Mohamed Hussien Ahmed1, Mohamed H Emara1, Amr Asem Elfert2, Aymen M. El-Saka3, Asem Ahmed Elfert4, Sherief Abd-Elsalam4 and Mohamed Yousef4..
1 Hepatology, Gastroenterology and Infectious Diseases Department, Kafrelsheikh University, Kafrelsheikh, Egypt.
2 Department of Pathology, College of Medicine, University of Illinois at Chicago, Chicago, IL, United States.
3 Department of Pathology, Faculty of Medicine, Tanta University, Egypt.
4 Department of Tropical Medicine, Faculty of Medicine, Tanta University, Tanta, Egypt.
Published: May 1, 2021
Received: January 17, 2021
Accepted: April 11, 2021
Mediterr J Hematol Infect Dis 2021, 13(1): e2021033 DOI
10.4084/MJHID.2021.033
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background and Aims: Human
schistosomiasis is one of the most important and unfortunately
neglected tropical diseases. The aim of the current study was to
investigate the prevalence and characteristics of colonic
schistosomiasis among symptomatic rural inhabitants of the Middle
Northern region of the Egyptian Nile delta.
Patients and Methods:
This study recruited 193 inhabitants of the rural community in the
Egyptian Nile Delta referred for colonoscopy because of variable
symptoms. After giving written informed consent, they were exposed to
thorough history, clinical examination, stool analysis, abdominal
ultrasonography, and pan-colonoscopy with biopsies.
Results: Twenty-four
cases out of the 193 patients had confirmed active schistosomiasis with
a prevalence rate of 12.4%. Bleeding with stool was the predominant
manifestation of active Schistosoma infection among the cases either
alone or in combination with abdominal pain. On clinical examination,
most patients (n=17; 70.8%) did not have organomegaly, and 25% had
clinically palpable splenomegaly as far as 75% of them had
sonographically detected hepatic peri-portal fibrosis. Also, 66.6% of
patients have significant endoscopic lesions (polyps, ulcers, mass-like
lesions), and 16.6% of them had colonic affection beyond the
recto-sigmoid region.
Conclusion: Colonic
schistosomiasis is still prevalent among the Egyptian Nile Delta's
symptomatic rural inhabitants at a rate of 12.4%. Of them, 66.6% had
significant endoscopic colorectal lesions. This persistent transmission
of schistosomiasis in the Egyptian Nile Delta's rural community sounds
the alarm for continuing governmental efforts and plans to screen the
high-risk groups. The prevalence rate reported in the current study is
lower than the actual prevalence rate of schistosomiasis due to
focusing only on a subgroup of individuals.
|
Introduction
Human
schistosomiasis (Bilharziasis) is one of the most important neglected
tropical diseases currently not receiving enough public attention. It
is endemic in 77 countries in the tropical and subtropical communities,
with about 250 million individuals worldwide infected.[1] The Middle East and North Africa (MENA region) represented an endemic hot spot for schistosomiasis late in the 20thcentury.[2,3]
In Egypt, Schistosoma mansoni (S. mansoni)
has almost totally replaced Schistosoma haematobium in the Nile Delta
and spread to other regions of the country since the middle of the 20th construction century of the High Dam.[4]
Ongoing
control measures have markedly decreased the incidence of the disease.
The disease's characteristics have changed as a result of the
government-sponsored mass treatment campaigns, implemented over the
past decades, that succeeded in reducing the prevalence of infection
all over Egypt from 3% in 2003 to 0.3% in 2012.[5]
However,
the transmission may remain ongoing due to the widespread distribution
of the intermediate snail host, poor sanitation, lack of health
education, and decreased treatment availability, especially among the
high-risk groups.[6] Furthermore, the data about the newly acquired infections in Egypt is scarce.
Among the high-risk groups who may maintain schistosomiasis' ongoing transmission are the farmers and fishermen.[4,7-9]
The
farmers and fishermen in the Egyptian Nile Delta's geographic area
mainly reside in the rural countryside. They cultivate and fish in
brackish water, harboring the schistosoma snail intermediate host, and
they sometimes practice promiscuous defecation in the water.
Consequently, they act as both victims susceptible to be infected and
offenders disseminating the infection.[8-10]
The
aim of the current study was to investigate the prevalence and
characteristics of colonic schistosomiasis among symptomatic adult
rural inhabitants of the Middle Northern region of the Egyptian Nile
delta.
Patients and Methods
Study area.
Subjects of the current study were symptomatic inhabitants of the rural
community attending the endoscopy units of both the Department of
Hepatology, Gastroenterology and Infectious Diseases, Kafrelshiekh
University, Egypt, and the Tropical Medicine Department, Tanta
University, Egypt. Attendants to both units reside in Kafr-El-Sheikh
and Gharbia governorates. Both governorates are located in the Middle
and North of the Nile Delta. The Western and Eastern borders of this
geographic area are the Rosetta and Damietta branches of the River
Nile. The Northern border is the Mediterranean Sea (Figure 1). The area of the Nile delta is considered an endemic area for S. mansoni infection.[10]
 |
Figure 1. The geographic
study area. Note that this geographic area occupies the Middle and
Northern zone of the Nile Delta. The whole western border of this
geographic area is the Rosetta branch of the River Nile, while a
reasonable distance of its Eastern borders is the Damietta branch of
the River Nile.
|
Kafr-El-Sheikh
and Gharbia governorates are agricultural districts. In this rural
community of the Nile Delta, inhabitants are primarily farmers and, to
a lesser extent, fishermen. They practice agriculture and fishing in
potentially infected water supplies.
Ethical consideration.
Permission and official approval to carry out the study was obtained.
All patients signed a written informed consent prior to inclusion into
this study, and the institutional ethical committee in Tanta University
Faculty of Medicine approved the study. The study protocol conforms to
the ethical guidelines of the 1975 Declaration of Helsinki.
Study design. A cross-sectional study:
a)
Our study's primary end-point was determining the percentage of
symptomatic adult inhabitants in the rural community with active
colonic schistosomiasis.
b) Our study's
secondary end-points were to characterize clinical, sonographic
features of colonic schistosomiasis besides the extent of the schistosoma induced pathology in the colon.
c) Inclusion criteria (Figure 2): Patients with the following criteria were recruited
- Any gender
- Living in the rural countryside
- Patients referred for colonoscopy due to complaints related to colon affection
d) Exclusion criteria: These patients were excluded from the study
- Pregnant ladies
- Non-Adults (˂18 years)
- Patients with established liver cirrhosis
- Patients with inflammatory bowel diseases (IBD)
- Patients with known other organic bowel damage, e.g., bowel cancer
- Patients not willing to participate or failed to give consent
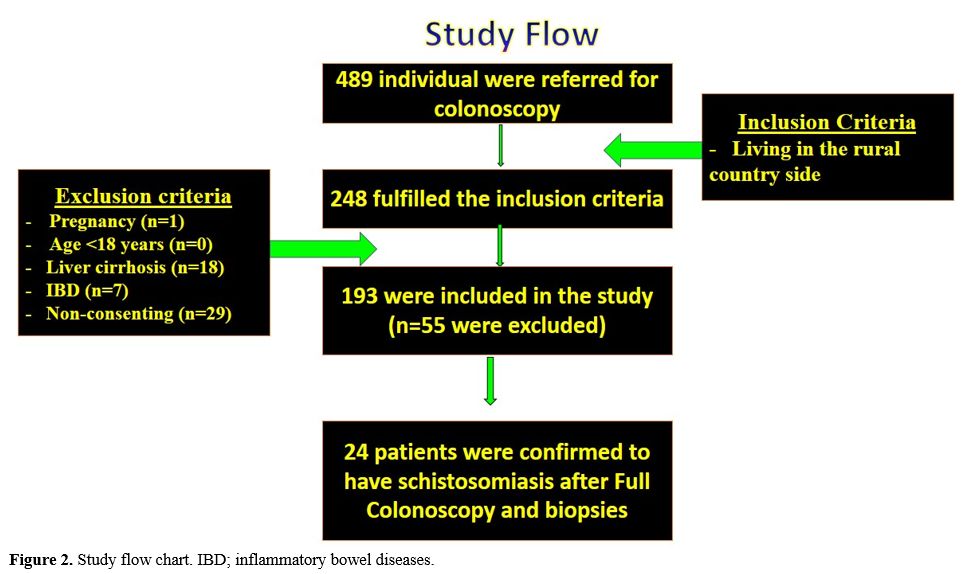 |
Figure 2. Study flow chart. IBD; inflammatory bowel diseases.
|
Study subjects.
They are histologically confirmed S. mansoni infected patients (n= 24).
This study was carried out over 18 months, between August 2018 and
January 2020. Four hundred eight-nine colonoscopies were performed
during the study period. Two hundred forty-eight fulfilled our
inclusion criteria, while 241 were excluded being non-inhabitants of
the rural community. Finally, 193 of them gave written informed consent
to participate in the study after explaining the concept, steps,
benefits, and possible adverse events of the investigation. Fifty-five
were excluded from the study due to: Failure to give consent (n=29),
Patients with IBD (n=7), pregnancy (n=1), and the presence of
established liver cirrhosis (n=18) (Figure 2).
Thorough history taking, clinical examination, stool analysis,
abdominal ultrasonography, and pan-colonoscopy evaluated all the
patients.
Confirmation of active Schistosoma infection. Detection of S. mansoni
eggs in stool samples was viewed as the gold standard for the infection
diagnosis. However, it has some limitations in cases of closed (due to
excess fibrosis) or light infections, [11] and that is why active schistosomiasis in this study was defined by detecting the S. mansoni eggs in the histopathology specimens obtained during colonoscopy (Figure 3).
Endoscopic specimens after proper processing were stained with
hematoxylin and eosin (H&E) and examined under a high power field.
At least five serial sections were examined before the specimen was
considered negative.
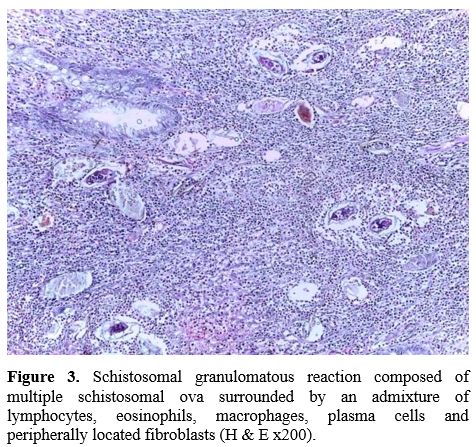 |
Figure 3. Schistosomal
granulomatous reaction composed of multiple schistosomal ova surrounded
by an admixture of lymphocytes, eosinophils, macrophages, plasma cells
and peripherally located fibroblasts (H & E x200).
|
Stool analysis. Stool samples were processed in the laboratory following the Kato–Katz procedure.[12]
Abdominal Ultrasonography.
Done on the day of colonoscopy or 7 days later at the same day of
receiving the histopathology reports. Patients were fasting and
examination with the greyscale ultrasound machine was done before being
examined by colonoscopy to avoid the masking effect of air insufflation
or one week later while patients were fasting. Grading of schistosomal
hepatic periportal fibrosis was carried out following the thickness of
three peripherally located portal tracts into three grades; I (mean
thickness from 3 to 5 mm), II (mean thickness from >5 to 7 mm), and
III (mean thickness from >7 mm).[13]
Colonoscopy examination.
Pan-ileocolonoscopy was planned for all rural residents. The
examination was done following a one-day bowel preparation using the
polyethylene glycol electrolyte solution as 4 sachets (MOVIPREP,
Norgine Limited, UK, or the comparable local products when
unavailable). Patients were prepared with one liter of Moviprep (2
sachets) in the evening before and one liter of Moviprep (2 sachets) in
the early morning of the colonoscopy. The examination was done under
conscious sedation most of the time. Examining the whole colon was
possible with a 100% caecal intubation rate; however, terminal ileum
intubation was possible only in 76.4% (n=19) of patients. The
meticulous colonic examination was done during scope withdrawal, and
mucosal biopsies were taken from the entire colon segments as well as
the morphologically detected lesions.
Rectal snip examination.
Four rectal snips were obtained at the time of colonoscopy. Two were
sent with the histopathology specimens, and 2 were examined under the
microscope to confirm the presence or absence of schistosoma eggs
(Crush biopsy or squash technique).[14,15]
Patient management. All patients with confirmed S. mansoni
infection in this study were treated with praziquantel 600 mg tabs in a
single oral dose given after a heavy (fatty) meal (40 mg/kg body
weight).[16] A second dose was given 4 weeks later to achieve the presumed 95-100% efficacy in parasite eradication.[17] Among our patients, 8 patients were scheduled for follow-up colonoscopy after 3 months from the index colonoscopy.
Data analysis.
The data were analyzed using SPSS, version 23 (SPSS Inc., Chicago,
Illinois, USA). Data were expressed in number (No), percentage (%) mean
(x̅) and standard deviation (SD).
Results
Study populations and clinical characteristics. In this study, 24 out of the 193 symptomatic rural inhabitants examined were infected with active S. mansoni
as confirmed by colonoscopy and biopsies with a prevalence rate of
12.4% (with 95% CI 12.1%, - 25.6%). Patients were diagnosed both in
the Department of Hepatology, Gastroenterology and Infectious Diseases,
Kafrelshiekh University (n=13) and in the endoscopy unit of the
Tropical Medicine Department, Tanta University (n=11). The clinical
characteristics of the cases are shown in Table 1.
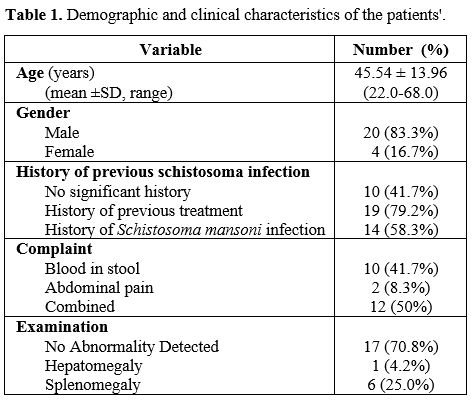 |
Table
1. Demographic characteristics for 320 admissions for febrile children
with sickle cell disease.
|
In
this cohort of patients, either the elderly and young adults were
infected; the age range was 22 to 68 years, while the mean age was
45.54 ± 13.96 years, with a high male predominance (83.3%). In this
study, only 11 out of 24 patients (45.8%) were positive for S. mansoni
eggs in their stool samples.
All patients with confirmed active
schistosoma infection in this study were referred to our endoscopy
units due to colon affection manifestations. Overt bleeding with stool
was the predominant manifestation of active S. mansoni infection among the cases either alone or in combination with abdominal pain (Table 1). Of note, 58.3% gave a history of prior S. mansoni infection
confirmed by stool examination at a time point over the last 10 years.
However, 41.7% of the patients, and at the best of their knowledge, did
not report any history of previous schistosomal infection. However,
most of them (n=19, 79.2%) received oral praziquantel treatment; for
their prior S. mansoni
infection (n=14, 58.3%) or during the mass treatment campaigns
implemented by the primary health care. On clinical examination, most
patients (n=17; 70.8%) did not have organomegaly, and 25% had
clinically palpable splenomegaly.
The infected patients'
laboratory data showed that 10 cases (41.7%) had the profile of
microcytic hypo-chromic anemia with hemoglobin levels variable from 8.8
to 10.7 gm/dl with a mean of 9.53±0.71. However, among the 6 patients
(25%) with clinically palpable splenomegaly, the pattern of anemia was
normocytic normochromic with a mean hemoglobin concentration of
10.18±0.34.
Ultrasonic Features.
When these cases were examined by abdominal ultrasonography, the
frequency of organomegaly (splenomegaly and hepatomegaly) increased to
50.0% versus 29.2% on clinical assessment, respectively (Table 2).
 |
Table 2. Demographic characteristics for 320 admissions for febrile children
with sickle cell disease.
|
The
most dangerous sequela of intestinal schistosomiasis is the development
of schistosomal hepatic peri-portal fibrosis. Unfortunately, 75.0% of
the infected rural inhabitants had schistosomal hepatic fibrosis,
although it was of grade I (mild form) in half of the cases (50%).
Endoscopic Features. The adult S. mansoni worms
migrate against blood flow to the pelvic venous plexus to lay eggs, and
that is consistent with this study's findings where on colonoscopy, the
recto-sigmoid region was always involved. Furthermore, 16.6% of lesions
showed extension beyond the recto-sigmoid region to involve the entire
left colon (Table 2).
The
most commonly encountered morphologic feature during colonoscopy was
mucosal erythema and congestion (Supplementary video I) either alone (n=7, 29.2%) or associated
with mucosal ulcerations (n=5, 20.8%). Colonic schistosomal polyps
either as the sole manifestation of colonic schistosomiasis (n=3,
12.5%) or in combination with erythema and ulcerations (n=3, 12.5%)
where it is limited to the rectum and sigmoid regions (Figure 4).
An interesting finding of the current study is that 2 patients (8.3%)
were diagnosed with mass-like lesions confused with cancer (Table 2).
 |
Figure 4. Demographic characteristics for 320 admissions for febrile children
with sickle cell disease.
|
All
cases with Schistosomal polyps (n=6) were snared successfully during
endoscopy without significant adverse events, while the two patients
with mass-like lesions were referred to surgical resection, and benign
schistosomal nature was histologically confirmed. Colonoscopy follow-up
for 8 patients (polyps n=6, mass-like lesions n=2) showed complete
resolution of the morphologic features associated with schistosomiasis.
Furthermore, surgical margins were free from any apparent lesions. From
the eight patients, multiple rectal and sigmoid biopsies were negative
for schistosomiasis.
Histologic Features. From the histopathologic point of view (Figure 3), all patients showed active S. mansoni
infection features with the living ova surrounded by the characteristic
eosinophilic granuloma containing macrophages, plasma cells, and
lymphocytes and variable degrees of fibrosis (100%). A group of cases
(15.5%) harbored both living and dead S. mansoni
ova. The differentiation between living and dead schistosomal ova was
feasible by noticing the transparency of the eggs, its internal
structures, the shell, the presence of calcification, and granuloma.
Rectal snips directly examined by the crush technique demonstrated the S. mansoni
ova in 21 patients (87.5%). The three patients negative for Schistosoma
ova by rectal snips were the 2 patients with mass-like lesions and one
patient with colonic polyp as the sole finding of colonic
schistosomiasis.
Discussion
Schistosomiasis
has plagued the Egyptian population since the ancient Egyptians. The
disease's prevalence has tremendously decreased but, unfortunately, the
awareness and index of suspicion. As at present, there is only one drug
available for individual treatment, and preventive mass chemotherapy,
and no vaccine, the infection's resurgence is to be feared.[18]
The
spread of schistosomiasis among the rural community inhabitants has
long been investigated in Egyptian[8,9,19] and international
research.[20,21]
The prevalence of S. mansoni
among the symptomatic inhabitants of the rural community in the Middle
and Northern Nile Delta, according to the current study, is 12.4%, a
number lower than the reported rates not only from Egypt but also from
other endemic hot spots in rural communities as in Nigeria (17.8%)[21]
and in Brazil (> 20.5%).[22] Indeed, this rate is lower than the
actual prevalence rates due to focusing on a subset of populations. In
Egypt, among the Nile Delta inhabitants, the same region investigated
in the current study, the prevalence was reported to be 37.7% in the
year 2000.[4] Furthermore, the prevalence rate is lower than the rates
among other high risk groups. Among fishermen in Brazil, it is 13.9%,
while among those of Manzala Lake in Egypt, the prevalence was 24.6%.
The lower prevalence rates reported in the current study can be
explained by health education and mass treatment campaigns practiced in
the country over the last years. In our study, previous Egyptian[8,9]
and international studies,[20,21] males predominate; because they were
more exposed. In our community, males are responsible for the family
earnings most of the time; they have greater employment in agricultural
work and higher contact with water.[23]
Intestinal manifestations
such as diarrhea and colicky pain and dysenteric features may pass
unnoticed, and asymptomatic forms of the infection are more
common.[24] With the infection progression, the chronic sequelae set up
with hepato-splenic affections development, established portal
hypertension occurs, and anemia becomes normochromic.[25] All patients
with confirmed schistosomal infection studied had a history of bleeding
per rectum and/or abdominal pain and consequent hypochromic sideropenic
anemia, and that was why they were referred for pan-colonoscopy.
In addition, 29.2% of them had clinically palpable hepatomegaly and/or
splenomegaly, which points to two crucial issues. First, patients are
symptomatic probably due to a severe infection, and patients had a
neglected ongoing long-term infection, which is why they had
hepato-splenic affection.[25]
Other studies reported different
rates of hepatomegaly and splenomegaly among inhabitants of S. mansoni
high-risk regions both in Egypt[4] and in Tanzania,[26] with
percentages of 22.3%, 20.8%, and 59.70%, 13.73%, respectively. These
figures are different from our figures of 4.2% and 25% due to the
advanced stage of the disease in our cohort with established periportal
fibrosis
We reported, 75% of them had schistosomal periportal
fibrosis, although half (50%) were of the mild form. This rate is much
higher than that of 13.79% found by Mazigo et al. in 2015[26] in
Tanzania. The difference is attributable to both the number and the
nature of participants. We enrolled only 193 high-risk sub-group with
clinical manifestations in our study compared to 1671 individuals
described as permanent inhabitants in their study.[26] Furthermore,
high prevalence rates of schistosomal periportal fibrosis were reported
from different subgroups and geographic locations in Egypt. In Gharbia
Governorate, a prevalence rate of >50% was reported,[19] while
remote governorates, e.g., Ismailia, reported a rate of 43%,[27] and
the pooled data from 5 governorates in lower Egypt reported a rate of
50.3%.[4] However, our higher prevalence rates were due to targeting a
high-risk symptomatic group of small sample size compared to the
general populations in the studies mentioned above.
In fact, in Egypt, hepatic periportal fibrosis is commonly seen with complicated S. mansoni
infection. However, confusion may occur with the presence of liver
cirrhosis.[28] Consequently, following our exclusion of patients with
established liver cirrhosis who may be confused for periportal
fibrosis, we can assume that our patients had schistosomal hepatic
periportal fibrosis.
Zaher et al. in 2011[29] encountered eggs of S. mansoni in
stool samples of 99 persons (0.33%) out of the 30,000 outpatients in
Egypt, while Gad et al. in 2011[30] found stool examination positive
for ova in 25 (9.83%) patients only out of 205 biopsy-positive
schistosomiasis cases in Egypt. Hence, we alarm practitioners in
endemic areas not to rely solely upon stool analysis for diagnosing
colonic schistosomiasis. They can ask for rectal sip examination or
colonoscopy and biopsy due to high positivity rates of 87.5% and 100%,
respectively, compared to 45.8% positivity of stool analysis as
reported in the current study.
Severe chronic intestinal
schistosomiasis may result in colonic or rectal polyposis, stenosis, or
present as an inflammatory mass that may be even confused with
cancer.[31-33] One of the crucial findings of the current study is its
ability to assess the colon both morphologically (by endoscopy) and
pathologically (by histology) to investigate the extent of S. mansoni
induced colon affection. On complete colonoscopy examination, 66.6% of
patients have significant lesions (polyps, ulcers, mass-like lesions)
while 33.4% had mild mucosal erythema and granularity.
Acute and
chronic inflammatory changes could be observed in the same colon
segment of chronic active schistosomal colitis patients.[23] This is
consistent with the findings of the current study, the majority of
patients (66.7%) had mucosal erythema and congestion consistent with
acute active infection, and at the same time, endoscopic features of
chronicity as polyps and masses were also observed.
An interesting
Egyptian study by Gad et al. in 2011[30] reported the prevalence of
colorectal schistosomiasis among patients with different gut symptoms
to be 20.83% by colonoscopy and biopsy. In this study, the authors
biopsied any suspected schistosomal lesion (n= 66) with additional 2
biopsies from the apparently normal rectal mucosa (n=139). The latter
two were examined by crush biopsy, while the other biopsies (average
3-6 per patient) were examined by histopathology. S. mansoni was detected in 205 patients out of 984 patients by colonoscopy.
Endoscopic
findings of the 205 confirmed cases reported by Gad et al.[30] with
schistosomiasis included patchy mucosal congestion (n=39, 19%), patchy
mucosal petechiae (n=11, 5.4%), patchy mucosal erosions +ulcers (n=5,
2.4%), patchy telangiectasia (n=5, 2.4%), sessile mucosal polyps at the
sigmoid colon (n=6,2.2.42%), and apparently normal mucosa (n=139). When
these figures were compared with our reported figures, we reported that
66.6% of patients have significant lesions (polyps, ulcers, mass-like
lesions), which means that our cohort suffered more. Furthermore, we
reported a finding lacking from their cohort; the mass-like lesions
related to schistosomiasis.
In the study of Gad et al.,[30] the
squash technique established the diagnosis of schistosomiasis in all
endoscopically apparently normal cases by demonstrating the
schistosomiasis ova with its characteristic lateral spine. In our
study, the squash technique demonstrated the schistosoma ova in 19
cases. In the two cases with mass-like lesions, the rectal snips were
negative.
In the study of Gad et al.[30] schistosomiasis
affected the rectum (n=25, 12.2%), sigmoid colon (n=26, 12.7%) or
rectum and colon (n=154, 75.1%), while in our study the figures were
12.5%, 25%, and 62.5% respectively. In addition, we reported lesions
beyond the recto-sigmoid in 18.2% of cases. The obvious differences
between our study and that of Gad et al. are probably related to the
nature of patients recruited. Patients of the current study were
high-risk group inhabiting a highly endemic area.
The presence of
colonic schistosomal polyposis does not appear to predispose patients
to significant bowel malignancy development.[33,34] There is agreement
among authors that S. mansoni
is not related to cancer colon. The reported cases of schistosomiasis
in patients with bowel malignancy are no more than epidemiological
association,[33-36] and this is consistent with findings of the current
study.
The high frequency of schistosomal polyps reported in the
current study (25%) is consistent with literature reports of high
frequency of schistosomal colonic polyps in Egypt compared with other
endemic regions like Brazil.[25] These findings of prevalent
schistosomal colon polyps should alarm the endoscopists working in
endemic areas to consider schistosomiasis in the differential diagnosis
of left-sided colonic polyps.[33]
One of the most important
retrospective studies[36] that focused on colonic schistosomiasis was
carried out in Saudi Arabia and recruited 216 patients with
schistosomal colonic disease out of 2458 who had sigmoidoscopy or
colonoscopy over 10 years, diagnosed by endoscopic biopsies (prevalence
rate of 8.8%). The colonoscopic appearance was suggestive of
schistosomiasis in 98 of these patients (45.37%), S. mansoni
ova in stool was detected in only 24 of these 216 patients (11.11%).
The most common histopathological finding in these patients' colonic
biopsies was S. mansoni ova
in the colonic mucosa with no or mild inflammatory cell infiltrates.
The most common symptoms were abdominal pain or distention reported in
84 patients (38.88%). Sixty-five patients (30.09%) had hepatosplenic
schistosomiasis. Eight patients (3.7%) had schistosomal polyps, and two
patients had colonic malignancy in which no association between their
malignancy and S. mansoni
infection was established. The authors concluded that colonoscopic
examination is valuable in colonic schistosomiasis as it can show
characteristic colonic lesions, and colonic biopsies are diagnostic and
correlate with histological findings.
Although we have some
agreements with this study[36] regarding the importance of colonoscopy
in diagnosing colonic schistosomiasis, the low yield of stool ova
detection, and schistosomal's benign nature colonic affection, we have
disagreements on other points. We have a higher frequency rate of
colonic schistosomiasis (12.4% vs. 8.8%); contrary to abdominal pain
and distention, our patients mainly presented with rectal bleeding,
they reported lower prevalence rates of hepato-splenic affection, and
also we had a higher frequency of schistosomal polyps (25% vs 3.7%). We
believe these differences are related to the endemicity of
schistosomiasis in Egypt compared to Saudi Arabia.
The safety of
endoscopic polypectomy for schistosomal colonic polyps among Egyptian
patients has long been documented as early as 1983.[38] This was
emphasized in the current study and in many previous
publications[29,30,36] with minimal risk of adverse events, similar to
the reports for the current study.
The current study showed the
histologic features of active schistosomiasis with the schistosomal
granulomatous reaction composed of multiple schistosomal ova surrounded
by an admixture of lymphocytes, eosinophils, macrophages, plasma cells,
and peripherally located fibroblasts (Figure 1)
and variable degrees of fibrosis. Many studies reported different
activity patterns and fibrosis,[30,36] which seems correlated with the
stage of infection, either acute or chronic.
This study had its
limitations, first, targeting only symptomatic individuals. The aim was
to determine the prevalence among complaining patients to highlight a
daily clinical practice and alarm clinicians in the area for the
persistence of this disease despite the great governmental efforts to
control it. The second limitation was targeting only a specific patient
group, and hence the prevalence rates reported in the current study
cannot be applied to the whole community. Third, the small sample size
of the affected patients; this probably related to targeting only a
specific category of patients. Fourth, it may underestimate the
prevalence among symptomatic rurals due to the exclusion of patients
with IBD and liver cirrhosis patients. However, this was valuable to
achieve the secondary end-points of the study. IBD and liver cirrhosis
are associated with morphological changes in the colon, which may
obscure the S. mansoni induced colon pathology. Furthermore, liver
cirrhosis may confuse the diagnosis of Schistosomal periportal
fibrosis. Furthermore, patients with structural colon damage as IBD and
liver cirrhosis may be excluded from the regular agricultural and
fishing activity in the area.
Conclusions
Out
of the 193 symptomatic rural inhabitants recruited to the current
study, we reported active schistosomiasis in 12.4%, with 66.6% of
patients had significant endoscopic colorectal lesions. All these data
points to the persistent transmission of schistosomiasis in the
Egyptian Nile Delta's rural community. However, the great success in
controlling this infection achieved by governmental efforts over the
past 4 decades with the collaboration of the health care system and
stakeholders should not discontinue screening programs, particularly
for high-risk groups, e.g., farmers, fishermen, etc. If not detected
and effectively treated, these individuals will represent a great
challenge for the diseases' resurgence because they are suitable for
breeding schistosomiasis through water channels and the snail
intermediate host.[3,7]
References
- Hotez PJ, Savioli L, Fenwick A. Neglected tropical
diseases of the Middle East and North Africa: review of their
prevalence, distribution and opportunities of control. PLoS Negl Trop
Dis 2012; 6:e1475-e1482. https://doi.org/10.1371/journal.pntd.0001475 PMid:22389729 PMCid:PMC3289601
- Hotez
PJ, Alvarado M, Basanez M-G, et al. The Global Burden of Disease Study
2010: interpretation and implications for the neglected tropical
diseases. PLoS Negl Trop Dis 2014; 8:e2865-e2873. https://doi.org/10.1371/journal.pntd.0002496 PMid:24873825 PMCid:PMC4038631
- El
Sharazly BM, Abou Rayia DM, Antonios SN, et al. Current status of
Schistosoma mansoni infection and its snail host in three rural areas
in Gharbia governorate, Egypt. Tanta Med J 2016; 44:141-50. https://doi.org/10.4103/1110-1415.201724
- El-Khoby
T, Galal N, Fenwick A, et al. The epidemiology of schistosomiasis in
Egypt: summary findings in nine governorates. Am J Trop Med Hyg 2000;
62:88-99. https://doi.org/10.4269/ajtmh.2000.62.88 PMid:10813505
- Barakat
MR, El-Morshedy H, Farghaly A. Human schistosomiasis in the Middle East
and North Africa region. In: McDowell MA, Rafati S, editors. Neglected
tropical diseases − Middle East and North Africa. Wien:
Springer-Verlag; 2014. 23-57. https://doi.org/10.1007/978-3-7091-1613-5_2
- Olveda DU, Li Y, Olveda RM, et al.. Bilharzia: Pathology, Diagnosis, Management and Control. Trop Med Surg. 2013 20;1(4):135. https://doi.org/10.4172/2329-9088.1000135 PMid:25346933 PMCid:PMC4208666
- Barakat RM. Epidemiology of Schistosomiasis in Egypt: Travel through Time: Review. J Adv Res. 2013;4(5):425-32. https://doi.org/10.1016/j.jare.2012.07.003 PMid:25685449 PMCid:PMC4293883
- Mohamed
AM, el-Sharkawi FM, el-Fiki SA. Prevalence of schistosomiasis among
fishermen of Lake Maryut. Egypt J Bilharz. 1978;5(1-2):85-90.
- Taman
A, El-Tantawy N, Besheer T, et al. Schistosoma mansoni infection in a
fishermen community, the Lake Manzala region-Egypt, As Pac J Trop
Dis,2014; 4(6): 463-468. https://doi.org/10.1016/S2222-1808(14)60607-1
- Haggag
AA, Rabiee A, AbdElaziz KM, et al. Mapping of Schistosoma mansoni in
the Nile Delta, Egypt: Assessment of the prevalence by the circulating
cathodic antigen urine assay. Acta Trop. 2017;167:9-17. https://doi.org/10.1016/j.actatropica.2016.11.038 PMid:27965144
- Doenhoff
MJ, Chiodini PL, Hamilton JV. Specific and sensitive diagnosis of
schistosome infection: can it be done with antibodies? Trends Parasitol
2004; 20:35-39. https://doi.org/10.1016/j.pt.2003.10.019 PMid:14700588
- Katz
N, Chaves A, Pellegrino J. A simple device for quantitative stool thick
smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo
1972; 14:397-400.
- Abdel-Wahab MF, Esmat
G, Farrag A, et al. Grading of hepatic schistosomiasis by the use of
ultrasonography. Am J Trop Med Hyg. 1992;46(4):403-8. https://doi.org/10.4269/ajtmh.1992.46.403 PMid:1575286
- Harries
AD, Speare R. Rectal snips in the diagnosis of hepatosplenic
schistosomiasis, Transactions of The Royal Society of Tropical Medicine
and Hygiene, 1988; 82(5): 720. https://doi.org/10.1016/0035-9203(88)90213-1
- Shipkey FH. Squash technique for rapid identification of schistosoma ova. Ann Saudi Med. 1986;6:71-2. https://doi.org/10.5144/0256-4947.1986.71 PMid:21164245
- Gray DJ, Ross AG, Li YS, et al. diagnosis and management of schistosomiasis. BMJ. 2011 17;342:d2651. https://doi.org/10.1136/bmj.d2651 PMid:21586478 PMCid:PMC3230106
- Li
Y, Sleigh AC, Williams GM, et al. Measuring exposure to Schistosoma
japonicum in China. III. Activity diaries, snail and human infection,
transmission ecology and options for control. Acta Trop 2000;75:279-89.
https://doi.org/10.1016/S0001-706X(00)00056-5
- Othman AA, Soliman RH. Schistosomiasis in Egypt: A never-ending story? Acta Trop. 2015; 148:179-90. https://doi.org/10.1016/j.actatropica.2015.04.016 PMid:25959770
- El-Hawey
AM, Amer MM, Abdel Rahman AH, et al. The epidemiology of
schistosomiasis in Egypt: Gharbia Governorate. Am J Trop Med Hyg 2000;
62:42-48. https://doi.org/10.4269/ajtmh.2000.62.42 PMid:10813499
- Melo,
Andrea Gomes Santana de, Irmão, José Jenivaldo de Melo, Jeraldo,
Verónica de Lourdes Sierpe, et al. Schistosomiasis mansoni in families
of fishing workers of endemic area of Alagoas. Escola Anna Nery, 2019;
23(1), e20180150. https://doi.org/10.1590/2177-9465-ean-2018-0150
- Dawaki
S, Al-Mekhlafi HM, Ithoi I, et al. PREVALENCE AND RISK FACTORS OF
SCHISTOSOMIASIS AMONG HAUSA COMMUNITIES IN KANO STATE, NIGERIA. Rev
Inst Med Trop Sao Paulo. 2016 11;58:54. https://doi.org/10.1590/S1678-9946201658054 PMid:27410914 PMCid:PMC4964323
- Conceição
MJ, Carlôto AE, de Melo EV, et al. Prevalence and Morbidity Data on
Schistosoma mansoni Infection in Two Rural Areas of Jequitinhonha and
Rio Doce Valleys in Minas Gerais, Brazil. ISRN Parasitol. 2013
19;2013:715195. https://doi.org/10.5402/2013/715195 PMid:27335859 PMCid:PMC4890927
- El
Malatatwy A., El Habashy A., LechineN.,et al. Selective population
chemotherapy among school children in Beheira Governate: the
UNICEF/Arab Republic of Egypt/WHO Schistosomiasis Contrl Project. Bull
World Health Organization. 1992;70:47-56.
- Elbaz T, Esmat G. Hepatic and intestinal schistosomiasis: review. J Adv Res. 2013;4(5):445-52. https://doi.org/10.1016/j.jare.2012.12.001 PMid:25685451 PMCid:PMC4293886
- Da Silva LC, Chieffi PP, Carrilho FJ.Schistosomiasis mansoni -- clinical features. Gastroenterol Hepatol. 2005;28(1):30-9. https://doi.org/10.1157/13070382 PMid:15691467
- Mazigo
HD, Dunne DW, Morona D, et al. Periportal fibrosis, liver and spleen
sizes among S. mansoni mono or co-infected individuals with human
immunodeficiency virus-1 in fishing villages along Lake Victoria
shores, North-Western, Tanzania. Parasit Vectors. 2015 7;8:260. https://doi.org/10.1186/s13071-015-0876-4 PMid:25948238 PMCid:PMC4424565
- Nooman
ZM, Hasan AH, Waheeb Y, et al. The epidemiology of schistosomiasis in
Egypt: Ismailia governorate. Am J Trop Med Hyg. 2000;62(2 Suppl):35-41.
https://doi.org/10.4269/ajtmh.2000.62.35 PMid:10813498
- Abdel-Kader
S, Amin M, Hamdy H, et al. Causes of minimal hepatic periportal
fibrosis present in Egypt. J Egypt Soc Parasitol. 1997;27(3):919‐924.
- Zaher
T, Abdul-Fattah M, Ibrahim A, et al. Current Status of Schistosomiasis
in Egypt: Parasitologic and Endoscopic Study in Sharqia Governorate.
Afro-Egypt J Infect Endem Dis 2011; 1(1):9-11. https://doi.org/10.21608/aeji.2011.8754
- Gad
YZ, Ahmad NA, El-Desoky I, et al. Colorectal schistosomiasis: Is it
still endemic in delta Egypt, early in the third millennium?. Trop
Parasitol 2011;1:108-10. https://doi.org/10.4103/2229-5070.86948 PMid:23508170 PMCid:PMC3593472
- Ross AGP, Bartley PB, Sleigh AC, et al. schistosomiasis. N Eng J Med 2002;346:1212-9. https://doi.org/10.1056/NEJMra012396 PMid:11961151
- Gryseels B, Polman K, Clerinx J, et al. Human schistosomiasis. Lancet 2006; 368:1106-18. https://doi.org/10.1016/S0140-6736(06)69440-3
- Elbatee HE, Emara MH, Zaghloul MS, et al. Huge bilharzial polyp mimicking colon cancer. JGH Open. 2019 12;4(2):280-283. https://doi.org/10.1002/jgh3.12181 PMid:32280778 PMCid:PMC7144792
- Barsoum H. Cancer in Egypt: its incidence and clinical forms. Acta Uni Intern Con Can. 1953;9:241-250.
- Salim
HO, Hamid HK, Mekki SO, et al. Colorectal carcinoma associated with
schistosomiasis: a possible causal relationship. World J Surg Oncol.
2010;8:68. https://doi.org/10.1186/1477-7819-8-68 PMid:20704754 PMCid:PMC2928231
- Mohamed AR, al Karawi M, Yasawy MI. Schistosomal colonic disease. Gut. 1990;31(4):439-42. https://doi.org/10.1136/gut.31.4.439 PMid:2110925 PMCid:PMC1378420
- Emara
MH, Ahmed MH, Mahros AM, et al. No part of the colon is immune from
large Bilharzial polyps. Eur J Gastroenterol Hepatol.
2020;32(7):896‐897. https://doi.org/10.1097/MEG.0000000000001727 PMid:32472818
- Bessa SM, Helmy I, El-Kharadly Y. Colorectal schistosomiasis. Endoscopic polypectomy. Dis Colon Rectum. 1983;26(12):772-4.) https://doi.org/10.1007/BF02554745 PMid:6641458
[TOP]



