The first of the three CML patients we report is a 88 years old female patient living at a nursing home and receiving imatinib treatment because of chronic myeloid leukemia (CML) for 7 years. Her nasal swab was found to be COVID-19 PCR positive in the course of routine screening in April 2020. Another COVID-19 PCR was positive two weeks later again, though no other signs or clinical symptoms were found aside from profound weakness. Imatinib treatment was continued unchanged during COVID-19 infection. Since then, two further CML patients treated at our hematological center were found to have acquired COVID-19 infection. The main clinical characteristics of these three patients are summarized in Table 1.
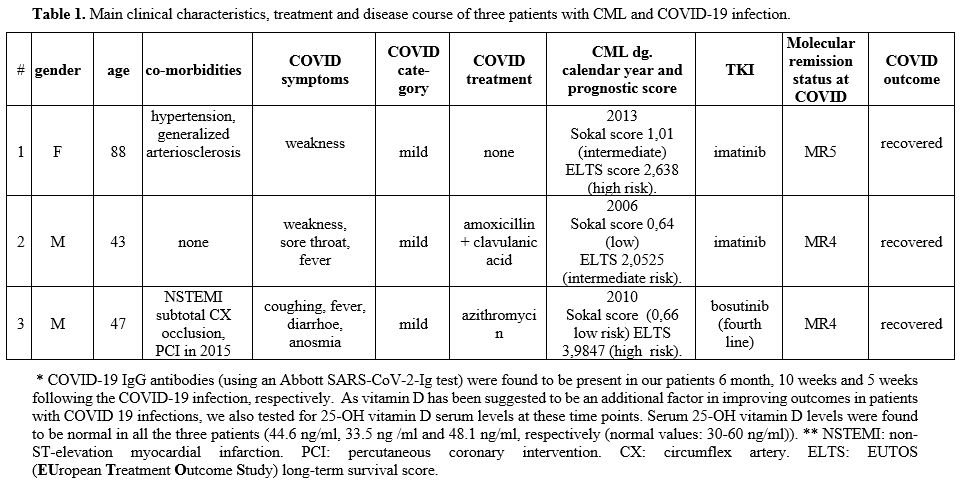 |
Table
1. Main clinical characteristics, treatment and disease course of three patients with CML and COVID-19 infection. |
The second patient, a barber, tested PCR positive for Covid-19 five days after the first symptoms, he had no symptoms 10 days later anymore, but his PCR was still positive and was negative 15 days after the first test, allowing him to return to work. The third patient might have acquired Covid-19 from his companion, a nurse who suffered Covid 19-infection. At the diagnosis of CML in 2010, this patient was treated with imatinib first line, was switched later to nilotinib second line and dasatinib third line without achieving major molecular response (no mutation was identified). Thus we started treatment with 500 mg bosutinib/day in March 2015 without side effects, and the patient got into deep molecular response (DMR). In November 2015, anginal symptoms developed with elevated cardiac biomarkers of necrosis in the absence of persistent ST-segment elevation (NSTEMI), a coronarography proved subtotal circumflex artery (CX) occlusion, a percutaneous coronary intervention (PCI) was performed. His cardiological follow-up is uneventful; he performs moderate sport activities and is in continuous DMR. Two weeks after his positive COVID-19 PCR test, he was symptom-free, his Covid PCR was still positive (it became negative further two weeks later).
Though coronavirus disease 2019 (COVID-19) pandemic poses several challenges to managing patients with leukemia, CML patients treated with TKIs seem to represent a unique patient population in this respect. In May 2020, Abruzzese et al. reported the relatively mild clinical course of COVID-19 infection in a patient who continued full-dose dasatinib therapy when diagnosed with COVID-19. The authors suggested that the incidence and severity of SARS- CoV-2 virus infection may not be worse in CML patients treated with TKIs than in virus victims without an underlying CML diagnosis and that CML patients who contract SARS-CoV-2 may even be protected by TKI therapy.[2] A previous in vitro research - aimed at repurposing approved drugs for the treatment of emerging coronaviruses - has shown that imatinib's anti-coronavirus activity occurs at the early stages of infection, after internalization and endosomal trafficking, by inhibiting fusion of the virions at the endosomal membrane. The authors specifically identified the imatinib target, Abelson tyrosine-protein kinase 2 (Abl2), as required for efficient SARS-CoV (and MERS-CoV replication) in vitro.[3] Ongoing studies intend to evaluate the efficacy and safety of oral administration of imatinib in hospitalized patients with COVID-19 (ClinicalTrials.gov Identifier: NCT04394416) and the potential of oral imatinib to prevent pulmonary vascular leak in COVID-19 patients with severe disease (https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-001236-10/NL).
There are few other published reports on the clinical course of COVID-19 infection in CML patients. Sorá et al. described a case of life-threatening COVID-19 infection complicated by severe immune hemolytic anemia and a „cytokine storm” in a patient with a history of polyarthritis and ulcerative colitis treated with salazopyrin, as well as CML treated with imatinib.[4] The first case series came from the Hubei Province and reported relatively few COVID-19+ CML patients.[5] Breccia et al. reported that in 6,883 CML patients observed at 51 Italian centers, only 12 cases (0,17%) were SARS-CoV-2 positive.[6] Lately, serologic testing of 161 CML patients showed the prevalence of infection in CML patients to be similar to that of the overall population, suggesting that the patients are capable of mounting appropriate antibody response against SARS-CoV-2 (Claudiani, 2020).[7] The CANDID study, a study of the International CML Foundation (iCMLf) characterizing COVID-19 in CML, included 110 cases of COVID-19 reported to iCMLf from 20 countries. In this study, the mortality rate from COVID-19 in the 87 evaluable CML patients was 13.7%.[8] Though age and imatinib therapy were found to be associated with a higher mortality rate, the authors conclude that imatinib may represent a confounder instead of a true adverse prognostic predictor given the strong link between imatinib treatment and advanced age in their global cohort study.[8]
Our patients' common clinical feature is that they were in deep molecular remission at the time of acquiring Covid-19 infection, that TKI treatment was continued unchanged during the infection and none of the patients had to be hospitalized or needed intensive care. These observations lend further support for the potential protective effect of TKIs regarding the course of Covid-19 infection in CML.