Monia Marchetti1, Robert Peter Gale2 and Giovanni Barosi3.
1 Hematology Department, Azienda Ospedaliera Antonio e Biagio e Cesare Arrigo, Alessandria, Italy.
2 Haematology Research Centre, Department of Immunology and Inflammation, Imperial College London, London, UK.
3 Center for the Study of Myelofibrosis, IRCCS Policlinico S. Matteo Foundation, Pavia, Italy.
Correspondence to: Monia Marchetti, MD, PhD. Hematology
Department, Az. Osp. Antonio e Biagio e Cesare Arrigo, via Venezia 16,
15121 Alessandria, Italy. P +39 3668377191 E-mail:
moniamarchettitamellini@gmail.com
Published: May 1, 2021
Received: January 20, 2021
Accepted: April 12, 2021
Mediterr J Hematol Infect Dis 2021, 13(1): e2021034 DOI
10.4084/MJHID.2021.034
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Considerable
data indicate post-transplant lenalidomide prolongs progression-free
survival and probably survival after an autotransplant for multiple
myeloma (MM). However, optimal therapy duration is unknown,
controversial and differs in the EU and US. We compared outcomes
and cost-effectiveness of 3 post-transplant lenalidomide strategies in
EU and US settings: (1) none; (2) until failure; and (3) 2-year fixed
duration. We used a Markov decision model, which included six
health states and informed by published data. The model estimated
the lenalidomide strategy given to failure achieved 1.06
quality-adjusted life years (QALYs) at costs per QALY gained of €29,232 in the EU and $133,401 in the US settings. Two-year fixed-duration lenalidomide averted €7,286 per QALY gained in the EU setting and saved 0.84 QALYs at $60,835 per QALY gained in the US setting. These highly divergent costs per
QALY in the EU and US settings resulted from significant differences in
post-transplant lenalidomide costs and 2nd-line therapies driven by
whether post-transplant failure was on or off-lenalidomide. In
Monte Carlo simulation analyses which allowed us to account for the
variability of inputs, 2-year fixed-duration lenalidomide remained the
preferred strategy for improving healthcare sustainability in the EU
and US settings.
|
Introduction
High-dose chemotherapy, typically with melphalan followed by a haematopoietic cell autotransplant, is the global standard-of-care in persons < 65-70 years with multiple myeloma (MM).[1-5]
Substantial data indicate post-transplant lenalidomide prolongs
post-transplant progression-free survival (PFS) and probably survival
without reducing quality-of-life (QoL) or increasing interval-to-progression after starting subsequent anti-MM therapy/ies.[6-15]
Based on these data, post-transplant lenalidomide is approved in the EU
and US by the European Medicines Agency (EMA) and US Food and Drug
Administration (FDA).
Precisely how long to continue lenalidomide
post-transplant is controversial. Two considerations, besides
therapy-outcome and cost, affect this calculus. First, some data,
albeit controversial, suggest an increased risk of new cancers in
persons receiving continuous post-transplant lenalidomide leading some
experts, especially in the EU, to recommend giving post-transplant
lenalidomide for 1 or 2 years.[8] In contrast,
the strategy in the US is to give post-transplant lenalidomide until
failure. These strategies are not compared in randomized trials,
so there is no evidence-based way to decide which is better.
The 2nd consideration is cost. On 1st
examination giving continuous post-transplant lenalidomide seems more
expensive than the no or fixed duration lenalidomide strategies.
However, this conclusion fails to consider other critical confounding
issues. Because high-dose chemotherapy with autotransplant is not
curative, most, if not all, recipients relapse or progress. Their
subsequent anti-MM therapy will depend on circumstances of therapy
failure. For example, persons failing whilst receiving
post-transplant lenalidomide are likely to be treated with drugs other
than lenalidomide. In contrast, a person failing after no or after
stopping fixed duration post-transplant lenalidomide is likely to
receive lenalidomide-based therapies. Consequently, a critical
economic analysis must consider the cost not only of post-transplant
lenalidomide but also costs of drugs used to treat therapy failure and
their anticipated clinical outcomes.
We compared
consequences of 3 potential post-transplant interventions: (1) no
intervention; (2) 2-year fixed-duration lenalidomide; and (3)
lenalidomide until failure (relapse or progression). These
strategies were compared in EU and US cost settings. Our analysis
considered not only clinical outcomes such as interval from
autotransplant to first progression or death from any cause (PFS1), the
interval from autotransplant to second progression or death (PFS2) and
interval from the start of rescue therapy to second progression or
death (2nd PFS), survival and costs but also costs of subsequent therapy/ies.
Methods
Decision problem and scope. We interrogated the problem of assessing the cost-for-value of
2-year fixed-duration or continuous post-transplant lenalidomide in
persons with MM by comparing these strategies with no post-transplant
intervention. The economic assessment is conducted from the
perspective of the third-party payers in the EU and US.
Model details.
We used a 6-state Markov model, which allowed us to follow the monthly
evolution of subjects from progression-free on-lenalidomide to
progression-free off-lenalidomide, 1st subsequent therapy, 2nd subsequent therapy and death (Figure 1).
We modelled subjects with a median age of 58 years based on data from
randomized trials included in the meta-analysis providing baseline
PFS1.[6] Subjects should have had a partial or complete response 90 days after their autotransplant.
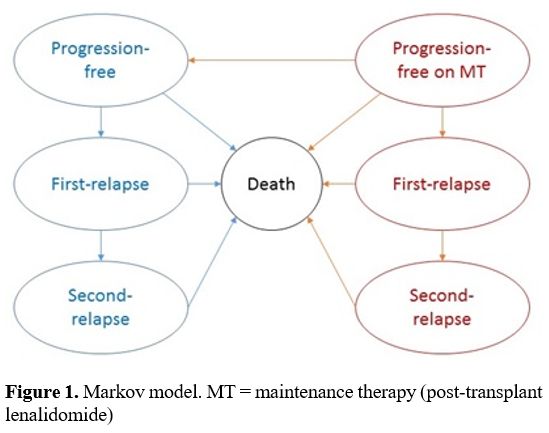 |
Figure 1. Markov model. MT = maintenance therapy (post-transplant lenalidomide)
|
The
progression rate in subjects receiving no post-transplant lenalidomide
was assessed in two-time intervals based on PFS1 curves reported in a
meta-analysis.[6] An exponential parametric assumption was made to allow model reproducibility.
The
rate of progression in subjects on post-transplant lenalidomide was
estimated by adapting the hazard ratio reported by the above
intention-to-treat meta-analysis6 since we considered the possibility
post-transplant lenalidomide might be stopped because of an adverse
event(s) (Table 1),[6,16,18-23,25,26] progression or planned interruption because of a 2-year fixed-duration post-transplant lenalidomide strategy.
The
relative risk of relapse or progression in subjects stopping
lenalidomide for reasons other than relapse or progression was returned
to 1 if post-transplant lenalidomide duration was < 12 months,
whereas it was decreased progressively as post-transplant lenalidomide
duration lengthened beyond 12 months (Table 1) as reported in a retrospective study[16] and a randomized trial.[34] Probabilities of 2nd and 3rd progression were obtained from recent clinical trials (Table 1). The fatality rate was estimated to be 12, 40 and 60 per cent at 1st, 2nd and 3rd failure.[24]
We
assumed subjects relapsing or progressing post-transplant would next
receive a therapy based on carfilzomib or daratumumab. Lenalidomide
triplets were allowed for subjects failing off post-transplant
lenalidomide. A 1:1 ratio was assumed in assigning subjects to a
daratumumab- or carfilzomib-based treatment. Nighty per cent of
subjects with a 1st relapse or progression were assumed to receive a 2nd therapy, and 80% of subjects with a 2nd relapse to receive a 3rd line therapy.[17]
Subjects were assigned 1:1 to a pomalidomide-based or a daratumumab- or
carfilzomib-based therapy according to prior therapy. The modelled
strategies were reported in Supplementary Table 1.
Utilities. Utilities were adapted from a study mapping EORTC QLO-30 and an MM-specific quality-of-life (QoL)
questionnaire to EQ5D-based utilities.25 We also considered the
impact of being on-therapy, including post-transplant lenalidomide.[25]
Costs.
Costs were considered in EU and US settings. We used a third payer
perspective and included only direct medical costs given in 2018 EU and
US euros and dollars. Anti-MM therapies were valued according to ex-factory drug costs for EU and wholesale US cost (Table 2).[27-33] A 3 per cent additional cost was considered for parenteral drugs.[31-33]
Theoretical drug costs were reduced by 10 per cent because of treatment
schedules and therapy-free months between progression and start of
subsequent therapy/ies (Table 2). Post-transplant lenalidomide's monthly cost was calculated for a 21 of 28-day schedule at 10 mg per day.
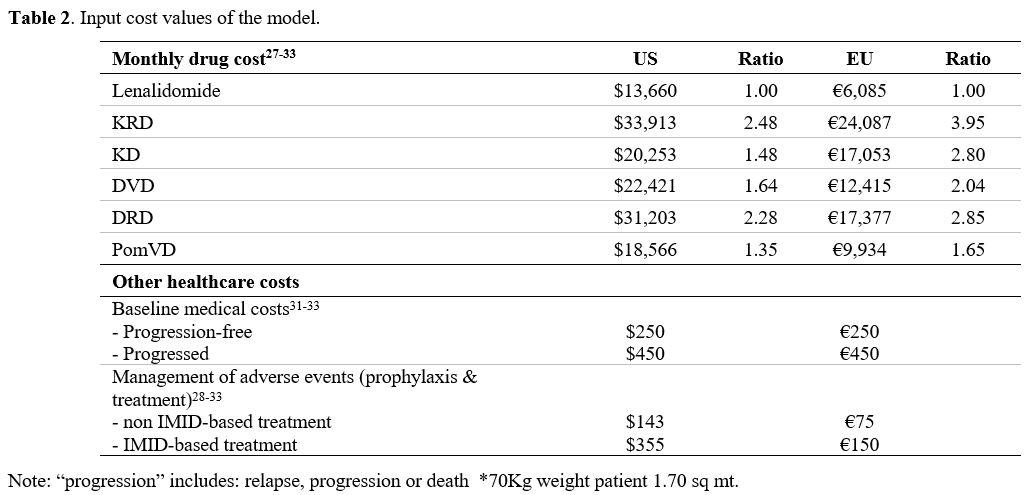 |
Table 2. Input cost values of the model.
|
Analyses. Mean costs and mean effectiveness were calculated as discounted costs and discounted quality-adjusted years-of-life (QALYs)
associated with each clinical state. Analysis of life years and
costs was limited to a 20-year time horizon which is ≥ twice the median
survival reported for persons not receiving post-transplant
lenalidomide.6 According to international guidelines, life years and
costs were discounted by 3 per cent per year.[15]
First-order sensitivity analyses were run for all input co-variates and
for ratios amongst co-variates. Furthermore, scenario analyses explored
extreme ranges for key variables. Second-order sensitivity analysis was
run for each paired comparison; 10,000 Monte Carlo simulations were run
by sampling log-normal distributions for hazard ratios, beta
distributions for utilities, and gamma distributions for cost.
Results
Model validation.
The model forecasted 70%, 52%, and 29% of persons assigned to
continuous lenalidomide remained on-therapy after 12, 24 and 48
months. The median therapy duration was 25 months, and the mean
duration of therapy 30 months in a 79-month time horizon (39 months in
a 20-year horizon). Corresponding rates in a meta-analysis were
70%, 54% and 15% and the mean post-transplant therapy duration 28
months at a median follow-up of 79 months.[6] The
model also forecasted mean lenalidomide duration in the 2-year
fixed-duration cohort was 18 months like that reported for Arm A1 in
the GMMG-MM5 randomized trial.[34]
The model
predicted median PFS1 like data from the meta-analysis for no
intervention and continuous lenalidomide strategies, 23 and 52 months.[6]
Notably, the model did not over-estimate long-term outcomes, which was
an 80-month PFS of 31% and survival of 67% for persons receiving
continuous lenalidomide. The model also forecasted a 5-year PFS of 36%
and survival of 76% for persons receiving 2-year fixed-duration
lenalidomide like data from the GMMG-MM5 trial (arms A1 and A2).[34]
Second
PFS was estimated to be 23 and 36 months for persons failing on- or
off-lenalidomide, respectively. Similarly, median survival after
the first failure was estimated as 45 and 60 months,
respectively. These survival rates are like those reported in the
GMMG-MM5 trial and in a recent pooled analysis of randomized trials,
including continuous post-transplant lenalidomide.[34,39] Finally, the model estimated median survival after 2nd
failure of 28 months. Median PFS2 was 84 months for continuous
lenalidomide, 82 months for 2-year fixed duration lenalidomide and 63
months for no post-transplant therapy. These figures are higher than
reported by the McCarthy meta-analysis because of the assumption
currently available highly effective 2nd-line therapies are prescribed.[6]
Baseline analysis.
At baseline analyses, continuous and 2-year fixed-duration
post-transplant lenalidomide prolonged median survival from 97 to 119
and 113 months, indicating a 6-month advantage for the continuous
strategy compared with fixed-duration. Mean life-years and
quality-adjusted life-years for the three strategies are displayed in Table 3:
continuous post-transplant lenalidomide prolonged mean survival by 21.5
months and fixed-duration by 16.0 months. After adjusting for quality
of life, the two strategies' gain was 17.2 and 12.7 quality-adjusted
months, respectively.
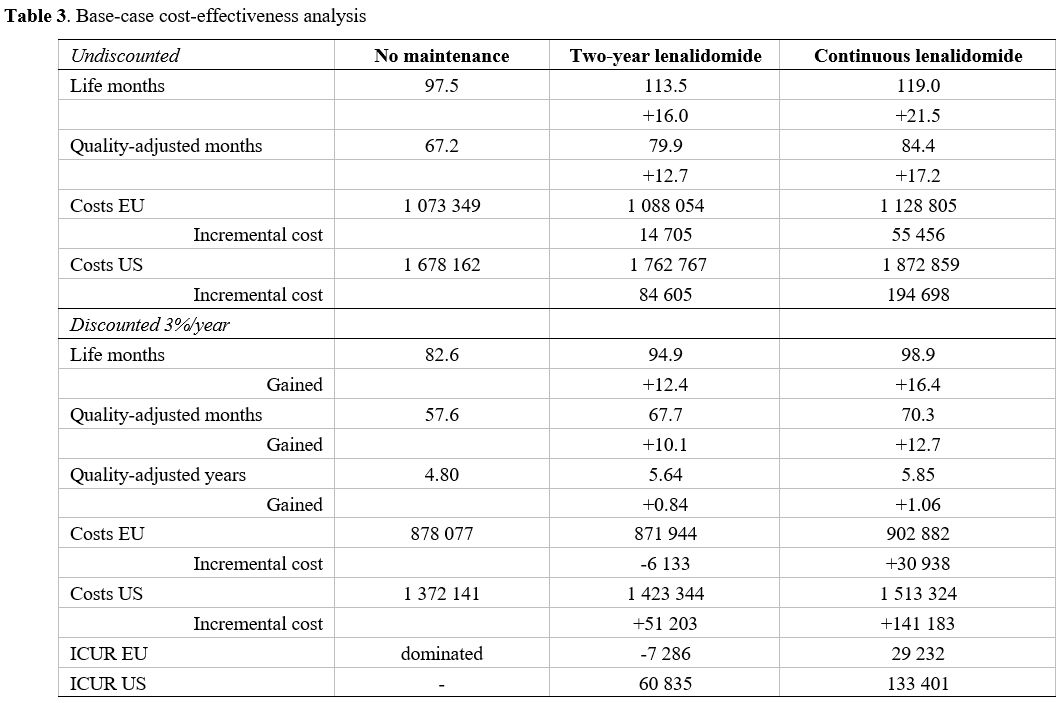 |
Table 3. Base-case cost-effectiveness analysis
|
Discounting
of future life years further reduced the gain of post-transplant
strategies to 12.7 and 10.1 months, respectively, which is about a 40%
decrease of the gain.
Cumulative health-care costs for managing
post-transplant MM ranged from €1,073,349 to €1,128,805 in EU and from
$1,678,162 to $1,872,859 in US in the 20 year time horizon chosen for
the analysis. Breakdown of costs (Supplementary Figure 1)
reported that 16% (EU) and 22% (US) of the overall healthcare costs of
the continuous post-transplant lenalidomide strategy were from to costs
of lenalidomide. The same rates were 10% (EU) and 15% (US) for 2-year
fixed duration lenalidomide. 3rd-line therapies accounted for 17-20% of
overall costs, whereas 2nd-line therapies accounted for 59-77% of
overall costs. By avoiding some 1st
failures, 2-year fixed duration strategy saved $146,045 (€88,112) and
continuous lenalidomide saved $194,705 (€117,010). Continuous
lenalidomide avoided > $200,000 (€120,000) of further therapy costs,
but this is 54% and 73% of the post-transplant lenalidomide drug
cost. Fixed-duration post-transplant lenalidomide avoided >
$150,000 dollars and > €110,000 in the US and EU settings.
These are 62% and 104% of the drug cost for post-transplant
lenalidomide. Consequently, post-transplant lenalidomide's
resulting incremental cost was especially favourable for the 2-year
fixed-duration strategy and even more favourable in the EU setting
because the largest part of post-transplant costs was offset by avoided
2nd-line costs.
Future healthcare costs discounting further
reduced incremental costs of 2-year fixed-duration post-transplant
lenalidomide because more subjects assigned to this strategy receive
higher-cost drug triplets at 1st failure. Consequently, in the EU
setting, 2-year fixed-duration post-transplant lenalidomide reduced net
healthcare cost and avoided €7,286 in costs for every QALY saved.
In contrast, continuous post-transplant lenalidomide achieved 1 QALY at
the cost of €29,232. In the US setting, 2-year fixed-duration
post-transplant lenalidomide increased discounted healthcare costs by
$60,835 per QALY saved, whereas continuous post-transplant lenalidomide achieved each QALY at the cost of $133,401.
Sensitivity analyses. We tested the results' sensitivity to different time horizons and multiple input co-variates (Figure 2).
Results were highly sensitive to the time horizon, the monthly cost of
lenalidomide, and the cost of 2nd-line and subsequent
therapy/ies. However, 2-year fixed-duration lenalidomide
maintained a favourable incremental cost per
QALY gained < €50,000 in the EU setting even in persons with a low
risk of early relapse or progression, such as individuals achieving a
complete post-transplant response.[38,40] Similarly, in the US setting, 2-year fixed-duration lenalidomide maintained an incremental cost per QALY gained < $150,000 despite extreme-range sensitivity analysis.
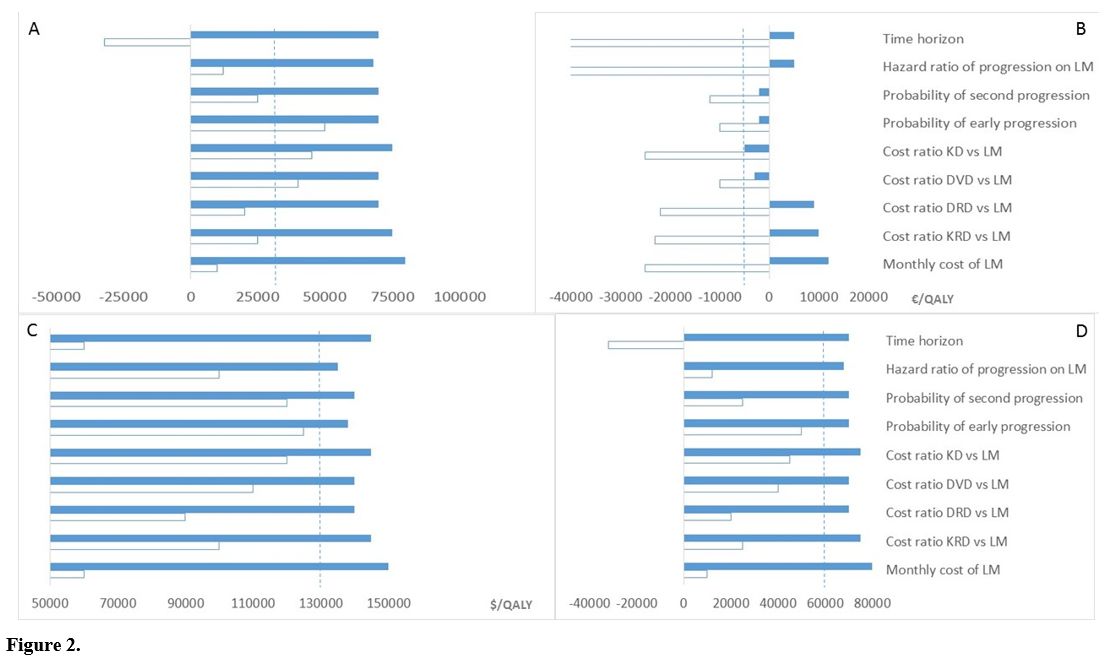 |
Figure 2
|
Relative costs were the major driver of the incremental cost per
QALY saved: the higher the ratio between 2nd-line lenalidomide-based
therapies versus post-transplant lenalidomide, the greater the economic
benefit of post-transplant lenalidomide. For cost ratios of
carfilzomib, lenalidomide, dexamethasone (KRD) > 4.1 and
daratumumab, lenalidomide, dexamethasone (DRD) > 3.0 continuous
post-transplant lenalidomide was cost saving in the EU setting.
Similarly, for cost ratios of DRD > 2.8 and KRD >
3.1, 2-year fixed-duration lenalidomide was cost-saving in the US
setting.
Two-way sensitivity analysis display chances for
post-transplant strategies to be cost-effective (incremental cost <
$100,000 per QALY) derive from the interplay between lenalidomide monthly cost and the cost ratio of 2nd-line therapies (Figure 3). Therefore,
continuous lenalidomide is potentially cost-effective for lower monthly
lenalidomide cost and higher KRD and DRD cost ratios, as happens in the
EU setting. In contrast, 2-year fixed duration lenalidomide may
be cost-effective even at higher lenalidomide cost and lower KRD and
DRD cost ratios, as in the US setting.
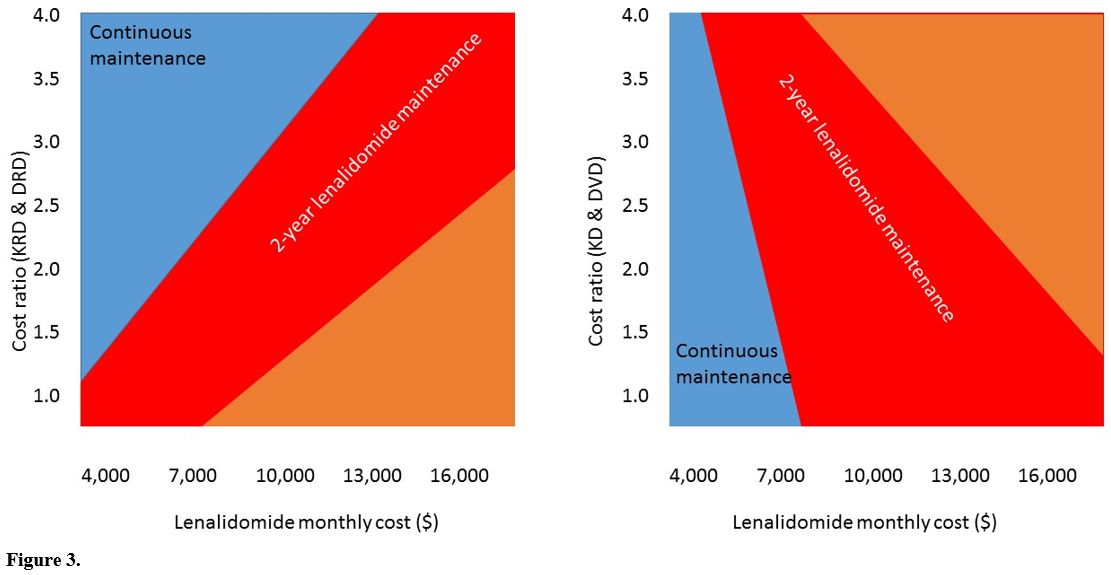 |
Figure 3
|
Our
study tested different post-transplant strategies in cohorts of
subjects in whom individual probabilities of post-transplant failure
are unknown and for whom we have only estimated with reasonably wide 95
per cent confidence intervals. However, different persons in these
cohorts have different probabilities of post-transplant failure. If
these probabilities could be accurately predicted on the subject-level,
it would be possible to predict the most cost-effective strategy for
that person. Monte Carlo simulation analysis (10,000 runs) allowed
us to simultaneously assess multiple input variables' effect on the
results and track several individual outcomes as displayed by the
scatterplots in Supplementary Figure 2. Continuous
post-transplant lenalidomide had a 62% probability of achieving a QALY
at a cost < €50,000 in the EU setting, whereas in the US, the
probability of achieving one QALY at < $100,000 was only
42%. 2-year fixed-duration lenalidomide had an 81% probability of
achieving a QALY at a cost < €50,000 in the EU setting and a 69%
probability of achieving a QALY at a cost < $100,000 in the US
setting.
Scenario analyses.
We tested the sensitivity of the results to extreme variations of five
input variables in order to test the variability of the results
according to different settings, namely patient age and therapeutic
choices for second and third line. Based on different survival rates in
patients younger than 50 years,45 we modelled patients younger than 50
years by decreasing fatality rates by 50% and patients older than 65
years by increasing fatality rates by 50%. Table 4
shows that, as expected, both continuous post-transplant lenalidomide
maintenance and two-year lenalidomide have a markedly better
cost-utility in younger patients: despite a better cost-utility profile
of two-year maintenance, continuous lenalidomide maintenance was also
cost-saving in this clinical subgroup.
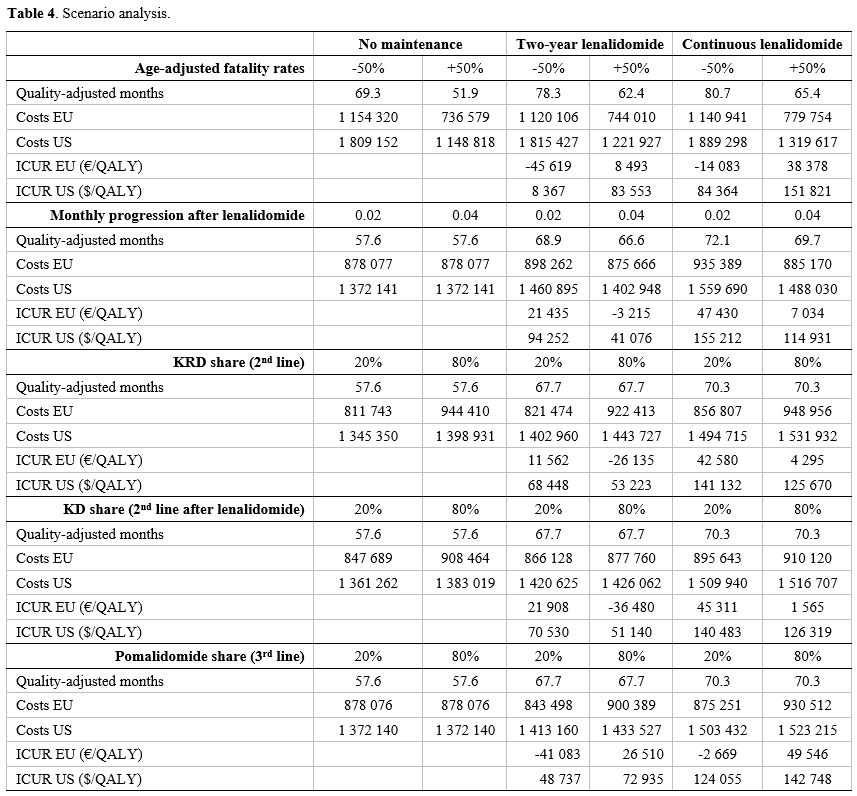 |
Table 4. Scenario analysis.
|
We also tested extremely low (20%) and extremely high (80%) shares of KRD, KD and pomalidomide in the second and third line. Table 4
shows that the two maintenance strategies might report a better
cost-utility in case of a lower carfilzomib share in the second line
and a lower pomalidomide share in the third line.
Finally, we
tested whether a strongly shorter PFS2 after lenalidomide might change
the results: a PFS2 of 18 months, corresponding to a monthly rate of
progression of 0.04 ameliorates the cost-utility profile of both the
maintenance strategies. Therefore, continuous lenalidomide might still
be a cost-effective option in those patients for whom a shorter PFS2 is
expected.
Discussion
In
persons with MM receiving an autotransplant, giving post-transplant
lenalidomide until relapse or progression prolongs median PFS and
survival by about 2 years.[6] Put otherwise, about 5
persons need to receive post-transplant lenalidomide for 2 years to
avoid one relapse or progression over a 5-year horizon. Achieving
this gain involves the cost of post-transplant lenalidomide and
subsequent therapy/ies.[31,32,41]
However, analyzing the cost of post-transplant lenalidomide is
complex. Issues include: (1) numbers needed to treat to avoid
failure; (2) duration; and (3) post-failure outcomes and
interventions.
Post-transplant maintenance's optimal
duration is unknown: direct and indirect data from prospective studies
report a prolonged failure-free period after stopping post-transplant
lenalidomide in persons receiving it failure-free for > 2 years.[16,34,39]
These data suggest a fixed-duration strategy of post-transplant
lenalidomide might be as effective at a lower cost compared with
continuous post-transplant lenalidomide. Because of this
possibility, we compared the cost-effectiveness of different
post-transplant strategies: (1) no intervention; (2) continuous
post-transplant lenalidomide; and (3) 2-year fixed-duration
lenalidomide. The model was based on simplified modelling of
failure rates and costs but calibrated to provide survival rates and
mean post-transplant lenalidomide durations like published randomized
trials.[6,34]
Outputs of our model indicate continuous lenalidomide is cost-effective in the EU setting but costs more than $100,000 per
QALY in the US setting. 2-year fixed-duration lenalidomide
significantly prolonged PFS and quality-adjusted survival at an
acceptable cost per life-year
gained in EU and US settings. In the EU setting, 2-year fixed-duration
lenalidomide reduced overall healthcare costs in the baseline 20-year
horizon. Different costs between the EU and US settings resulted
predominately from cost ratios for 2nd-line and subsequent therapy/ies
compared with post-transplant lenalidomide cost.[42]
Sensitivity
analyses of the model highlighted some interesting issues. First,
economic advantages driven by the lower rate of failure while receiving
post-transplant lenalidomide were more evident in shorter time
horizons. In the long-term, advantages were partially balanced by
the healthcare costs for subsequent therapy/ies. Second, the
incremental cost per QALY gained by post-transplant lenalidomide versus
no intervention was highly dependent on subsequent therapy/ies
costs. Higher costs for therapies containing lenalidomide or
pomalidomide in persons failing after stopping post-transplant
lenalidomide favoured giving post-transplant lenalidomide whereas
higher costs for subsequent therapy(ies) without lenalidomide or
pomalidomide in persons failing while receiving lenalidomide were
against post-transplant lenalidomide (Figure 2, Figure 3). Third, there was an increase in the cost-for-benefit ratio of post-transplant lenalidomide as the rate of 2nd
failure increased in persons previously failing off-lenalidomide. We
also tested other lenalidomide fixed-durations, including 1- and 3-year
fixed-durations with no substantial change in our conclusions.
Our analysis focused on cost-effectiveness, typically expressed as cost per
QALY. However, this widely accepted approach does not consider the
economic value of a quality life saved, termed the value of a
statistical life (VSL), which is about €225,000 ($250,000) per
year. In our analysis, lenalidomide given until failure saves more
lives than 2-year fixed-duration lenalidomide but at a considerable
cost per QALY saved. The
2-year fixed duration strategy in the EU saves substantial health care
costs. In the US setting, it results in substantially less cost per
QALY. Neither calculation is adjusted for VSL saved, which may be an
important offset to some patients, families, physicians, policy-makers,
and societies.
Our study has several limitations. 1st, the results
have no universal value because they depend on the time horizon adopted
and country-specific drug costs.[43] 2nd, our analyses used a 3rd-
party payer perspective but did not consider indirect costs from
productivity loss, a relevant social burden for young persons with MM.[44] 3rd, unit costs of treatments resembled ex-factory
costs and not true acquisition costs. This could result in relevant
mismatches. Finally, we did not cover model costs of palliative and
end-of-life care.
Conclusions
Our modelling indicates the most favourable value-for-cost of
post-transplant lenalidomide in persons with MM is associated with a
2-year fixed-duration strategy. However, continuous lenalidomide
maintenance showed an acceptable cost-utility in younger patients and
in those for whom a shorter PFS2 is expected. Definite conclusions
require validation in controlled clinical trials, which consider
safety, efficacy, and cost.
We compared our results with other published clinical and economic outcomes of continuous post-transplant lenalidomide (Supplementary Figure 2 and Table 2). These
studies used partitioned survival but considered different health
states and comparators. All studies included survival data from
the CALBG 100104 study, whereas 2 studies included data from the IFM
trial or other studies (Supplementary Table 3). Time
horizons were also different, ranging from 10 years to a
lifetime. Consequently, incremental life-years gained ranged from
1 to 3.64 years. Overall incremental costs ranged from €147,707 to
$476,690 and incremental cost per QALY from €30,709 to €277,456.
References
- Mikhael J, Ismaila N,
Cheung N, et al. treatment of multiple myeloma: ASCO and CCO joint
clinical practice guideline. J Clin Oncol. 2019;37:1228-1263. doi:
10.1200/JCO.18.02096 https://doi.org/10.1200/JCO.18.02096
PMid:30932732
- Jain T, Sonbol MB,
Firwana B, et al. High-dose chemotherapy with early autologous stem
cell transplantation compared to standard dose chemotherapy or delayed
transplantation in patient with newly diagnosed multiple myeloma: a
systematic review and meta-analysis. Biol Blood Marrow Transplant.
2019;25(2):239-247. doi: 10.1016/j.bbmt.2018.09.021 https://doi.org/10.1016/j.bbmt.2018.09.021
PMid:30244101
- Su B, Zhu X, Jiang Y, et
al. A meta-analysis of autologous transplantation for newly diagnosed
multiple myeloma in the era of novel agent. Leuk Lymphoma.
2019;60(6):1381-1388. doi: 10.1080/10428194.2018.1543874. https://doi.org/10.1080/10428194.2018.1543874
PMid:30516074
- Dhakal B, Szabo A,
Chhabra , et al. Autologous transplantation for newly diagnosed
multiple myeloma in the era of novel agent induction: a systematic
review and meta-analysis. JAMA Oncology. 2018;4(3):343-350. doi:
10.1001/jamaoncol.2017.4600. https://doi.org/10.1001/jamaoncol.2017.4600
PMid:29302684 PMCid:PMC5885822
- Barosi G, Gale RP. Is
lenalidomide the standard-of-care after an autotransplant for multiple
myeloma? Leukemia. 2019;33(3):588-596. doi: 10.1038/s41375-019-0383-2. https://doi.org/10.1038/s41375-019-0383-2
PMid:30692596
- McCarthy PL, Holtein SA,
Petrucci MT, et al. Lenalidomide maintenance after autologous stem-cell
transplantation in newly diagnosed multiple myeloma: a meta-analysis. J
Clin Oncol. 2017:35(29):3279-3289. doi: 10.1200/JCO.2017.72.6679 https://doi.org/10.1200/JCO.2017.72.6679
PMid:28742454 PMCid:PMC5652871
- Holstein SA, Jung SH,
Richardson PG, et al. Updated analysis of CALBG (Alliance) 100104
assessing lenalidomide versus placebo maintenance after single
autologous stem-cell transplantation for multiple myeloma: a
randomized, double-blind, phase 3 trial. Lancet Haematol.
2017;4(9):e431-e442. doi: 10.1016/S2352-3026(17)30140-0. https://doi.org/10.1016/S2352-3026(17)30140-0
- Diamond B, Mclachlan K,
Chung DJ, et al. Maintenance therapy and need for cessation studies in
multiple myeloma: focus on the future. Best Pract Res Clin Hematol.
2002:33(1):101140. doi: 10.1016/j.beha.2020.101140. https://doi.org/10.1016/j.beha.2020.101140
PMid:32139006
- Mian I, Milton DR, Shah
N, et al. Prolonged survival with a longer duration of maintenance
lenalidomide after autologous hematopoietic stem cell transplantation
for multiple myeloma. Cancer. 2016;122(24):3831-3837. doi:
10.1002/cncr.30366 https://doi.org/10.1002/cncr.30366
PMid:27680710 PMCid:PMC5138119
- Jagannath S, Abonous R,
Durie BGM, et al. impact of post-ASCT maintenance therapy on outcomes
of patients with newly diagnosed multiple myeloma in Connect MM. Blood
Adv. 2018;2(13):1608-1615. doi: 10.1182/bloodadvances.2018017186 https://doi.org/10.1182/bloodadvances.2018017186
PMid:29986853 PMCid:PMC6039656
- Fonseca R, Parikh K,
Ung B, Ni Q, Argawal A. Maintenance after lenalidomide, bortezomib, and
dexamethasone induction with newly diagnosed multiple myeloma and
high-risk cytogenetics: an enhanced medical record analysis.
HemaSphere. 2018;2:230-231.
- Cherniawsky H, Sandhu
I, Chu M, et al. Lenalidomide maintenance chemotherapy: an analysi of
real world data in multiple myeloma patient treated with autologous
stem cell transplant and bortezomib-based induction. HemaSphere
2018;2(supplement 2): 968
- Tay J, Vij R, Norkin M.
Impact of active maintenance treatment (MT) compared with no MT on the
quality of life (QOL) of patients with multiple myeloma (MM) following
first autologous stem cell transplant (ASCT). Blood. 2017; 130
(Supplement 1).
- Kumar SK, Dispenzeri A,
Fraser R, et al. Early relapse after autologous hematopoietic cell
transplantation remains a poor prognostic factor in multiple myeloma
but outcomes have improved over time. Leukemia. 2018;32(4):986-995.
doi: 10.1038/leu.2017.331 https://doi.org/10.1038/leu.2017.331
PMid:29263438 PMCid:PMC5871538
- Attema AE, Brouwer WBF,
Claxton K. Discounting in economic evaluations. Pharmacoeconomics.
2018;36(7):745-758. doi: 10.1007/s40273-018-0672-z https://doi.org/10.1007/s40273-018-0672-z
PMid:29779120 PMCid:PMC5999124
- Amsler IG, Jeker B,
Taleghani BM, et al. Prolonged survival with increasing duration of
lenalidomide maintenance after autologous transplant for multiple
myeloma. Leukemia Lymphoma. 2019;60(2):511-514. doi:
10.1080/10428194.2018.1473577 https://doi.org/10.1080/10428194.2018.1473577
PMid:30616438
- Offidani M, Morè S,
Corvatta L, et al. Factors associated with the probability to skip
subsequent lines of therapy: analysis of 321 multiple myeloma (MM)
patients in a single tertiary centre. Clin Lymphoma Myeloma Leuk.
2019;19 (supplement 10): e275 https://doi.org/10.1016/j.clml.2019.09.453
- Dimopoulos MA,
San-Miguel J, Belch A, et al. Daratumumab plus lenalidomide and
dexamethasone versus lenalidomide and dexamethasone in relapsed or
refractory multiple myeloma: updated analysis of POLLUX. Haematologica.
2018;103(12):2088-96. doi: 10.3324/haematol.2018.194282 https://doi.org/10.3324/haematol.2018.194282
PMid:30237262 PMCid:PMC6269302
- Spencer A, Lentzsch S,
Weisel K, et al. Daratumumab plus bortezomib and dexamethasone versus
bortezomib and dexamethasone in relapsed or refractory multiple
myeloma: updated analysis of CASTOR. Haematologica. 2018;103(12):
2079-2087. doi: 10.3324/haematol.2018.194118 https://doi.org/10.3324/haematol.2018.194118
PMid:30237264 PMCid:PMC6269293
- Stewart AK, Rajkumar
SV, Dimopoulos MA, et al. Carlfizomib, lenalidomide, and dexamethasone
for relapsed multiple myeloma. N Engl J Med 2015;372(2):142-152. doi:
10.1056/NEJMoa1411321 https://doi.org/10.1056/NEJMoa1411321
PMid:25482145
- Dimopoulos MA, Moreau
P, Palumbo A, et al. Carlfizomib and dexamethasone versus bortezomib
and dexamethasone for patients with relapsed or refractory multiple
myeloma (ENDEAVOUR): a randomized, phase 3, open-label, multicenter
study. Lancet Oncol. 2016;17(1):27-38. doi:
10.1016/S1470-2045(15)00464-7 https://doi.org/10.1016/S1470-2045(15)00464-7
- Richardson PG, Oriol A,
Beksac M, et al. Pomalidomide, bortezomib, and dexamethasone for
patients with relapsed or refractory multiple myeloma previously
treated with lenalidomide (OPTIMISMM): a randomized, open-label, phase
3 trial. Lancet Oncol. 2019;20(6):781-94. doi:
10.1016/S1470-2045(19)30152-4 https://doi.org/10.1016/S1470-2045(19)30152-4
- Moreau P, Joshua D,
Chng W-J, et al. impact of prior treatment on patients with relapsed
multiple myeloma treated with carfilzomib and dexamethasone vs
bortezomib and dexamethasone in the phase 2 ENDEAVOR study. Leukemia.
2017;31(1):115-122. doi: 0.1038/leu.2016.186 https://doi.org/10.1038/leu.2016.186
PMid:27491641 PMCid:PMC5220137
- Felix J, Aragao F,
Almeida JM, et al. Time-dependent endpoints as predictors of overall
survival in multiple myeloma. BMC Cancer. 2013;13:122.
10.1186/1471-2407-13-122. https://doi.org/10.1186/1471-2407-13-122
PMid:23497363 PMCid:PMC3607860
- Proskorovsky I, Lewis
P, Williams CD, et al. Mapping EORTC QLQ-C30 and QLQ-MY20 to EQ-5D in
patients with multiple myeloma. Health Qual Life Outcomes. 2014;12:1-9.
doi: 10.1186/1477-7525-12-35. https://doi.org/10.1186/1477-7525-12-35
PMid:24618388 PMCid:PMC4007827
- Acaster S, Gaugris S,
Velikova G, Yong K, Lloyd A. Impact of the treatment-free interval on
health-related quality of life in patients with multiple myeloma: a UK
cross-sectional survey. Supportive Care Cancer. 2013;21(2):599-607.
doi: 10.1007/s00520-012-1548-y https://doi.org/10.1007/s00520-012-1548-y
PMid:22886429
- Carlson JJ, Guzauskas
GF, Chapman RH, et al. Cost-effectiveness of drugs to treat
relapsed/refractory multiple myeloma in the United States. J Manag Care
Spec Pharm. 2018;24(1):29-38. doi: 10.18553/jmcp.2018.24.1.29. https://doi.org/10.18553/jmcp.2018.24.1.29
PMid:29290170
- Hollmann S, Moldaver D,
Goyert N, Grima D, Maiese EM. A US cost analysis of triplet regimens
for patients with previously treated multiple myeloma. J Manag Care
Spec Pharm. 2019;25(4):449-59. doi: 10.18553/jmcp.2019.25.4.449. https://doi.org/10.18553/jmcp.2019.25.4.449
PMid:30917078
- Zhang TT, Wang S, Wan
N, et al. Cost-effectiveness of daratumumab-based triplet therapies in
patients with relapsed or refractory multiple myeloma. Clin Ther.
2018;40(7):1122-1139. doi: 10.1016/j.clinthera.2018.05.012 https://doi.org/10.1016/j.clinthera.2018.05.012
PMid:30006069
- Jakubowiak AJ, Houisse
I, Majer I, et al. Cost-effectiveness of carlfizomib plus dexamethasone
compared with bortezomib plus dexamethasone for patients with relapsed
or refractory multiple myeloma in the United States. Expert Rev
Hematol. 2017;10(2):1107-1119. doi: 10.1080/17474086.2017.1391088. https://doi.org/10.1080/17474086.2017.1391088
PMid:29027825
- Gonzalez-McQuire S,
Young K, Leleu H, et al. Healthcare resource utilization among patients
with relapsed multiple myeloma in the UK, France, and Italy. J Med
Econ. 2018;21(5):450-467. doi: 10.1080/13696998.2017.1421546 https://doi.org/10.1080/13696998.2017.1421546
PMid:29278014
- Ashcroft J, Judge D,
Dhanasiri S, Taylor-Stokes G, Middleton C. Chart review across EU5 in
MM post-ASCT patients. Int J Hematol Oncol. 2018;7:IJH05 https://doi.org/10.2217/ijh-2018-0004
PMid:30302236 PMCid:PMC6176952
- Pelligra CG, Parikh K,
Abouzaid S, Ailawadi S. Cost-effectiveness of pomalidomide,
carlfizomib, and daratumumab for the treatment of patients with heavily
pretreated relapsed-refractory multiple myeloma in the United States.
Clin Ther. 2017;398(10):1986-2005. Doi: 10.1016/j.clinthera.2017.08.010
https://doi.org/10.1016/j.clinthera.2017.08.010
PMid:28967482
- Goldschmidt H, Mai EK,
Durig J, et al. Response-adapted lenalidomide maintenance in newly
diagnosed myeloma: results from the phase III GMMG-MM5 trial. Leukemia
2020 (in press https://doi.org/10.1038/s41375-020-0724-1) https://doi.org/10.1038/s41375-020-0724-1
PMid:32034285
- Olry de Labry Lima A,
Gimeno-Ballester V, Rios Tamayo R, et al. Cost-effectiveness of
lenalidomide maintenance in patients with multiple myeloma who have
undergone autologous transplant of hematopoietic progenitor cells. Bone
Marrow Transplant. 2019;54(11):1908-1919. doi:
10.1038/s41409-019-0574-5 https://doi.org/10.1038/s41409-019-0574-5
PMid:31150015
- Zhou Z-Y, Parikh K,
Chai X, et al. cost-effectiveness analysis of lenalidomide for
maintenance therapy after autologous stem cell transplant (ASCT) in
newly diagnosed multiple myeloma (NDMM) patients: a United States payer
perspective. Blood. 2018;132 (suppl 1). https://doi.org/10.1182/blood-2018-99-112942
- Uyl-de Groot CA, Ramsen
R, Boersma J, et al. Lenalidomide as maintenance treatment for patients
with newly diagnosed multiple myeloma post-autologous stem cell
transplantation: a pharmacoeconomic assessment in the Netherlands.
Blood. 2018;132 (suppl 1). https://doi.org/10.1182/blood-2018-99-112826
- Lehners N, Becker N,
Benner A, Pritsh M, Lopprich M, Mai EK. Analysis of long-term survival
in multiple myeloma after first-line autologous stem cell
transplantation: impact of clinical risk factors and sustained
response. Cancer Med. 2018;7(2): 307-316. doi: 10.1002/cam4.1283. https://doi.org/10.1002/cam4.1283
PMid:29282899 PMCid:PMC5806105
- Bonello F, Pulini S,
Ballanti S, et al. Lenalidomide maintenance with or without prednisone
in newly diagnosed myeloma patients: a pooled analysis. Cancers
(Basel). 2019;11(11):E1735. doi: 10.3390/cancers11111735. https://doi.org/10.3390/cancers11111735
PMid:31694338 PMCid:PMC6896192
- Usmani SZ, Hoering A,
Cavo M, et al. Clinical predictors of long-term survival in newly
diagnosed transplant eligible multiple myeloma - an IMWG Research
project. Blood Cancer J. 2018;8(12): 123. Doi:
10.1038/s41408-018-0155-7. https://doi.org/10.1038/s41408-018-0155-7
PMid:30470751 PMCid:PMC6251924
- Boccadoro M, Usmani SZ,
Chari A, et al. A global treatment standard in multiple myeloma (MM)
remains elusive despite advances in care over 15 years first results
from insight mm, the largest global perspective, observational MM
study. HemaSphere. 2018;2; supplement 2 (591-)
- Hewitt C, Foxon G,
Craddy P, Chunara F. ICERs are not all the same. How cost-effectiveness
estimates differ beween the UK and US. Value in Health. 2018; 21;
supplement 1 (S20-) https://doi.org/10.1016/j.jval.2018.04.189
- Marchetti M. Value of
innovation for haematologic malignancies. J Med Econ.
2016;19(5):487-489. doi: 10.3111/13696998.2015.1133429 https://doi.org/10.3111/13696998.2015.1133429
PMid:26706602
- Jackson G, Galinsky J,
Alderson DEC, et al. Productivity losses in patients with newly
diagnosed multiple myeloma following stem cell transplantation and the
impact of maintenance therapy. Eur J Haematol. 2019;103(4):393-401.
doi: 10.1111/ejh.13298 https://doi.org/10.1111/ejh.13298
PMid:31325331 PMCid:PMC6899492
- Kristinsson SY,
Anderson WF, Landgren O. Improved long-term survival in multiple
myeloma up to the age of 80 years. Leukemia. 2014; 28: 1346-1348. doi:
doi:10.1038/leu.2014.23 https://doi.org/10.1038/leu.2014.23
PMid:24418994
Supplementary Data
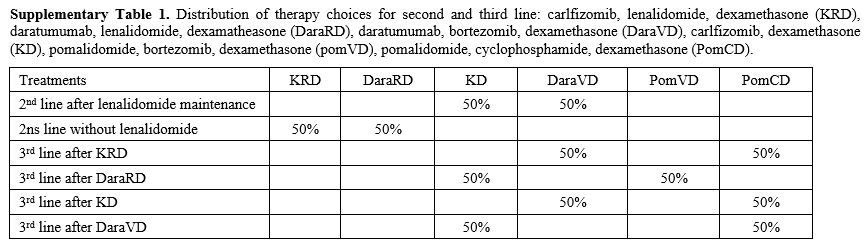 |
Supplementary Table 1. Distribution of
therapy choices for second and third line: carlfizomib, lenalidomide,
dexamethasone (KRD), daratumumab, lenalidomide, dexamatheasone
(DaraRD), daratumumab, bortezomib, dexamethasone (DaraVD), carlfizomib,
dexamethasone (KD), pomalidomide, bortezomib, dexamethasone (pomVD),
pomalidomide, cyclophosphamide, dexamethasone (PomCD). |
 |
Supplementary Table 2. Literature search strategy. |
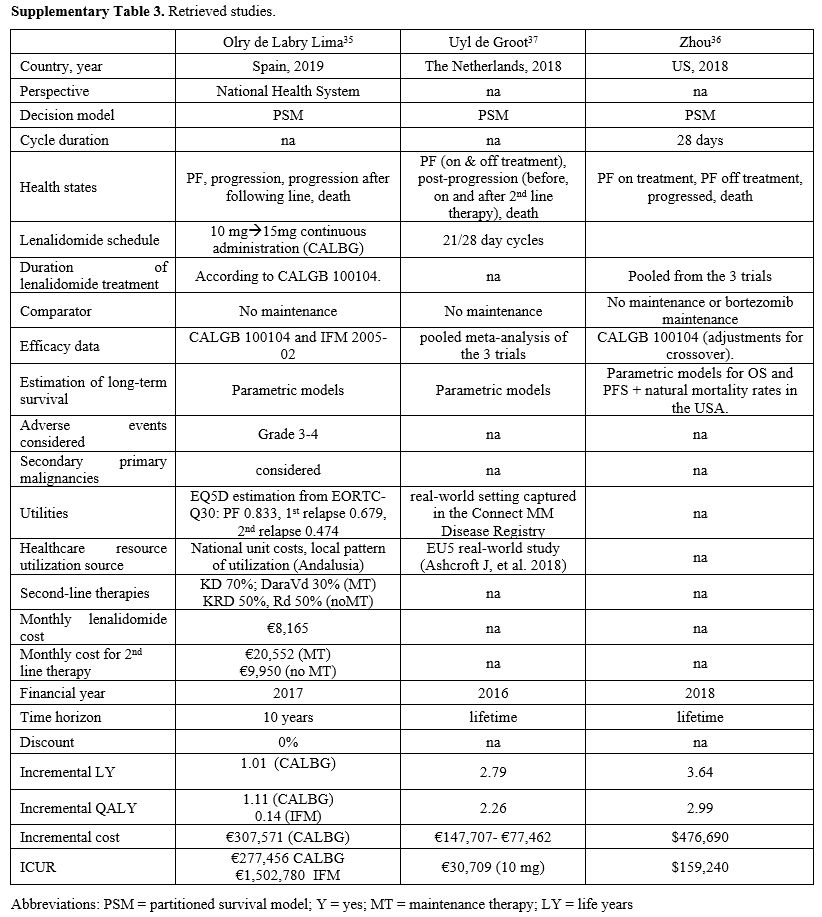 |
Supplementary Table 3. Retrieved studies. |
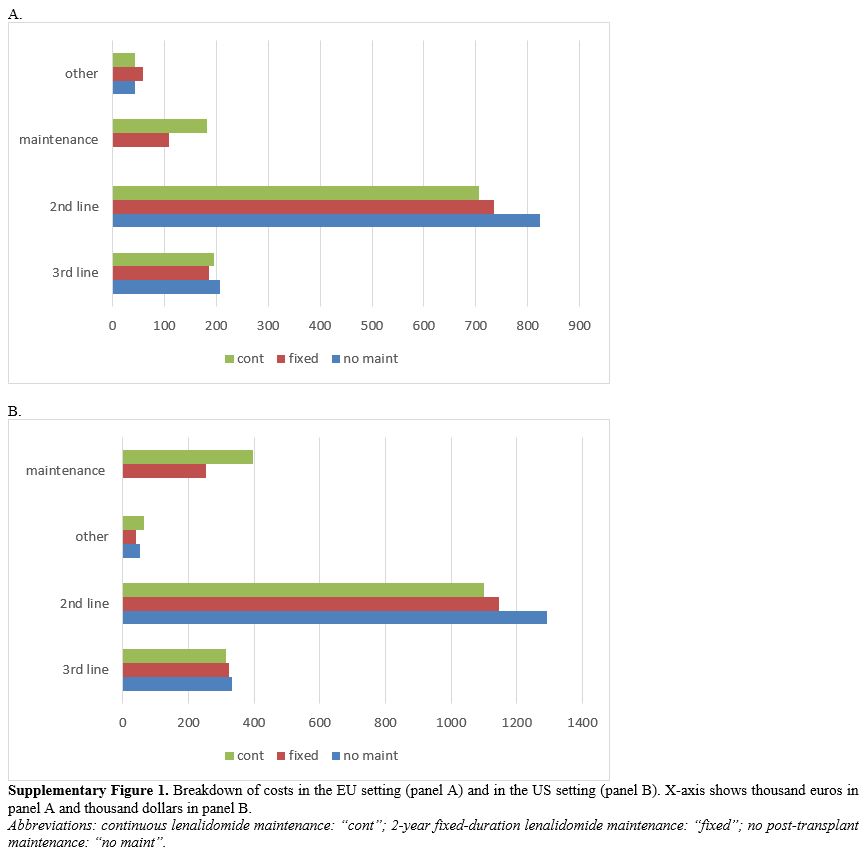 |
Supplementary Figure
1. Breakdown of costs in the EU setting (panel A) and in the US
setting (panel B). X-axis shows thousand euros in panel A and thousand
dollars in panel B.
Abbreviations:
continuous lenalidomide maintenance: “cont”; 2-year fixed-duration
lenalidomide maintenance: “fixed”; no post-transplant maintenance: “no
maint”.
|
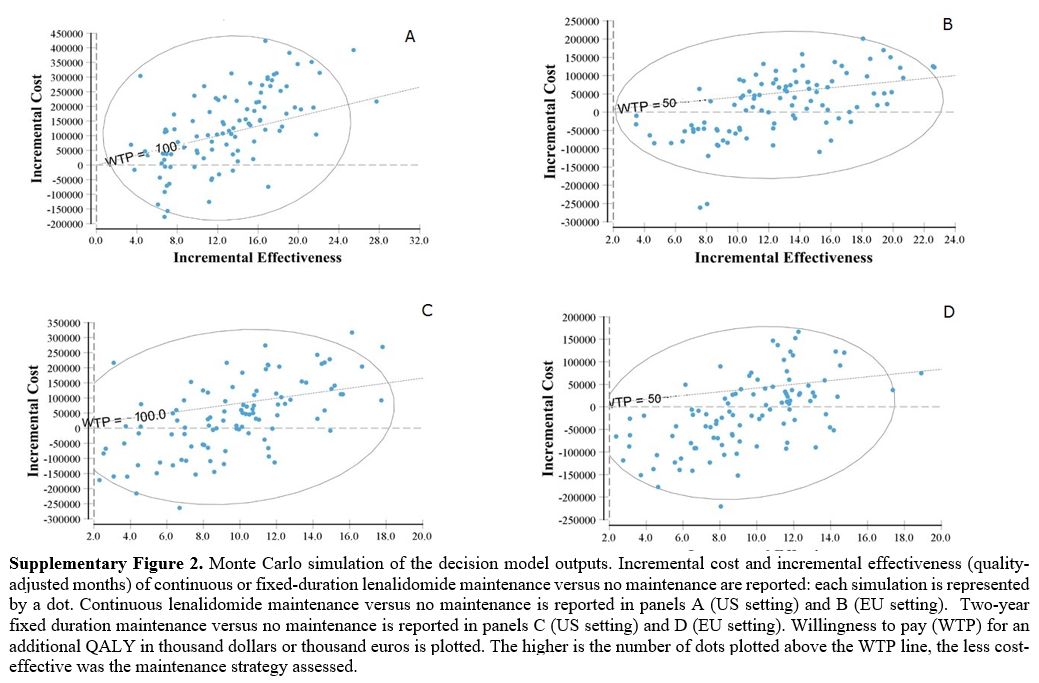 |
Supplementary Figure 2. Monte
Carlo simulation of the decision model outputs. Incremental cost and
incremental effectiveness (quality-adjusted months) of continuous or
fixed-duration lenalidomide maintenance versus no maintenance are
reported: each simulation is represented by a dot. Continuous
lenalidomide maintenance versus no maintenance is reported in panels A
(US setting) and B (EU setting). Two-year fixed duration maintenance
versus no maintenance is reported in panels C (US setting) and D (EU
setting). Willingness to pay (WTP) for an additional QALY in thousand
dollars or thousand euros is plotted. The higher is the number of dots
plotted above the WTP line, the less cost-effective was the maintenance
strategy assessed. |
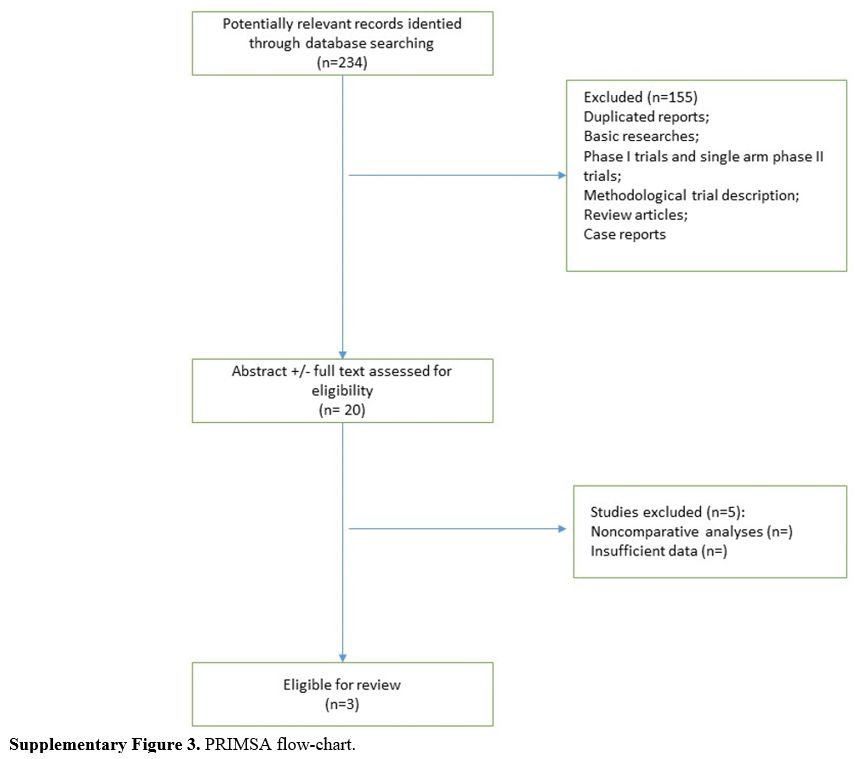 |
Supplementary Figure 3. PRIMSA flow-chart. |
[TOP]