Abdolreza
Sotoodeh Jahromi1, Mohammad Kargar1*,
Farshid Kafilzadeh1, Marzieh Jamalidoust2
and Maliheh Moradzadeh3*.
1
Department of Microbiology, Jahrom Branch, Islamic Azad University,
Jahrom, Iran
2
Department of Clinical Virology, Clinical Microbiology Research Center,
Shiraz University of Medical Sciences, Namazi Hospital, Shiraz, Iran.
3 Golestan Rheumatology Research Center, Sayad
Shirazi Hospital, Golestan University of Medical Sciences, Gorgan, Iran.
* Both corresponding authors contributed and
supervised equally to this research work and manuscript.
Correspondence to: Mohammad Kargar, Department of
biology,
Jahrom Branch, Islamic Azad University, Jahrom, Iran Tel: 09173149203
Fax: 071 5437 2001 Email:
microkargar@gmail.com
Maliheh
Moradzadeh, Golestan Rheumatology Research Center, Sayad Shirazi
Hospital, Gorgan, Iran Telefax: + 98 17 32239791 Email:
Moradzadeh63@yahoo.com
Published: July 1, 2021
Received: February 13, 2021
Accepted: June 18, 2021
Mediterr J Hematol Infect Dis 2021, 13(1): e2021049 DOI
10.4084/MJHID.2021.049
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
As a major carotenoid in saffron, crocin demonstrates potent
anti-cancer impacts. However, its anti-lymphoma effects remain vague,
especially in the human EBV-associated B-cell lymphoproliferative
disorders. This study examined crocin's apoptogenic potential and its
underlying mechanism in CO 88BV59-1 cell line vs. normal human
peripheral blood B cells.
Methods:
CO 88BV59-1 cells were treated with crocin alone or in combination with
vincristine for up to 72 h. The cell viability was examined using a
resazurin assay. Flow cytometry using annexin V and propidium iodide
labeling was performed to detect apoptotic cells. Also, the expression
levels of genes and proteins involved in apoptosis (CASP3, CASP8,
CASP9, P53, Bax, and Bcl-2) were respectively determined via real-time
PCR and Western blot analysis.
Results:
Crocin concentration-dependently reduced cell viability in CO 88BV59-1
cells with no significant toxicity toward normal B cells. Similar to
vincristine, crocin significantly increased apoptosis in these cells
during 72 h of incubation. Furthermore, the combination of crocin (80
μM) and vincristine (1 μM) enhanced apoptosis in CO 88BV59-1 cells.
Therefore, this synergistic effect was detected in human
EBV-transformed B-lymphocyte. CASP3, CASP9, P53, and Bax/Bcl-2 ratio
expressions were significantly raised in CO 88BV59-1 cells, whereas
CASP8 was unaltered. It was proposed that crocin promoted apoptosis in
CO 88BV59-1 cells in a time- and concentration-dependent manner via the
induction of the intrinsic pathway.
Conclusion:
The results suggest that crocin may serve as a good
alternative/coadjuvant to vincristine in EBV-associated B-cell
lymphoproliferative disorders.
|
Introduction
B-cell
lymphomas associated with Epstein-Barr virus (EBV) infection are common
in patients who have immunodeficiency states, e.g., organ
transplantation and human immunodeficiency virus (HIV) infection.
Reports show that 1.8% of global cancer deaths in 2010 are related to
EBV-attributable malignancies (Approximately 142,979).[1]
The three main types of B cell malignancy associated with EBV are the
Burkitt, Hodgkin, and diffuse large B-cell lymphomas (respectively BL,
HL, and DLBCL). These pre-neoplastic/neoplastic lesions appearing in
transplant patients are collectively referred to as post-transplant
lymphoproliferative disorders (PTLD). The majority of PTLD cases are B
cell, but 5-10% T and NK cell lymphoma have been described.[2]
EBV inflicts >95% of the world's population and leads to
lifelong
asymptomatic infection. Its ability to induce oncogenesis may occur
because of the suppression of the immune system or uncontrolled
proliferation. The virus increases the activity of the B cell lymphoma
2 (Bcl-2) gene, which promotes cell proliferation by curbing apoptosis.
The upregulation of the Bcl-2 gene can decrease the activity of tumor
suppressor genes, including P53.[3]
Apoptosis is
an evolutionary conserved, intrinsic program of cell death that occurs
in various physiological and pathological states. The underlying
mechanisms for initiating an apoptosis response upon cytotoxic therapy
may depend on the individual stimulus and damage to DNA; however,
damage to DNA or other critical molecules is considered a common
initial event propagated by the cellular stress response. Apoptosis
pathways can be initiated through different entry sites, for example,
at the plasma membrane by death receptor ligation (named receptor or
extrinsic pathway) or at the mitochondria (mitochondrial or intrinsic
pathway). Bcl-2, P53, Bax, Caspase-9 (CASP9) are involved in the
intrinsic pathway, and CASP8 in the extrinsic pathway.[4]
Introducing
rituximab plus cyclophosphamide, doxorubicin, vincristine, and
prednisone (R-CHOP) to treat B-cell lymphoma can markedly improve
patients' survival rate. Nevertheless, resistance to these drugs and
their toxicity are significant obstacles to the course of treatment.
Therefore, there is an urgent need for the development of novel
therapeutic medications.[5]
Researchers held that
dietary phytochemical agents might affect chemotherapy and contribute
to the treatment of cancer patients.[6]
According to some studies, phytochemicals isolated from medicinal
plants inhibited cell proliferation and induced apoptosis.[7]
At present, some of these plant-derived compounds are widely applied in
chemotherapy for cancer treatment. For instance, taxol analogs, vinca
alkaloids (vincristine and vinblastine), and podophyllotoxin analogs
have greatly contributed to cancer treatment.[8]
Plant-derived compounds such as carotenoids play a pivotal role against
cancer, providing a valuable source of anti-cancer agents. Increasing
evidence suggests that crocin, a carotenoid isolated from the saffron
plant (Crocus sativus
L), has anti-cancer effects in various types of
cancer.[9] Like vincristine,
crocin's anti-proliferative activity involves targeting microtubules[10] or p53- dependent and -independent
mechanisms in cancer cells.[11,12]
In our laboratory, we observed that crocetin, a hydrolyzed form of
crocin, exerts anti-proliferative, pro-apoptotic, and
pro-differentiating impacts on human leukemia cells by inhibiting
protein kinase B (Akt)-mediated pro-survival cascades, raising the
intracellular Bcl-2-like protein 4 (Bax)/Bcl-2 ratio, and reducing
Tyrosyl-DNA phosphodiesterase 1 (TDP1) enzyme activity and the
expressions of promyelocytic leukemia-retinoic acid receptor alpha
(PML-RARα), Histone deacetylase 1 (HDAC1), and Multidrug resistance
(MDR)-associated proteins.[13,14]
Our laboratory
has been interested in crocin's application in lymphoma and leukemia.
Still, the exact mechanism of its action against EBV-associated B-cell
lymphomas remains unknown. Thus, herein, a series of experiments were
designed to examine crocin's apoptogenic potential and its underlying
mechanisms in CO 88BV59-1 EBV-transformed B-lymphocyte vs. normal human
B cells.
Materials and Methods
Cell
line and reagents. Human CO 88BV59-1 EBV-transformed
B-lymphocyte (CRL-10624™)
was purchased from ATCC (USA). The high-glucose Roswell Park Memorial
Institute medium (RPMI 1640), penicillin-streptomycin, and fetal bovine
serum (FBS) were obtained from Gibco BRL Life Technologies (USA).
Moreover, 7-hydroxy-3H-phenoxazin-3-one-10-oxide (resazurin), crocin
(>95%), vincristine (>95%), Fluorescein isothiocyanate
(FITC)
annexin V antibody, and propidium iodide (PI) were procured from
Sigma-Aldrich (USA). TRIzol was obtained from Invitrogen (USA). A
real-time PCR Master Mix and a cDNA synthesis Kit were also purchased
from Roche Diagnostic (Switzerland) and Fermentas (Lithuania).
Moreover, an enhanced chemiluminescence (ECL) detection kit and
polyvinylidene difluoride (PVDF) membranes were purchased from GE
Healthcare (UK) and Millipore (USA), in that order. Primary antibodies
for β-actin, Bcl-2, Bax, P53, cleaved caspases (3, 8, and 9), and
secondary antibodies were obtained from Cell Signaling Technology
(USA). Fluorescein isothiocyanate-conjugated antibody against CD19 was
also purchased from BioLegend (USA). Finally, a human B cell isolation
kit was obtained from Miltenyi Biotec (Germany).
Human
normal B cell isolation and cell culture.
Human peripheral blood mononuclear cells (PBMCs) were obtained from
fresh blood samples taken from healthy volunteers by Ficoll-density
(Pharmacia, Sweden) gradient centrifugations. Subsequently, B-cells
were isolated from PBMC via a B-cell isolation kit. Then, the purity of
the cellular preparation was tested via FITC-conjugated anti-human CD19
antibody staining in the MACS analysis, and it was found to be
>97%
pure (Figure 1).[15]
CO 88BV59-1 and normal B cells were also cultured in the RPMI medium
containing 10% (v/v) FBS, 100 units/ml penicillin, and 100 μg/ml
streptomycin maintained at 37 ºC in a humidified atmosphere (90%)
containing 5% CO2.
The cells were
subsequently incubated with different concentrations of vincristine
(0.05-50 μM) and crocin (0.2-200 μM) up to 72 h. All the treatments
were conducted in triplicate.
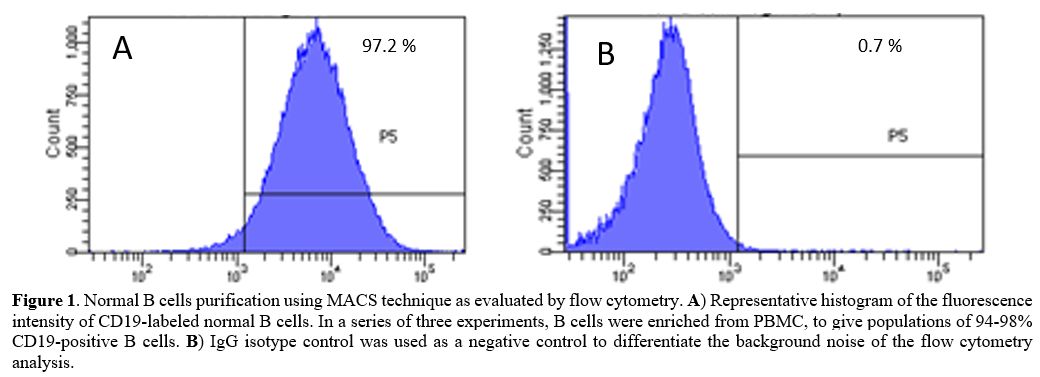 |
Figure
1. Normal B cells purification using MACS technique as evaluated by
flow cytometry. A)
Representative histogram of the fluorescence intensity of CD19-labeled
normal B cells. In a series of three experiments, B cells were enriched
from PBMC, to give populations of 94-98% CD19-positive B cells. B) IgG isotype
control was used as a negative control to differentiate the background
noise of the flow cytometry analysis.
|
Cell
viability assay. We determined cell viability by the
resazurin reagent. For this purpose, CO 88BV59-1 and normal B cells
(1×105)
were added to each well in 96-well culture plates treated with
vincristine (0.05-50 μM) and crocin (0.2-200 μM) up to 72 h. Next, 20
μl of the resazurin reagent was added to each well, and the plates were
incubated for 4 h. The fluorescence intensity of the product resorufin,
proportional to the number of viable cells per well, was measured via a
fluorescence Victor X5 2030 Multilabel Plate Reader (Perkin Elmer,
Shelton, Connecticut) with excitation at 530 nm and emission at 590 nm.[16]
Cell
apoptosis assay.
Crocin's apoptosis effect on CO 88BV59-1 cells was assessed by FITC
annexin V/PI staining. The cells were treated with crocin (175, 112,
and 80 µM) and vincristine (35, 23, and 1 µM) according to IC50 values
for different durations (24, 48, and 72 h), respectively. Also, we
assessed the combination of crocin (80 µM) and vincristine (1 µM) on
these cells for 72 h. After the treatment, the cells were incubated
with the FITC annexin V antibody and analyzed by a flow cytometer (BD
Biosciences, USA). The FlowJo software (TreeStar Inc.) was employed for
data analysis.[17]
Real-time
PCR quantification with SYBR Green.
The CO 88BV59-1 cells were treated with crocin (175, 112, and 80 µM)
alone or in combination with vincristine (35, 23, and 1 µM) up to 72
h., and then RNA extraction was done using TRIzol according
to
the manufacturer's instruction. RNA concentration and purity were
evaluated via spectrophotometry. For each sample, the complementary DNA
(cDNA) was synthesized from the total RNA (100 ng) via a cDNA synthesis
kit with the random hexamer primer. Primers (Bcl-2, Bax, P53, CASP3,
CASP8, and CASP9 genes) were designed using the Beacon software
(Applied Biosystems; Table
1).
Gene expression changes were determined using SYBR Green-based
real-time PCR technology by the Applied Biosystems Step One Plus
Detection System (ABI, USA). The reaction mixture comprised 1 μl of the
primers (100 pmol), 2 μl of cDNA (250-400 ng), 10 μl of 2x master mix,
and dH2O
to bring the volume to 20
μl. The optimized parameters utilized for the thermocycler included a
short hot-start at 95 °C for 15 min, followed by 40 cycles, each
consisting of denaturing at 95 °C for 15 secs, annealing at 60 °C for 1
min, and extension at 72 °C for 20 sec. Melting curves were
used
from 60 to 90 °C rising by 0.3 °, as the final step of the SYBR Green
real-time PCR. Gene expressions were normalized to Glyceraldehyde
3-phosphate dehydrogenase (GAPDH) as the housekeeping gene. The samples
were run in triplicate, and the fold difference of expression in the
treated and untreated samples was calculated using the 2-ΔΔCt method.[18]
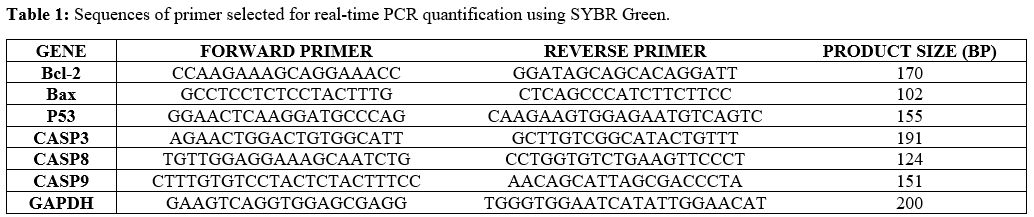 |
Table
1. Sequences of primer selected for real-time PCR quantification using
SYBR Green.
|
Western
blot analysis.
Following 72 h of treatment with crocin (80 μM) alone or in combination
with vincristine (1 μM), the cells were lysed using the lysis buffer
and centrifuged at 18000 g, at 4 °C for one h, and then the supernatant
was collected. Next, the lysates were run on 10% sodium dodecyl sulfate
(SDS)-polyacrylamide gel and then transferred onto nitrocellulose
membranes. After blocking with 2% Bovine serum albumin (BSA), the blots
were exposed to the primary antibody for one h at room temperature. In
the next step, they were washed and incubated with the corresponding
horseradish peroxidase-conjugated to the secondary antibody for two h.
Finally, membrane visualization was performed using an ECL detection
kit. The reactions were revealed and documented by Gel-Doc (Syngene,
Cambridge, UK), and the images were quantified using the Image J
software (version 1.46).[19]
Statistical
analysis.
The data are represented as mean ± SD and were analyzed using one-way
analysis of variance (ANOVA) with Tukey's multiple comparisons post-hoc
test in the Graph Pad PRISM software (Version 6, Graph Pad Software,
CA). A p-value <0.05 was considered statistically significant.
Results
Crocin
concentration-dependently reduced the viability of CO 88BV59-1 cells.
Crocin at concentrations of 100 and 200 μM significantly decreased the
viability of CO 88BV59-1 cells at 48 and 72 h (p < 0.05; Figure 2A).
Similarly, a significant drop in viability was observed in these cells
incubated for 48 and 72 h with 25 and 50 μM of vincristine (p <
0.05). However, crocin did not affect the viability of normal B cells
at concentrations of 0.2-200 μM (Figure
2B). Table 2
presents the IC50 values of crocin and vincristine in CO 88BV59-1 cells
for 24, 48, and 72 h of incubation.
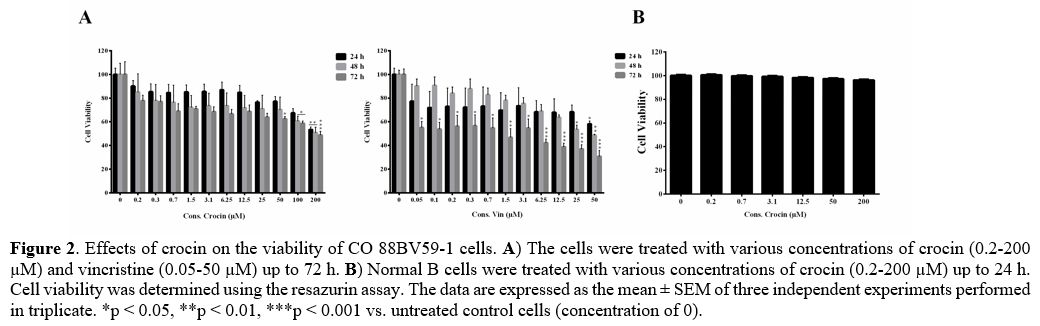 |
Figure 2.
Effects of crocin on the viability of CO 88BV59-1 cells. A) The cells were
treated with various concentrations of crocin (0.2-200 µM) and
vincristine (0.05-50 µM) up to 72 h. B)
Normal B cells were treated with various concentrations of crocin
(0.2-200 µM) up to 24 h. Cell viability was determined using the
resazurin assay. The data are expressed as the mean ± SEM of three
independent experiments performed in triplicate. *p < 0.05, **p
<
0.01, ***p < 0.001 vs. untreated control cells (concentration of
0). |
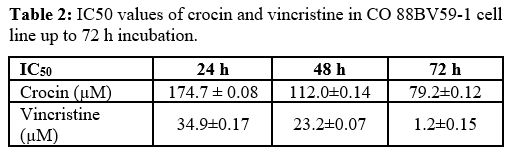 |
Table
2. IC50 values of crocin and vincristine in CO 88BV59-1 cell line up to
72 h incubation. |
Crocin
time-dependently induced apoptosis in CO 88BV59-1 cells. Figure 3
displays the impacts of crocin on the apoptosis of CO 88BV59-1 cells,
assessed by annexin V and PI double staining. Similar to vincristine,
crocin (175, 112, and 80 µM) significantly and time-dependently spiked
the apoptosis rate of these cells (p < 0.001). Furthermore, the
combination of crocin (80 μM) and vincristine (1 μM) remarkably
enhanced apoptosis by up to 68% in CO 88BV59-1 cells (p <
0.001).
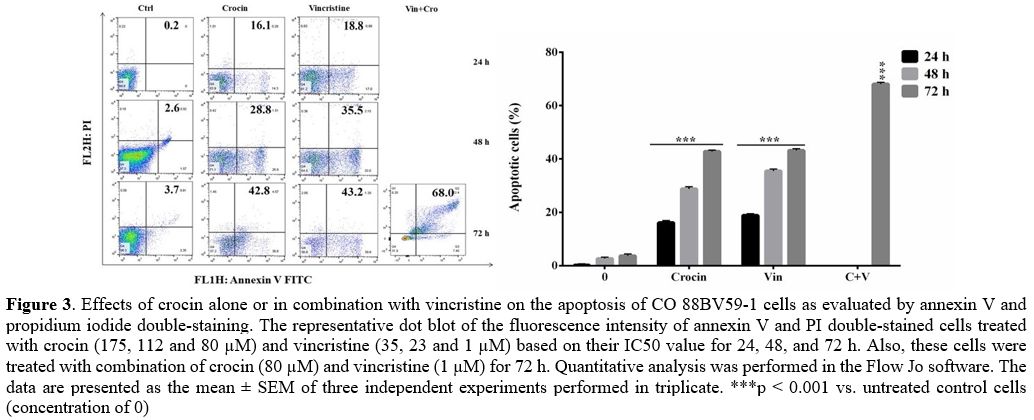 |
Figure
3. Effects of
crocin alone or in combination with vincristine on the apoptosis of CO
88BV59-1 cells as evaluated by annexin V and propidium iodide
double-staining. The representative dot blot of the fluorescence
intensity of annexin V and PI double-stained cells treated with crocin
(175, 112 and 80 µM) and vincristine (35, 23 and 1 µM) based on their
IC50 value for 24, 48, and 72 h. Also, these cells were treated with
combination of crocin (80 µM) and vincristine (1 µM) for 72 h.
Quantitative analysis was performed in the Flow Jo software. The data
are presented as the mean ± SEM of three independent experiments
performed in triplicate. ***p < 0.001 vs. untreated control
cells
(concentration of 0)
|
Crocin
modulated genes involved in survival and apoptosis in CO 88BV59-1 cells.
Figure 4
depicts the effects of crocin alone or in combination with vincristine
on the expression of genes involved in survival (Bcl-2) and apoptosis
(P53, Bax, CASP3, CASP8, and CASP9) in CO 88BV59-1 cells up to 72 h.
The expressions of P53, CASP3, CASP8, and CASP9 were significantly
raised in these cells treated with either crocin or vincristine (p
<
0.001 compared with control cells). In addition, crocin alone or in
combination with vincristine significantly increased the Bax/Bcl-2
ratio to 4.4 ± 0.16 and 5.7 ± 0.20-fold in the cells during 72 h,
respectively (p < 0.001 compared with control cells; Figure 4).
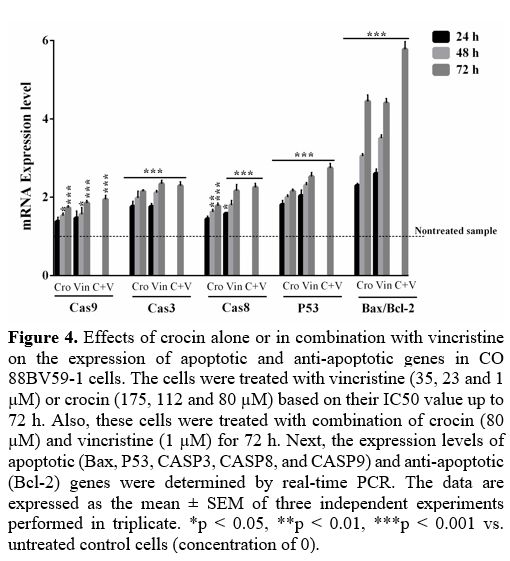 |
Figure
4. Effects of
crocin alone or in combination with vincristine on the expression of
apoptotic and anti-apoptotic genes in CO 88BV59-1 cells. The cells were
treated with vincristine (35, 23 and 1 µM) or crocin (175, 112 and 80
µM) based on their IC50 value up to 72 h. Also, these cells were
treated with combination of crocin (80 µM) and vincristine (1 µM) for
72 h. Next, the expression levels of apoptotic (Bax, P53, CASP3, CASP8,
and CASP9) and anti-apoptotic (Bcl-2) genes were determined by
real-time PCR. The data are expressed as the mean ± SEM of three
independent experiments performed in triplicate. *p < 0.05, **p
<
0.01, ***p < 0.001 vs. untreated control cells (concentration of
0).
|
To further
assess the impact of crocin on apoptotic genes, their protein levels
were evaluated by western blot analysis (Figure 5).
Treatment of cells with crocin increased the protein expressions of
P53, Bax, CASP3, and CASP9 but reduced the Bcl-2 expression. On the
contrary, the expression of CASP8 protein did not change in CO 88BV59-1
cells following treatment with crocin (Figure 5);
however, crocin combined with vincristine raised the expression of
CASP8 (p < 0.001). The levels of Bax/Bcl-2 protein was
significantly
increased in crocin (80 μM) alone or in combination with vincristine (1
μM)-treated cells compared to untreated cells (4.09 ± 0.18 and 5.4 ±
0.11, respectively; p < 0.001), thereby confirming the
synergistic
effect of crocin on CO 88BV59-1 cells. A similar surge was also
observed in the cells incubated with 1 μM of vincristine (4.2 ± 0.11, p
< 0.001). It was demonstrated that crocin significantly
increased
Bax/Bcl-2 ratio, approximately equal to vincristine.
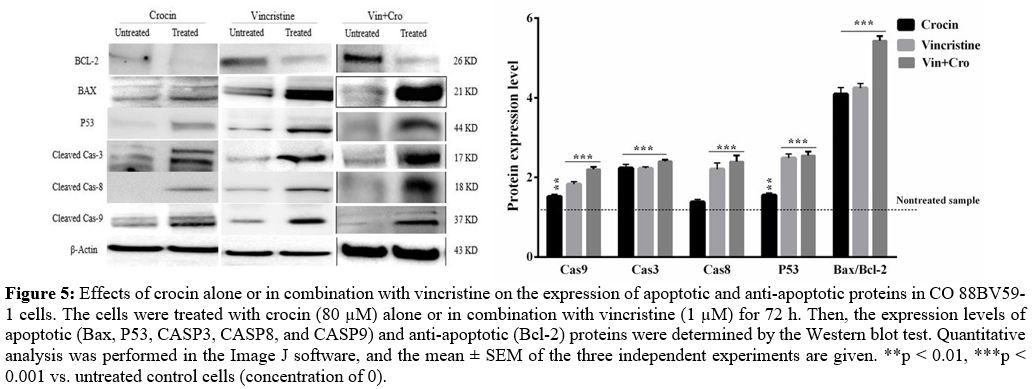 |
Figure
5. Effects of
crocin alone or in combination with vincristine on the expression of
apoptotic and anti-apoptotic proteins in CO 88BV59-1 cells. The cells
were treated with crocin (80 µM) alone or in combination with
vincristine (1 µM) for 72 h. Then, the expression levels of apoptotic
(Bax, P53, CASP3, CASP8, and CASP9) and anti-apoptotic (Bcl-2) proteins
were determined by the Western blot test. Quantitative analysis was
performed in the Image J software, and the mean ± SEM of the three
independent experiments are given. **p < 0.01, ***p <
0.001 vs.
untreated control cells (concentration of 0).
|
Discussion
This
study was the first to examine the mechanism of apoptotic cell death
induced by crocin in EBV-transformed B-lymphocyte (CO 88BV59-1 cell
line), compared to the standard anti-lymphoma drug, vincristine, during
short-term treatment. Crocin effectively inhibited cell proliferation
and induced apoptosis at high concentrations in CO 88BV59-1 cells
during 72 h of treatment. The cytotoxic effect of crocin was more
noticeable against CO 88BV59-1 cells than against normal human B cells,
and these effects were comparable with those of vincristine.
Furthermore, crocin significantly up-regulated the expression P53,
CASP3, CASP9, and Bax/Bcl-2 ratio in CO 88BV59-1 cells at the mRNA and
protein level, whereas the CASP8 protein remained unchanged. The
present study's findings revealed that crocin induces apoptosis via the
intrinsic pathway in a concentration- and time-dependent manner in CO
88BV59-1 cells. Interestingly, the combination of crocin (80 μM) and
vincristine (1 μM) induced apoptosis in these cells via both pathways
(intrinsic and extrinsic). Therefore, this synergistic apoptotic effect
of crocin and vincristine was detected in these cells.
Patients
who have acquired or inherited immune incompetence demonstrate a high
incidence of lymphoma. A common factor in these patients seems to be
the impairment of immunoregulatory mechanisms involved in neoplastic
and/or viral surveillance.[20] The
incidence of EBV
among B-cell lymphoma patients is <5% in the United States and
European countries, but 10-15% in Latin American and Asian countries.[21,22]
However, EBV-related B-cell lymphomas in transplant recipients show
some additional characteristics; most notably, a large proportion of
these tumors tend to regress spontaneously upon immunosuppression
withdrawal or reduction, even though they are almost universally fatal
if they remain untreated.[23]
Several studies
reported that nontoxic natural agents could be useful either alone or
in combination with conventional therapeutics to prevent tumor
progression and/or treat human malignancies.[24]
The
fact that crocin is abundantly available in large quantities in food
products and is reportedly nontoxic makes its anti-cancer effect even
more attractive.[25] Furthermore,
since it also
possesses immunosuppressive characteristics, it can exert potent
anti-inflammatory effects in autoimmune diseases via inhibiting
cytokines.[26-29] Consistent with
our findings, a
study explored the effect of crocin on the proliferation and
differentiation of HL-60 cells during long-term (5 days) exposure.[30]
In another research, crocin inhibited proliferation and induced
apoptosis in leukemic cell lines (K562, HL-60, L1210, and P388):[31]
crocin also inhibited the proliferation of Jurkat and HL60 cells by
reducing cell growth and induced apoptosis by raising the Bax/Bcl-2
ratio.[32,33]
Some studies showed that the anti-proliferative activity of crocin like
vincristine involves targeting microtubules[10]
and p53-dependent and -independent mechanisms in colon cancer cells.[11,12]
Crocin
triggers apoptosis by increasing the Bax/Bcl-2 ratio and caspase
activation in human gastric adenocarcinoma without affecting human
normal fibroblast skin cells.[34]
Also, Luo et al.
reported that the combination of crocin with cisplatin exerts growth
suppression and apoptosis in gastric carcinoma cells.[35]
Crocetin (hydrolyzed crocin) induced p53-mediated cell death in
functional p53-expressing cancer cells through Bax and P53-induced
protein with a death domain (PIDD) caspase-2-t-BH3 interacting-domain
death (BID) pathway.[36] Another
study showed that
dimethyl-crocetin and crocin induced cytotoxicity on HL60 cells but did
not affect K562 cells. They suggested that dimethyl-crocetin could
disrupt DNA–protein interactions (e.g., topoisomerase II) and inhibit
nucleic acid synthesis.[37]
Controversially, the
other study showed that crocetin, unlike silymarin, retinoic acid, and
other drugs, was unable to prevent the neoplastic
transformation
of rat tracheal epithelial cells by Benzopyrene.[38]
Another study presented that crocin suppressed multiple human myeloma
growth through inhibition of STAT3-mediated gene products, including
BAX, Bcl-2, vascular endothelial growth factor (VEGF), CXC Chemokine
Receptor 4 (CXCR4), and cell cycle regulator (cyclin D1).[39]
Xu et al. showed that crocin could block HL-60 cells in the G₀/G₁ phase
and inhibit their proliferation. The suggested mechanism in these cells
may be related to the inhibition of Bcl-2 and activation of Bax.[40]
The
studies investigated the effect of crocin on the proliferation and
immune function of dendritic cells (DC) derived from the bone marrow of
children with acute leukemia. They concluded that crocin could
synergically promote the maturity of DC cooperating with recombinant
human granulocyte-macrophage colony-stimulating factor (them-CSF),
recombinant human IL-4 (rhIL-4), and recombinant human TNF-α (rhTNF-α).
The DC induced by crocin can particularly enhance the proliferation of
T cells.[41,42]
According to Molnar et al.,
crocin and crocetin were ineffective in reversing multidrug resistance
of lymphoma cells but inhibited the early tumor antigen expression of
adenovirus-infected mouse lymphoma cells.[43]
It is noteworthy that crocin demonstrates a significant antiviral
activity against HSV-1 and also HIV-1.[44]
Another study also showed that crocin concentration- and
time-dependently inhibited the proliferation and prolonged the lifespan
of Dalton's lymphoma-bearing animals through significant effects on
hematological parameters.[45] On
the other hand,
Khavari et al. concluded that a combination of DNA vaccine with crocin
did not potentiate protective and therapeutic effects compared to
mono-therapies for controlling papillomavirus-infected tumors.[46]
Based on another study, crocin exhibited low cytotoxic effects on the
MOLT-4 cell line, which might be mediated through the escalation of DNA
fragmentation.[47] Also, crocin
significantly and
concentration-dependently promoted T cell proliferation and IL-2 and
IL-4 secretion. Crocin itself caused no significant damage to T cells
but curbed DNA damage in T cells treated with cytarabine.[48]
Clinical experiments reported that healthy volunteers treated with
saffron tablets (200 mg/kg) did not demonstrate hematological or
biochemical toxicity.[49] In
addition, a
pharmacokinetic study suggested that orally administered crocins are
hydrolyzed to crocetin before or during intestinal absorption.[50] The LD50 of crocin has been
reported to be >3 g/kg.[51]
The
present study had some limitations partially due to a reduced financial
budget. This study was carried out on only one EBV-transformed B cell,
and only six genes and proteins (apoptotic and anti-apoptotic) were
evaluated in this research work. It should be better to utilize more
than two EBV-transformed B cells and study other apoptotic and
anti-apoptotic genes.
Conclusions
The
results showed that crocin promotes apoptosis in CO 88BV59-1 cells in a
time- and concentration-dependent manner via the induction of the
P53-dependent intrinsic pathway. Furthermore, crocin and vincristine
have a synergistic effect on these cells. Thus, it is suggested from
these preclinical studies to evaluate the effect of crocin alone or in
combination with vincristine in EBV-associated B-cell
lympho-proliferative disorders.
Ethical
consideration
This
study was approved by the Research Ethics Committee of Jahrom
University of Medical Sciences (ethic code: IR.JUMS.REC.1399.026).
Acknowledgments
We appreciate
the insightful comments of Dr. Saiedeh Erfanian, which greatly enhanced
an early version of this paper.
References
- Pei Y, Lewis AE and
Robertson ES. Current progress
in EBV-associated B-cell lymphomas. In: editors. Infectious Agents
Associated Cancers: Epidemiology and Molecular Biology. Springer; 2017.
p. 57-74. https://doi.org/10.1007/978-981-10-5765-6_5
PMid:29052132 PMCid:PMC6053051
- Shannon-Lowe
C, Rickinson AB and Bell AI. Epstein-Barr virus-associated lymphomas.
Philosophical Transactions of the Royal Society B: Biological Sciences
2017; 372: 20160271. https://doi.org/10.1098/rstb.2016.0271
PMid:28893938 PMCid:PMC5597738
- Fu
Q, He C and Mao ZR. Epstein-Barr virus interactions with the Bcl-2
protein family and apoptosis in human tumor cells. J Zhejiang Univ Sci
B 2013; 14: 8-24. https://doi.org/10.1631/jzus.B1200189
PMid:23303627 PMCid:PMC3542954
- Fulda
S and Debatin K-M. Extrinsic versus intrinsic apoptosis pathways in
anti-cancer chemotherapy. Oncogene 2006; 25: 4798-4811. https://doi.org/10.1038/sj.onc.1209608
PMid:16892092
- Coiffier
B and Sarkozy C. Diffuse large B-cell lymphoma: R-CHOP failure-what to
do? Hematology 2014, the American Society of Hematology Education
Program Book 2016; 2016: 366-378. https://doi.org/10.1182/asheducation-2016.1.366
PMid:27913503 PMCid:PMC6142522
- Wegiera
M, Smolarz HD, Jedruch M, Korczak M and Koproń K. Cytotoxic effect of
some medicinal plants from Asteraceae family on J-45.01 leukemic cell
line--pilot study. Acta poloniae pharmaceutica 2012; 69: 263-268.
- Shu
L, Cheung K-L, Khor TO, Chen C and Kong A-N. Phytochemicals: cancer
chemoprevention and suppression of tumor onset and metastasis. Cancer
and Metastasis Reviews 2010; 29: 483-502. https://doi.org/10.1007/s10555-010-9239-y
PMid:20798979
- Saklani A and Kutty SK.
Plant-derived compounds in clinical trials. Drug Discovery Today 2008;
13: 161-171. https://doi.org/10.1016/j.drudis.2007.10.010
PMid:18275914
- Khorasanchi
Z, Shafiee M, Kermanshahi F, Khazaei M, Ryzhikov M, Parizadeh MR,
Kermanshahi B, Ferns GA, Avan A and Hassanian SM. Crocus sativus a
natural food coloring and flavoring has potent anti-tumor properties.
Phytomedicine 2018; 43: 21-27. https://doi.org/10.1016/j.phymed.2018.03.041
PMid:29747750
- Hire
RR, Srivastava S, Davis MB, Kumar Konreddy A and Panda D.
Antiproliferative Activity of Crocin Involves Targeting of Microtubules
in Breast Cancer Cells. Scientific Reports 2017; 7: 44984-44984. https://doi.org/10.1038/srep44984
PMid:28337976 PMCid:PMC5364484
- Zhong
Y-j, Shi F, Zheng X-l, Wang Q, Yang L, Sun H, He F, Zhang L, Lin Y, Qin
Y, Liao L-c and Wang X. Crocetin induces cytotoxicity and enhances
vincristine-induced cancer cell death via p53-dependent and
-independent mechanisms. Acta Pharmacologica Sinica 2011; 32:
1529-1536. https://doi.org/10.1038/aps.2011.109
PMid:21986580 PMCid:PMC4010206
- Harchegani
AB, Khor A, Niha MM, Kaboutaraki HB, Shirvani H and Shahriary A. The
hepatoprotective and antioxidative effect of saffron stigma alcoholic
extract against vincristine sulfate induced toxicity in rats.
Interdiscip Toxicol 2019; 12: 186-191. https://doi.org/10.2478/intox-2019-0023
PMid:32461722 PMCid:PMC7247369
- Moradzadeh
M, Ghorbani A, Erfanian S, Mohaddes S, Rahimi H, Karimiani E, Mashkani
B, Chiang S, El-Khamisy S and Tabarraei A. Study of the mechanisms of
crocetin-induced differentiation and apoptosis in human acute
promyelocytic leukemia cells. Journal of cellular biochemistry 2018; https://doi.org/10.1002/jcb.27489
PMid:30203596
- Moradzadeh
M, Tabarraei A, Ghorbani A, Hosseini A and Sadeghnia HR. Short‐term in
vitro exposure to crocetin promotes apoptosis in human leukemic HL‐60
cells via intrinsic pathway. Acta Poloniae Pharm Drug Res 2018; 75:
445-451.
- Moore DK, Motaung B, du
Plessis
N, Shabangu AN, Loxton AG and Consortium S-I. Isolation of B-cells
using Miltenyi MACS bead isolation kits. PloS One 2019; 14: e0213832. https://doi.org/10.1371/journal.pone.0213832
PMid:30893384 PMCid:PMC6426237
- Mashkani
B, Tanipour MH, Saadatmandzadeh M, Ashman LK and Griffith R. FMS-like
tyrosine kinase 3 (FLT3) inhibitors: Molecular docking and experimental
studies. European Journal of Pharmacology 2016; 776: 156-166. https://doi.org/10.1016/j.ejphar.2016.02.048
PMid:26896780
- Rangarajan
P, Dharmalingam Subramaniam SP, Kwatra D, Palaniyandi K, Islam S,
Harihar S, Ramalingam S, Gutheil W, Putty S and Pradhan R. Crocetinic
acid inhibits hedgehog signaling to inhibit pancreatic cancer stem
cells. Oncotarget 2015; 6: 27661. https://doi.org/10.18632/oncotarget.4871
PMid:26317547 PMCid:PMC4695016
- A new mathematical
model for relative quantification in real-time RT-PCR. Nucleic acids
Research 2001; 29: e45. https://doi.org/10.1093/nar/29.9.e45
PMid:11328886 PMCid:PMC55695
- Moradzadeh
M, Tabarraei A, Sadeghnia HR, Ghorbani A, Mohamadkhani A, Erfanian S
and Sahebkar A. Kaempferol increases apoptosis in human acute
promyelocytic leukemia cells and inhibits multidrug resistance genes.
Journal of Cellular Biochemistry 2018; 119: 2288-2297. https://doi.org/10.1002/jcb.26391
PMid:28865123
- Marques-Piubelli
ML, Salas YI, Pachas C, Becker-Hecker R, Vega F and Miranda RN.
Epstein-Barr virus-associated B-cell lymphoproliferative disorders and
lymphomas: a review. Pathology 2020; 52: 40-52. https://doi.org/10.1016/j.pathol.2019.09.006
PMid:31706670
- Gibson
SE and Hsi ED. Epstein-Barr virus-positive B-cell lymphoma of the
elderly at a United States tertiary medical center: an uncommon
aggressive lymphoma with a nongerminal center B-cell phenotype. Human
Pathology 2009; 40: 653-661. https://doi.org/10.1016/j.humpath.2008.10.007
PMid:19144386
- Hoeller
S, Tzankov A, Pileri SA, Went P and Dirnhofer S. Epstein-Barr
virus-positive diffuse large B-cell lymphoma in elderly patients is
rare in Western populations. Human pathology 2010; 41: 352-357. https://doi.org/10.1016/j.humpath.2009.07.024
PMid:19913281
- Rossi AP and Klein CL.
Posttransplant malignancy. Surgical Clinics 2019; 99: 49-64. https://doi.org/10.1016/j.suc.2018.09.004
PMid:30471741
- Alavizadeh
SH and Hosseinzadeh H. Bioactivity assessment and toxicity of crocin: a
comprehensive review. Food and Chemical Toxicology 2014; 64: 65-80. https://doi.org/10.1016/j.fct.2013.11.016
PMid:24275090
- Moradzadeh
M, Kalani MR and Avan A. The antileukemic effects of saffron (Crocus
sativus L.) and its related molecular targets: A mini review. Journal
of Cellular Biochemistry 2019; 120: 4732-4738. https://doi.org/10.1002/jcb.27525
PMid:30644127
- Li
X, Jiang C and Zhu W. Crocin reduces the inflammation response in
rheumatoid arthritis. Bioscience, Biotechnology, and Biochemistry 2017;
81: 891-898. https://doi.org/10.1080/09168451.2016.1263145
PMid:28388359
- Nam
KN, Park Y-M, Jung H-J, Lee JY, Min BD, Park S-U, Jung W-S, Cho K-H,
Park J-H and Kang I. Anti-inflammatory effects of crocin and crocetin
in rat brain microglial cells. European Journal of Pharmacology 2010;
648: 110-116. https://doi.org/10.1016/j.ejphar.2010.09.003
PMid:20854811
- Tamaddonfard
E, Farshid A-A, Eghdami K, Samadi F and Erfanparast A. Comparison of
the effects of crocin, safranal and diclofenac on local inflammation
and inflammatory pain responses induced by carrageenan in rats.
Pharmacological Reports 2013; 65: 1272-1280. https://doi.org/10.1016/S1734-1140(13)71485-3
- Li
K, Li Y, Ma Z and Zhao J. Crocin exerts anti-inflammatory and
anti-catabolic effects on rat intervertebral discs by suppressing the
activation of JNK. International journal of molecular medicine 2015;
36: 1291-1299. https://doi.org/10.3892/ijmm.2015.2359
PMid:26648423 PMCid:PMC4601741
- Tarantilis
PA, Morjani H, POLISSIOU M and MANFAIT M. Inhibition of growth and
induction of differentiation of promyelocytic leukemia (HL-60) by
carotinoids from. Crocus Sativus 1994; 1913-1918.
- Morjani
H, Tarantilis P, Polissiou M and Manfait M. Growth inhibition and
induction of crythroid differentiation activity by crocin,
dimethylcrocetine and β-carotene on K562 tumor cells. Anticancer Res
1990; 10: 1398-1406.
- Sun Y, Wang Z, Wang
L, Wang L, Zang C and Sun L. The Effect and Mechanisms of Proliferative
Inhibition of Crocin on Human Leukaemia Jurkat Cells. The West Indian
Medical Journal 2015; 64: 473. https://doi.org/10.7727/wimj.2016.053
PMid:27398676 PMCid:PMC4961334
- Sun
Y, Xu H-J, Zhao Y-X, Wang L-Z, Sun L-R, Wang Z and Sun X-F. Crocin
exhibits antitumor effects on human leukemia HL-60 cells in vitro and
in vivo. Evidence-Based Complementary and Alternative Medicine 2013;
2013: https://doi.org/10.1155/2013/690164
PMid:23573146 PMCid:PMC3615578
- Hoshyar
R, Bathaie SZ and Sadeghizadeh M. Crocin triggers the apoptosis through
increasing the Bax/Bcl-2 ratio and caspase activation in human gastric
adenocarcinoma, AGS, cells. DNA and Cell Biology 2013; 32: 50-57. https://doi.org/10.1089/dna.2012.1866
PMid:23347444
- Luo
Y, Cui S, Tang F, Shen C, Qi Y, Lu D, Ma L, Yang Y, Li Y and Chen R.
The combination of crocin with cisplatin suppresses growth of gastric
carcinoma cell line BGC-823 and promotes cell apoptosis. Pakistan
Journal of Pharmaceutical Sciences 2017; 30.
- Ray
P, Guha D, Chakraborty J, Banerjee S, Adhikary A, Chakraborty S, Das T
and Sa G. Crocetin exploits p53-induced death domain (PIDD) and
FAS-associated death domain (FADD) proteins to induce apoptosis in
colorectal cancer. Scientific Reports 2016; 6: 1-11. https://doi.org/10.1038/srep32979
PMid:27622714 PMCid:PMC5020693
- Beljebbar
A, Sockalingum G, Morjani H, Angiboust J, Polissiou M and Manfait M.
Differential Interaction Modes of Dimethylcrocetin in K562 and HL60
Tumor Cells As Probed by Near Infrared FT-Raman Microspectroscopy. In:
editors. Spectroscopy of Biological Molecules. Springer; 1995. p.
475-476. https://doi.org/10.1007/978-94-011-0371-8_217
- Steele
VE, Kelloff GJ, Wilkinson BP and Arnold JT. Inhibition of
transformation in cultured rat tracheal epithelial cells by potential
chemopreventive agents. Cancer Research 1990; 50: 2068-2074.
- Kim
B, Lee KY and Park B. Crocin suppresses constitutively active STAT3
through induction of protein tyrosine phosphatase SHP‐1. Journal of
cellular biochemistry 2017; 118: 3290-3298. https://doi.org/10.1002/jcb.25980
PMid:28295507
- Xu
H-J, Zhong R, Zhao Y-X, Li X-R, Lu Y, Song A-Q, Pang X-Y, Yao R-Y and
Sun L-R. Proliferative inhibition and apoptotic induction effects of
crocin on human leukemia HL-60 cells and their mechanisms. Zhongguo shi
yan xue ye xue za zhi 2010; 18: 887-892.
- Xu
H-J, Zhang K-P, Zhong R, Zhao Y-X, Li X-R, Lu Y, Song A-Q, Pang X-Y and
Sun L-R. Influence of crocin on proliferation in vitro and function of
dendritic cells derived from bone marrow of children with acute
leukemia. Zhongguo shi yan xue ye xue za zhi 2012; 20: 57-61.
- Zhang
K, Zhong R, Xu H, ZHAO Y-x, LI X-r, LU Y, SONG A-q and SUN L-r. Effect
of crocin on culture and proliferation of dendritic cells derived from
children acute leukemia blood marrow in vitro. Prog Mod Biomed 2011;
24: 035.
- Molnar J, Szabo D,
Pusztai R,
Mucsi I, Berek L, Ocsovszki I, Kawata E and Shoyama Y. Membrane
associated antitumor effects of crocine-, ginsenoside-and cannabinoid
derivates. Anti-Cancer Research 2000; 20: 861-867.
- Soleymani
S, Zabihollahi R, Shahbazi S, Bolhassani A. Antiviral Effects of
Saffron and its Major Ingredients. Curr Drug Deliv. 2018;15(5):698-704.
doi: 10.2174/1567201814666171129210654. https://doi.org/10.2174/1567201814666171129210654
PMid:29189153
- Bakshi
HA, Sam S, Feroz A, Ravesh Z, Shah GA and Sharma M. Crocin from
Kashmiri saffron (Crocus sativus) induces in vitro and in vivo
xenograft growth inhibition of Dalton's lymphoma (DLA) in mice. Asian
Pac J Cancer Prev 2009; 10: 887-890.
- Khavari
A, Bolhassani A, Alizadeh F, Bathaie SZ, Balaram P, Agi E and Vahabpour
R. Chemo-immunotherapy using saffron and its ingredients followed by
E7-NT (gp96) DNA vaccine generates different anti-tumor effects against
tumors expressing the E7 protein of human papillomavirus. Archives of
Virology 2015; 160: 499-508. https://doi.org/10.1007/s00705-014-2250-9
PMid:25395243
- Rezaee
R, Mahmoudi M, Abnous K, Zamani Taghizadeh Rabe S, Tabasi N, Hashemzaei
M and Karimi G. Cytotoxic effects of crocin on MOLT-4 human leukemia
cells. Journal of Complementary and Integrative Medicine 2013; 10:
105-112. https://doi.org/10.1515/jcim-2013-0011
PMid:23934514
- Zhang
K, Wang L, Si S, Sun Y, Pei W, Ming Y and Sun L. Crocin improves the
proliferation and cytotoxic function of T cells in children with acute
lymphoblastic leukemia. Biomedicine & Pharmacotherapy 2018; 99:
96-100. https://doi.org/10.1016/j.biopha.2018.01.042
PMid:29329036
- Modaghegh
M-H, Shahabian M, Esmaeili H-A, Rajbai O and Hosseinzadeh H. Safety
evaluation of saffron (Crocus sativus) tablets in healthy volunteers.
Phytomedicine 2008; 15: 1032-1037. https://doi.org/10.1016/j.phymed.2008.06.003
PMid:18693099
- Xi
L, Qian Z, Du P and Fu J. Pharmacokinetic properties of crocin
(crocetin digentiobiose ester) following oral administration in rats.
Phytomedicine 2007; 14: 633-636. https://doi.org/10.1016/j.phymed.2006.11.028
PMid:17215113
- Bostan
HB, Mehri S and Hosseinzadeh H. Toxicology effects of saffron and its
constituents: a review. Iranian Journal of Basic Medical Sciences 2017;
20: 110.
[TOP]






