic disease. Blood.
2002;100(10):3776-3781. https://doi.org/10.1182/blood-2002-04-1260 PMid:12393428
- Pagani A, Nai A, Silvestri L, Camaschella C. Hepcidin and Anemia: A Tight Relationship. Front Physiol. 2019;10:1294. https://doi.org/10.3389/fphys.2019.01294 PMid:31649559 PMCid:PMC6794341
- Girelli
D, Ugolini S, Busti F, Marchi G, Castagna A. Modern iron replacement
therapy: clinical and pathophysiological insights. Int J Hematol.
2018;107(1):16-30. https://doi.org/10.1007/s12185-017-2373-3 PMid:29196967
- Moretti
D, Goede JS, Zeder C, Jiskra M, Chatzinakou V, Tjalsma H,
Melse-Boonstra A, Brittenham G, Swinkels DW, Zimmermann MB. Oral iron
supplements increase hepcidin and decrease iron absorption from daily
or twice-daily doses in iron-depleted young women. Blood.
2015;126(17):1981-1989. https://doi.org/10.1182/blood-2015-05-642223 PMid:26289639
- Stoffel
NU, Cercamondi CI, Brittenham G, Zeder C, Geurts-Moespot AJ, Swinkels
DW, Moretti D, Zimmermann MB. Iron absorption from oral iron
supplements given on consecutive versus alternate days and as single
morning doses versus twice-daily split dosing in iron-depleted women:
two open-label, randomised controlled trials. Lancet Haematol.
2017;4(11):e524-e533. https://doi.org/10.1016/S2352-3026(17)30182-5
- Avni
T, Bieber A, Grossman A, Green H, Leibovici L, Gafter-Gvili A. The
safety of intravenous iron preparations: systematic review and
meta-analysis. Mayo Clin Proc. 2015;90(1):12-23. https://doi.org/10.1016/j.mayocp.2014.10.007 PMid:25572192
- De
Franceschi L, Iolascon A, Taher A, Cappellini MD. Clinical management
of iron deficiency anemia in adults: Systemic review on advances in
diagnosis and treatment. Eur J Intern Med. 2017;42:16-23. https://doi.org/10.1016/j.ejim.2017.04.018 PMid:28528999
- Cappellini
MD, Comin-Colet J, de Francisco A, Dignass A, Doehner W, Lam CS,
Macdougall IC, Rogler G, Camaschella C, Kadir R, Kassebaum NJ, Spahn
DR, Taher AT, Musallam KM, Group IC. Iron deficiency across chronic
inflammatory conditions: International expert opinion on definition,
diagnosis, and management. Am J Hematol. 2017;92(10):1068-1078. https://doi.org/10.1002/ajh.24820 PMid:28612425 PMCid:PMC5599965
- Jimenez K, Kulnigg-Dabsch S, Gasche C. Management of Iron Deficiency Anemia. Gastroenterol Hepatol (N Y). 2015;11(4):241-250.
- Choe
YH, Kim SK, Son BK, Lee DH, Hong YC, Pai SH. Randomized
placebo-controlled trial of Helicobacter pylori eradication for
iron-deficiency anemia in preadolescent children and adolescents.
Helicobacter. 1999;4(2):135-139. https://doi.org/10.1046/j.1523-5378.1999.98066.x PMid:10382128
- Tolkien
Z, Stecher L, Mander AP, Pereira DI, Powell JJ. Ferrous sulfate
supplementation causes significant gastrointestinal side-effects in
adults: a systematic review and meta-analysis. PLoS One.
2015;10(2):e0117383. https://doi.org/10.1371/journal.pone.0117383 PMid:25700159 PMCid:PMC4336293
- Elli
L, Ferretti F, Branchi F, Tomba C, Lombardo V, Scricciolo A, Doneda L,
Roncoroni L. Sucrosomial Iron Supplementation in Anemic Patients with
Celiac Disease Not Tolerating Oral Ferrous Sulfate: A Prospective
Study. Nutrients. 2018;10(3). https://doi.org/10.3390/nu10030330 PMid:29522446 PMCid:PMC5872748
- Branchi
F, Locatelli M, Tomba C, Conte D, Ferretti F, Elli L. Enteroscopy and
radiology for the management of celiac disease complications: Time for
a pragmatic roadmap. Dig Liver Dis. 2016;48(6):578-586. https://doi.org/10.1016/j.dld.2016.02.015 PMid:27012449
- Vallurupalli M, Divakaran S, Parnes A, Levy BD, Loscalzo J. The Element of Surprise. N Engl J Med. 2019;381(14):1365-1371. https://doi.org/10.1056/NEJMcps1811547 PMid:31577880 PMCid:PMC7029838
- Parrott
J, Frank L, Rabena R, Craggs-Dino L, Isom KA, Greiman L. American
Society for Metabolic and Bariatric Surgery Integrated Health
Nutritional Guidelines for the Surgical Weight Loss Patient 2016
Update: Micronutrients. Surg Obes Relat Dis. 2017;13(5):727-741. https://doi.org/10.1016/j.soard.2016.12.018 PMid:28392254
- Albaramki
J, Hodson EM, Craig JC, Webster AC. Parenteral versus oral iron therapy
for adults and children with chronic kidney disease. Cochrane Database
Syst Rev. 2012;1:CD007857. https://doi.org/10.1002/14651858.CD007857.pub2 PMid:22258974
- Macdougall
IC, White C, Anker SD, Bhandari S, Farrington K, Kalra PA, McMurray
JJV, Murray H, Tomson CRV, Wheeler DC, Winearls CG, Ford I,
Investigators P, Committees. Intravenous Iron in Patients Undergoing
Maintenance Hemodialysis. N Engl J Med. 2019;380(5):447-458. https://doi.org/10.1056/NEJMoa1810742 PMid:30365356
- Dignass
AU, Gasche C, Bettenworth D, Birgegard G, Danese S, Gisbert JP,
Gomollon F, Iqbal T, Katsanos K, Koutroubakis I, Magro F, Savoye G,
Stein J, Vavricka S, European Cs, Colitis O. European consensus on the
diagnosis and management of iron deficiency and anaemia in inflammatory
bowel diseases. J Crohns Colitis. 2015;9(3):211-222. https://doi.org/10.1093/ecco-jcc/jju009 PMid:25518052
- Gordon
M, Sinopoulou V, Iheozor-Ejiofor Z, Iqbal T, Allen P, Hoque S, Engineer
J, Akobeng AK. Interventions for treating iron deficiency anaemia in
inflammatory bowel disease. Cochrane Database Syst Rev.
2021;1:CD013529. https://doi.org/10.1002/14651858.CD013529.pub2 PMid:33471939
,
[TOP]




