Xiao-peng Shang1, Jian-gang Wu2, Ying Cheng3 and Hong-bo Hu4.
1 Department of Infectious Disease, The first people’s Hospital of Guangshui, China.
2 Department of Laboratory, The first people’s Hospital of Guangshui, China.
3 Department of Pediatrics, Maternal and Child Health Hospital of Hubei Province, China
4 Department of Laboratory, Maternal and Child Health Hospital of Hubei Province, China.
Correspondence to: Hong-bo
Hu, Department of Laboratory, NO. 745 Wu Luo Road, Hongshan District,
Wuhan City, Hubei Province, P.R. China, 430070. E-mail:
hongbo1172@163.com
Published: May 1, 2021
Received: March 8, 2021
Accepted: April 21, 2021
Mediterr J Hematol Infect Dis 2021, 13(1): e2021037 DOI
10.4084/MJHID.2021.037
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
To the editor
Henoch-Schönlein
purpura (HSP) is the most common cause of vasculitis in children. The
incidence of HSP in China is 8.13–14.06/100000 children.[1,2]
The most challenging aspect of HSP is determining the specific trigger
for leukocytoclastic vasculitis. Associations with bacterial and viral
infection and immunisation have been reported.[1,3,4]
The most common cause of HSP is probably an infection of the respiratory tract.[1-4] Mycoplasma pneumonia (MP) is a common bacterial pathogen causing M. pneumoniae pneumonia (MPP) in children. In China, the rate of MPP is 30.3% - 37.5% in paediatric patients aged from 1 month to 18 years.[5]
Although there have been several case reports on MP-associated HSP,
data on the aetiology and epidemiology of children with HSP and MP
infection in developing countries are still insufficient.
The
participants in this study were recruited from all patients between the
ages of 2 and 15 years who were admitted to two hospitals in Hubei
province, China, from January 2015 and December 2019. We evaluated the
epidemiologic and clinical characteristics of those patients diagnosed
with MPP and HSP and 131 HSP patients with MPP, an association that was
rarely systematically described in the literature.
Methods
Patient selection.
Children with HSP, younger than 15 years old, were recruited for the
present study between January 2015 and December 2019. The diagnosis of
HSP was based on European League against Rheumatism endorsed consensus
criteria for HSP classification.[6] Children with HSP,
excluding infection as a trigger, were determined to serve as
non-infectious cases for direct comparison, as well as for
epidemiological interest. During the same period, children diagnosed
with MPP, regardless of HSP, were also surveyed. The diagnosis of
pneumonia was defined as follows: clinical manifestations (fever, cough
or wheezing), physical examination and chest imaging with infiltrates.[7] MPP is defined as pneumonia with MP infection, excluding other pathogen infections.
Laboratory tests for MP infection.
MP antibodies were detected using the passive agglutination method
(Serodia-Myco II, Fujirebio, Japan). Positive MP infection was defined
as single titres of serum MP antibody ⩾1:320 or seroconversion
(increased antibody titres ⩾4 fold).[8]
Exclusion criteria.
(1) Patients with impaired immune function or who were receiving
immunosuppressive therapy or were taking nephrotoxic drugs; (2)
Patients who received blood transfusions or other blood product
treatment in the past few months; (3) Patients with severe heart,
liver, kidney or other organ system diseases; (4) Patients with chronic
pulmonary disease that might affect the chest X-ray results, aspiration
pneumonia or interstitial lung disease. (5) Patients with incomplete
clinical data; (6) Patients with HSP associated with infections by
other pathogens.
Statistical analyses.
The statistical analyses were performed using SPSS ver. 21.0 software
(SPSS, Inc., Chicago, IL, USA). Normally distributed continuous data
are expressed as mean ± standard deviation. Comparisons of the
frequencies among groups were analysed using Chi-squared tests.
Comparisons of mean values between groups were performed using the
independent sample t-test. A P-value of less than 0.05 was considered
statistically significant.
Results
Frequency of MP-triggered HSP.
Among the 1437 children with HSP, 131 children were diagnosed with MPP,
and the incidence of MP-triggered HSP was 9.1% (131/1437).
Monthly or seasonal distribution of cases in HSP and MP infection.
In terms of seasonal frequency, MPP cases and HSP with MPP cases
occurred mainly in the autumn and winter seasons. No differences were
found in the repoted years. All cases occurred less frequently in
summer, and HSP cases occurred mainly in the winter (Figure 1A).
Age distribution in HSP and MPP.
In terms of age distribution, a bell-shape distribution pattern with a
peak prevalence mainly in the 4–10 years age range was observed in the
HSP and HSP with MPP cases. However, the peak ages were slightly
different across MPP cases (Figure 1B).
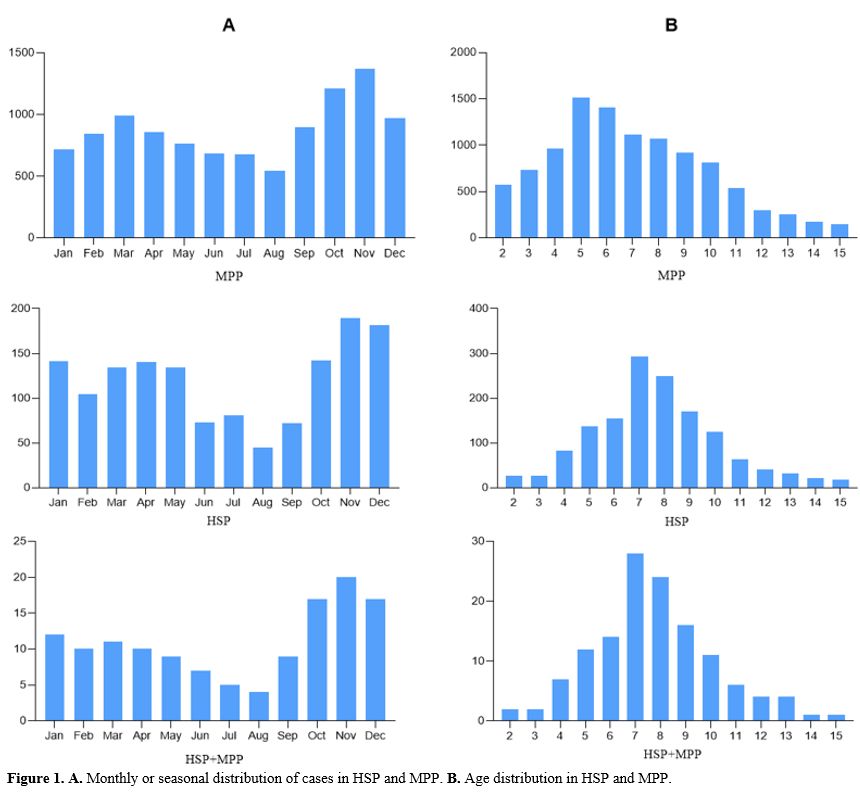 |
Figure 1. A. Monthly or seasonal distribution of cases in HSP and MPP. B. Age distribution in HSP and MPP.
|
Extrapulmonary manifestations of MPP. During the same period, 10519 children with MPP were diagnosed, of which HSP accounted for 1.2% (131/10519) (Figure 2A). In cases of MPP, a significantly greater frequency of MP-triggered HSP was found in different ages (χ2 = 39.340, p<0.001, Figure 2B).
Clinical manifestations of HSP with MP infection.
In the non-infectious group, there were 716 cases with purpura
(100.0%), 389 (54.3%) cases with arthritis/arthralgia, 373 (52.1%)
cases with abdominal pain and 146 (20.4%) cases with renal involvement.
MP-triggered HSP cases exhibited arthritis/arthralgia (77/131, 58.8%)
most frequently, followed by abdominal pain (55/131,42.0%) and renal
involvement (37/131, 28.2%). Compared with the non-infectious group,
infectious cases had a significantly higher frequency of renal
involvement (χ2 = 4.032, p = 0.045) and a lower frequency of abdominal
pain (χ2 = 4.528, p = 0.033) (Figure 2C).
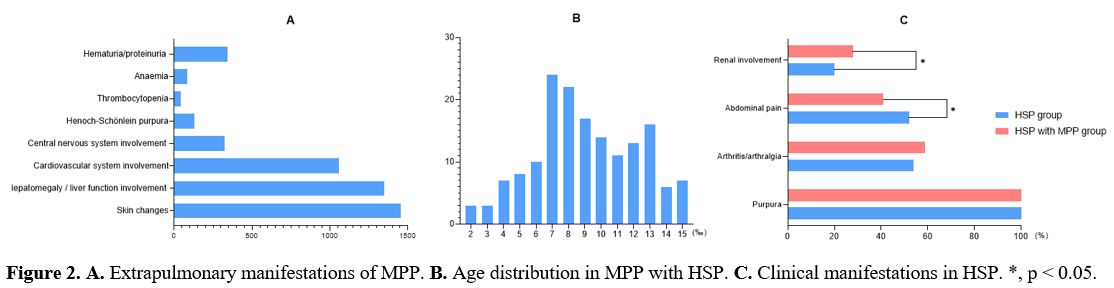 |
Figure 2. A. Extrapulmonary manifestations of MPP. B. Age distribution in MPP with HSP. C. Clinical manifestations in HSP. *, p < 0.05.
|
Therapeutic response. The therapeutic response of the non-infectious cases compared with infectious cases is presented in Table 1.
Significant differences were observed in the duration of purpura (t =
2.129, p = 0.034) and arthritis/arthralgia (t = 2.043, p = 0.042)
between the two groups.
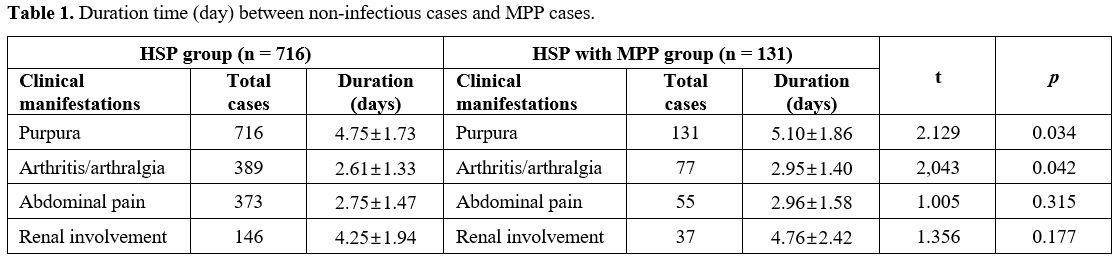 |
Table
1. Duration time (day) between non-infectious cases and MPP cases.
|
Discussion
MP
can cause milder upper respiratory tract infections (pharyngitis,
sinusitis) and severe lower respiratory tract infections (bronchitis,
pneumonia) and damage the extrapulmonary systems.[8]
The majority of extrapulmonary symptoms are associated with skin
changes such as exanthematous skin eruptions, urticaria, erythema
nodosum, Stevens-Jonson syndrome and HSP. Data on the prevalence of MP
infection in children with HSP are scarce. Timitilli et al.[9]
investigated the extrapulmonary manifestations of 92 children with MP
infection and found just one case with HSP. In the present study, 10519
children with MPP were diagnosed, of which HSP accounted for 1.2%.
MP stimulates the production of the interleukins and tumor necrosis factor α and can cause vasculitis.[10,11]
HSP is a leukocytoclastic vasculitis that affects small vessels.
Clinical manifestations of HSP typically include rash, arthritis, and
gastrointestinal and sometimes renal involvement. Immune complexes
activate cytokines, parts of complement and directly influence the
endothelium. MP was the common infectious agent identified by MP
antibodies in 131 cases (9.1%) from 1437 HSP cases in our study.
Moreover, 37 cases triggered by MP included renal involvement. Several
previous studies have reported direct evidence of HSP caused by MP.[11-13]
It
is known that the seasonality of HSP is determined by the factors of
these triggers and that age distribution and manifestations vary.[1]
The epidemiological characteristics of a disease can help determine the
aetiologic agents of the disease. For this reason, we evaluated and
compared the epidemiological characteristics among HSP, MPP and HSP
associated with MPP in children. In terms of seasonal frequency, MPP
cases and HSP with MPP cases occurred mainly in the autumn and winter
seasons. All cases occurred less frequently in summer, and HSP cases
occurred mainly in the winter. The epidemiological profiles in HSP with
MP infection were similar to those with HSP. In terms of age
distribution, a bell-shape distribution pattern with a peak prevalence
mainly in the 4–10 years age range was observed in the HSP and HSP with
MPP cases. However, the peak ages were slightly different across MPP
cases (Figure 1B). In cases of
MPP, a significantly greater frequency of MP-triggered HSP was found in
different ages (p<0.001). Children might have distinct immune responses
and clinical symptoms depending on their age.[14]
Compared
with the non-infectious group, a significantly higher frequency of
renal involvement in the infectious group (p = 0.045) was noted. PCR
and immunofluorescence failed to detect MP antigens in the renal
parenchyma in a previous study. However, other reports have noted that
failure to detect MP antigens does not necessarily rule out a role for
this microorganism because the pathogenesis of postinfectious renal
involvement is more likely to be based on immunologic mechanisms. An
immune reaction to the glomeruli could be suggested by
immunofluorescence detection of anti-MP antibodies in the glomeruli.
The antigen involved could be mycoplasmal or a cross-reacting renal
antigen.[15]
An objective of the present study
was to explore the therapeutic response between non-infectious cases
and infectious cases by analyzing the duration of the main clinical
manifestations. Significant differences were observed in the duration
time of purpura (p = 0.034) and arthritis/arthralgia (p = 0.042)
between the two groups. Although a higher frequency of renal
involvement was noted, the differences in the duration of renal
involvement do not attain statistical significance due to the limited
number of participants enrolled. Treatment with macrolides led to
remission of the disease.
Conclusions
We
report 131 paediatric cases of HSP children MPP with prolonged skin and
joint changes. The incidence of MP-triggered HSP was 9.1%, while MPP in
children with HSP was 1.2%. The epidemiological characteristics of HSP
with MPP were similar to those of HSP in terms of age distribution and
seasonal variations. We suggest that in cases of prolonged symptoms of
vasculitis due to HSP, MP infection could be a potential cause of
exacerbation of the disease.
References
- Wang JJ, Xu Y, Liu FF, et al. Association of the
infectious triggers with childhood Henoch-Schonlein purpura in Anhui
province, China. J Infect Public Health. 2020;13(1):110-117. https://doi.org/10.1016/j.jiph.2019.07.004 PMid:31337540
- Wang
X, Zhu Y, Gao L, Wei S, Zhen Y, Ma Q. Henoch-Schonlein purpura with
joint involvement: Analysis of 71 cases. Pediatr Rheumatol Online J.
2016;14(1):20. https://doi.org/10.1186/s12969-016-0080-x PMid:27029321 PMCid:PMC4815193
- Chen
JY, Mao JH. Henoch-Schonlein purpura nephritis in children: incidence,
pathogenesis and management. World J Pediatr. 2015;11(1):29-34. https://doi.org/10.1007/s12519-014-0534-5 PMid:25557596
- Hetland
LE, Susrud KS, Lindahl KH, Bygum A. Henoch-Schonlein Purpura: A
Literature Review. Acta Derm Venereol. 2017;97(10):1160-1166. https://doi.org/10.2340/00015555-2733 PMid:28654132
- Gao
LW, Yin J, Hu YH, et al. The epidemiology of paediatric Mycoplasma
pneumoniae pneumonia in North China: 2006 to 2016. Epidemiol Infect.
2019;147: e192. https://doi.org/10.1017/S0950268819000839 PMid:31364532 PMCid:PMC6518602
- Ozen
S, Pistorio A, Iusan SM, et al. EULAR/PRINTO/PRES criteria for
Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood
Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008.
Part II: Final classification criteria. Ann Rheum Dis. 2010; 69
(5):798-806. https://doi.org/10.1136/ard.2009.116657 PMid:20413568
- Hu YM, Jiang ZF. Zhu Futang's Practical Pediatrics. 7th Ed., China, People's Medical Publishing House. 2002;1175-1180.
- Respiratory
Branch of Chinese Pediatric Society of Chinese Medical Association.
Expert consensus on diagnosis and treatment of Mycoplasma pneumoniae
pneumonia in children. Chinese Journal of Applied Clinical Pediatrics.
2015;17: 1304 -1308.
- Timitilli A, Di
Rocco M, Nattero G, Tacchella A, Giacchino R. Unusual manifestations of
infections due to Mycoplasma pneumoniae in children. Infez Med.
2004;12(2):113-117.
- Wang Y, Zhang Y, Lu
W, Wang L. Serum Tumor Necrosis Factor- α and Interferon- γ Levels in
Pediatric Mycoplasma pneumoniae Pneumonia: A Systematic Review and
Meta-Analysis. Can Respir J. 2018; 2018:8354892. https://doi.org/10.1155/2018/8354892 PMid:30275916 PMCid:PMC6151362
- Kuźma-Mroczkowska
E, Pańczyk-Tomaszewska M, Szmigielska A, Szymanik-Grzelak H,
Roszkowska-Blaim M. Mycoplasma pneumoniae as a trigger for
Henoch-Schonlein purpura in children. Cent Eur J Immunol.
2015;40(4):489-492. https://doi.org/10.5114/ceji.2015.56976 PMid:26862316 PMCid:PMC4737748
- Yiallouros
P, Moustaki M, Voutsioti A, Sharifi F, Karpathios T. Association of
Mycoplasma pneumoniae infection with Henoch-Schonlein purpura. Prague
Med Rep. 2013;114(3):177-179. https://doi.org/10.14712/23362936.2014.20 PMid:24093818
- Lim CS, Lim SL. Henoch-Schönlein purpura associated with Mycoplasma pneumoniae infection. Cutis, 2011;87(6):273-276.
- Rhim
JW, Kang HM, Han JW, Lee KY. A Presumed Etiology of Kawasaki Disease
Based on Epidemiological Comparison with Infectious or Immune-Mediated
Diseases. Front Pediatr. 2019; 7:202. https://doi.org/10.3389/fped.2019.00202 PMid:31165053 PMCid:PMC6536658
- Siomou
E, Kollios KD, Papadimitriou P, Kostoula A, Papadopoulou ZL. Acute
nephritis and respiratory tract infection caused by Mycoplasma
pneumoniae: case report and review of the literature. Pediatr Infect
Dis J. 2003;22(12):1103-1106. https://doi.org/10.1097/01.inf.0000104531.58503.90 PMid:14688577
[TOP]


