Dina
Sameh A. Soliman1,3,4, Hesham El Sabah4,
Ibrahim Ganwo1, Aliaa Amer1,
Ruba Y. Taha5, Lajos Szabados6,
Mouhammad Sharaf Eldean2, Ahmad Al-Sabbagh2
and Feryal Ibrahim1.
1 Department
of Laboratory Medicine and Pathology, National Center for Cancer Care
and Research, Hamad Medical Corporation, Doha, Qatar.
2 Department of Laboratory Medicine and
Pathology, Hamad Medical Corporation, Doha, Qatar.
3 Weill Cornell Medicine-Qatar, Doha, Qatar.
4 Department of Clinical Pathology, National
Cancer Institute, Cairo, Egypt.
5
Department of Hematology and Medical Oncology, National Center for
Cancer Care and Research, Hamad Medical Corporation, Doha, Qatar.
6 PET/CT Center, Clinical Imaging, National
Center for Cancer Care and Research, Hamad Medical Corporation, Doha,
Qatar.
Correspondence to: Dina
Sameh A. Soliman. Department of Laboratory Medicine andPathology,
National Center for Cancer Careand Research, Hamad Medical Corporation,
PO Box 3050, 16060 Doha, Qatar. E-mail:
DSoliman@hamad.qa
Published: July 1, 2021
Received: March 31, 2021
Accepted: June 7, 2021
Mediterr J Hematol Infect Dis 2021, 13(1): e2021043 DOI
10.4084/MJHID.2021.043
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Plasma cell neoplasms can show aberrant expression of different
lineage-related antigens; however, co-expression of T-cell-associated
markers on malignant plasma cells is extremely rare.
Material and methods:
This report describes clinicopathologic characteristics of three
myeloma patients with emergent plasmablastic morphology and aberrant
acquisition of T-cell-associated markers diagnosed in our center. An
extensive literature search for similar cases was conducted, and the
relevant pathologic, clinical, and prognostic characteristics were
summarized.
Results: A
total of 22 cases of plasma cell neoplasm (including the three cases
reported here) showed aberrant co-expression of T-cell markers. We
found an evident association between aberrant expression of T-cell
markers on malignant plasma cells and extramedullary involvement,
aggressive morphologic features, high proliferative index ki67 >90%,
aggressive clinical course, an adverse outcome, and short survival.
Discussion & Conclusion:
Due to the rarity of this aberrant phenotype and scarcity of the
published data, the precise causative mechanism and its clinical
implications have not yet been elucidated.
|
Introduction
Plasma
cell neoplasms (PCN) is a clonal expansion of immunoglobulin secreting,
terminally differentiated mature effector B-cells that classically
secrete a single homogeneous monoclonal immunoglobulin (M protein).[1]
PCN can show aberrant expression of different lineages related to
antigens, but the expression of T-cell associated markers is
exceedingly rare. Herein, we describe three patients with plasma cell
myeloma (PCM) who relapsed with an aggressive disease with
plasmablastic morphology, extramedullary involvement, high ki-67, and
complex karyotype. Interestingly, these patients showed aberrant
acquisition of single or multiple T-cell associated markers, and two of
them died shortly after their latest presentation. Due to the rarity of
this aberrant phenotype and scarcity of the published data, the precise
causative mechanism and its clinical implications have not yet been
elucidated. Multiple theories have been proposed to explain the
aberrant expression of T-cell markers on plasma cells (PCs), being
terminally differentiated cells. Lineage infidelity is uncommon in
terminally differentiated B-cell lymphomas. An extensive literature
search for patients with similar findings was conducted, and the
relevant pathologic, clinical and prognostic characteristics were
summarized.
Methodology
This
report describes three myeloma patients with emergent plasmablastic
morphology and aberrant acquisition of multiple T‐cell associated
markers diagnosed in the National Centre for Cancer Care and Research
(NCCCR) in Qatar. The aberrant co-expression of T-cell associated
markers is confirmed by both flow cytometry (FCM) and
immunohistochemistry (IHC). Herein, we are documenting the detailed
clinicopathologic characteristics and cytogenetics features of these
patients. In addition, we conducted a systematic literature search
using PubMed, Google Scholar, and Scopus for patients with plasma cell
myeloma/ Plasma cell neoplasms and aberrant T-cell expression, using
pre-defined search terms and synonyms.
Results
Results of Patients diagnosed in our center (Table 1, first three cases).
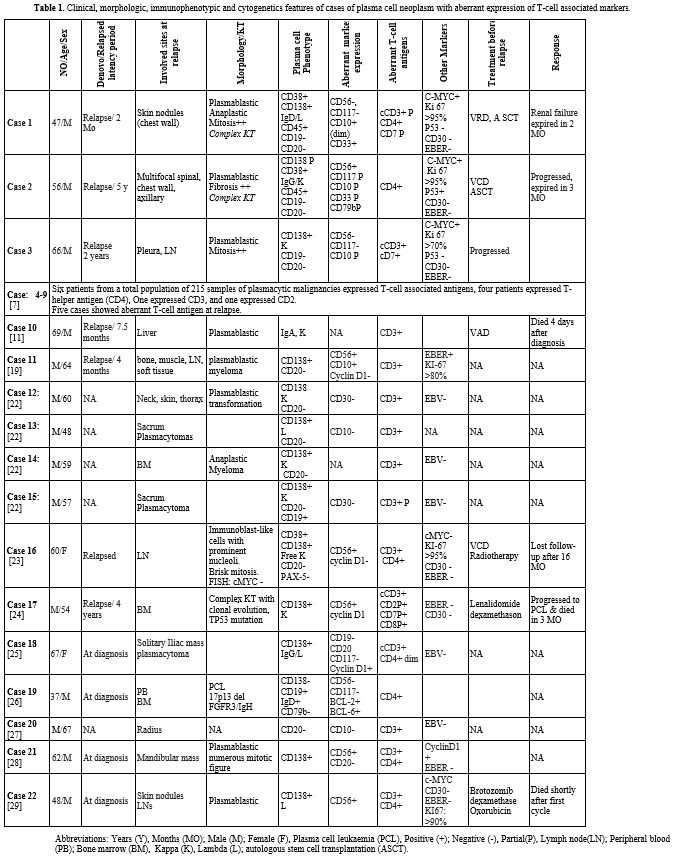 |
Table
1. Clinical, morphologic, immunophenotypic and cytogenetics features of
cases of plasma cell neoplasm with aberrant expression of T-cell
associated markers.
|
Case 1.
A 47-year-old male presented with multiple painless subcutaneous
swellings on the trunk, abdomen, and thigh, associated with significant
weight loss with no fever or night sweats. Serum protein
electrophoresis (SPE) showed two bands; IgD lambda and free lambda.
Free light chain (FLC) lambda was remarkably increased 1296 mg/L (5.71
- 26.30) with Kappa/Lambda (K/L) ratio of 0.01 (0.260 - 1.650). Skin
biopsy confirmed involvement by PCM with Lambda light chain restriction
and expression of BCL2 and c-MYC. FISH analysis was negative for C-MYC,
BCL2, BCL6, 17p deletion, and 14q32 rearrangements.
Bone marrow
(BM) examination confirmed the diagnosis of IgD-PCM; the plasma cells
mainly were mature looking mixed with few atypical forms, negative for
CD45, CD20, CD117, and CD56 with no immunophenotypic aberrancies
detected. The patient started on VRD chemotherapy (Bortezomib 1.5mg/m2
weekly/lenalidomide 25 mg for 21 days/Dexamethasone 20mg weekly) every
28 days cycle given for four cycles with complete resolution of skin
nodules. Then he underwent an Autologous stem cell transplant (ASCT).
Two
months later, the patient developed new skin nodules in the upper chest
wall and found a right renal mass. Peripheral blood (PB) revealed rare
circulating PCs detected on screening of peripheral smear. BM at
relapse revealed infiltration with many myeloma cells (78%) showing
marked pleomorphism including many forms with plasmablastic and
anaplastic morphology and increased mitotic figures (Figure 1; A and B). FCM (Figure 2)
showed a large population of lambda restricted monotypic PCs showing
variation in forward scatter (cell size), and side scatter (cytoplasm
complexity) and expressing CD45, CD38, CD138, dim CD10 with aberrant
expression of CD33 and CD4 with aberrant partial expression of CD7 and
cytoplasmic CD3. PCs were negative for CD19, CD20, CD117 & CD56. By
IHC (Figure 3). The PCs were
Lambda restricted and expressed cMYC, BCL-2 with confirmed aberrant
expression of CD4 and partial expression of CD7 and CD3. The PCs were
negative for CD20, CD56, CD117, P53, and EBV. The myeloma cells showed
a very high mitotic index reflected by KI-67 >90%. Cytogenetics
studies revealed complex karyotype: 47,X,Y, i(1)(q10), t(1;3)(p32;p13),
+der(3)t(1;3)(p32;p13), add(4)(q35), der(8)t(8;15)(q24;q11.2)
add(8)(p23), add(13)(q34), +20.[20]
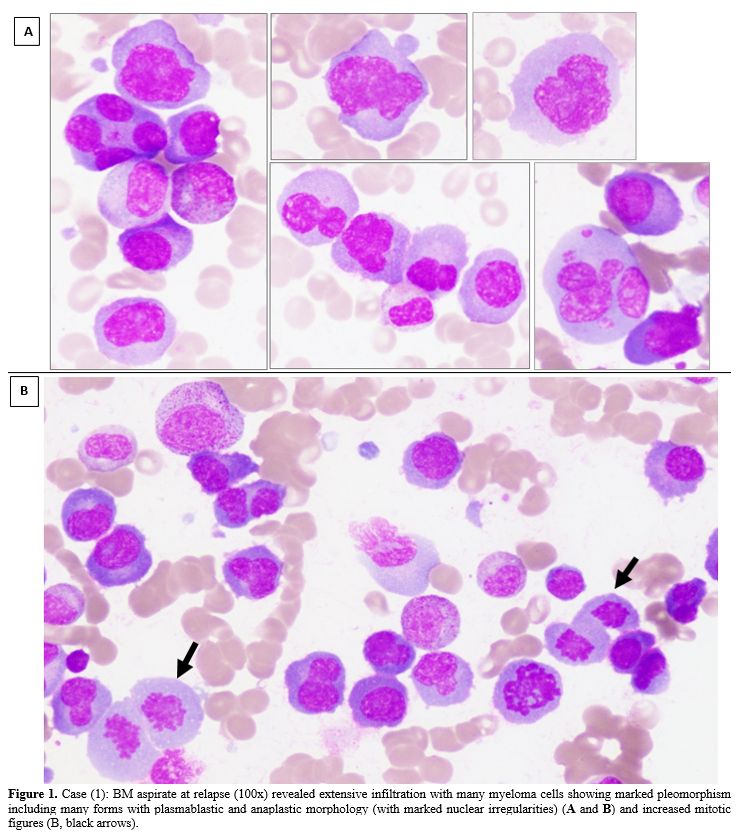 |
Figure 1. Case (1): BM
aspirate at relapse (100x) revealed extensive infiltration with many
myeloma cells showing marked pleomorphism including many forms with
plasmablastic and anaplastic morphology (with marked nuclear
irregularities) (A and B) and increased mitotic figures (B, black
arrows).
|
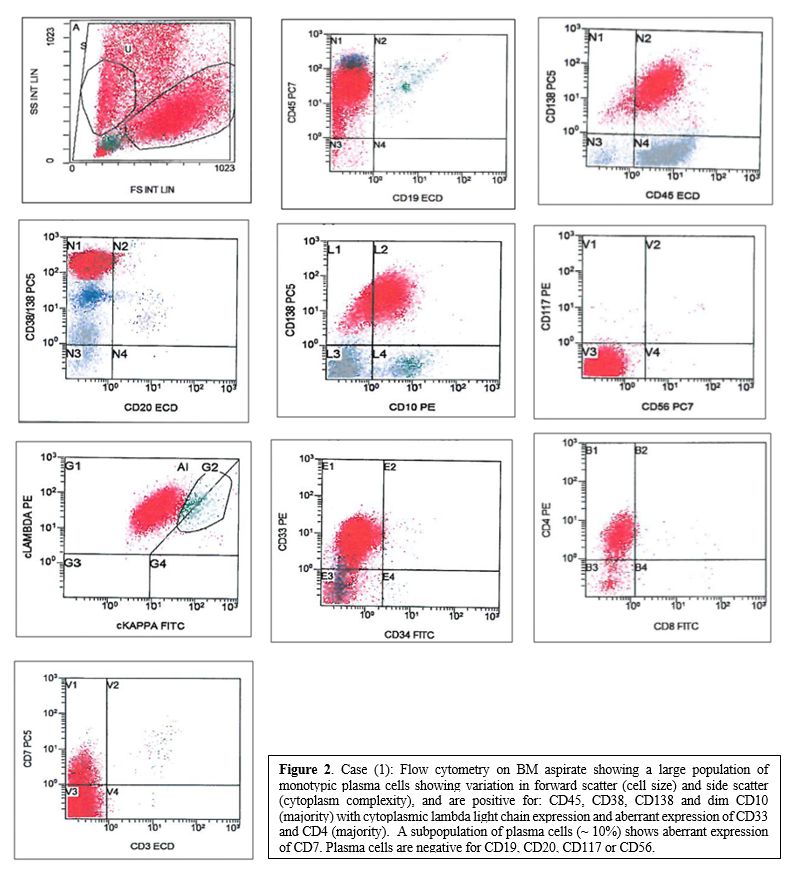 |
Figure 2. Case (1): Flow
cytometry on BM aspirate showing a large population of monotypic plasma
cells showing variation in forward scatter (cell size) and side scatter
(cytoplasm complexity), and are positive for: CD45, CD38, CD138 and dim
CD10 (majority) with cytoplasmic lambda light chain expression and
aberrant expression of CD33 and CD4 (majority). A subpopulation
of plasma cells (~ 10%) shows aberrant expression of CD7. Plasma cells
are negative for CD19, CD20, CD117 or CD56. |
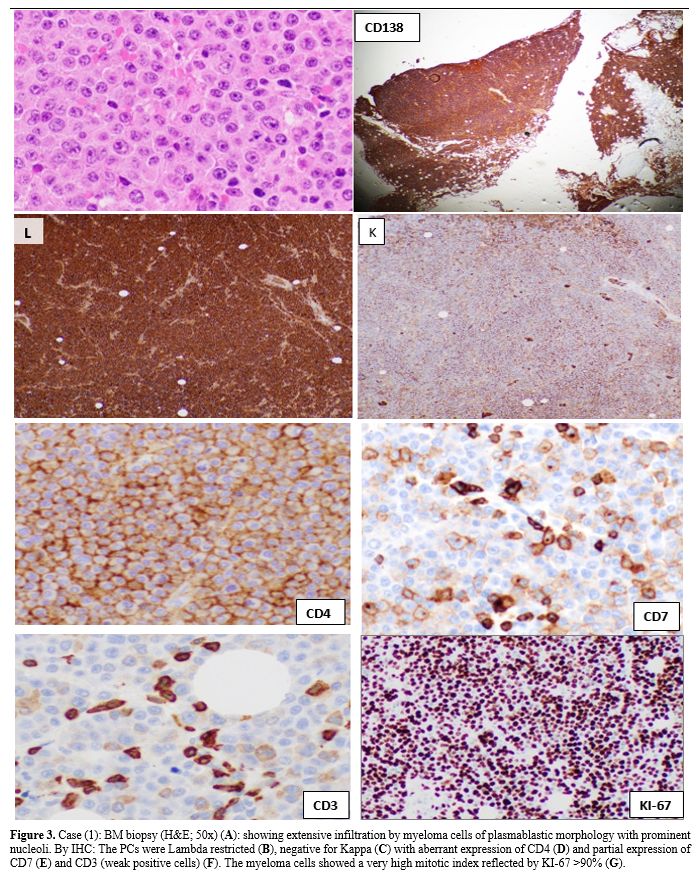 |
Figure 3. Case (1): BM
biopsy (H&E; 50x) (A): showing extensive infiltration by myeloma
cells of plasmablastic morphology with prominent nucleoli. By IHC: The
PCs were Lambda restricted (B), negative for Kappa (C) with aberrant
expression of CD4 (D) and partial expression of CD7 (E) and CD3 (weak
positive cells) (F). The myeloma cells showed a very high mitotic index
reflected by KI-67 >90% (G). |
PET/CT
showed multiple intramedullary lesions in bilateral humerus and femur,
multiple newly developed FDG-avid subcutaneous nodules in the right
upper chest wall, and right renal lesion (Figure 4).
The patient was started on second-line treatment (Carfilzomib 20 mg/m2) for two days; unfortunately, he progressively deteriorated and passed away.
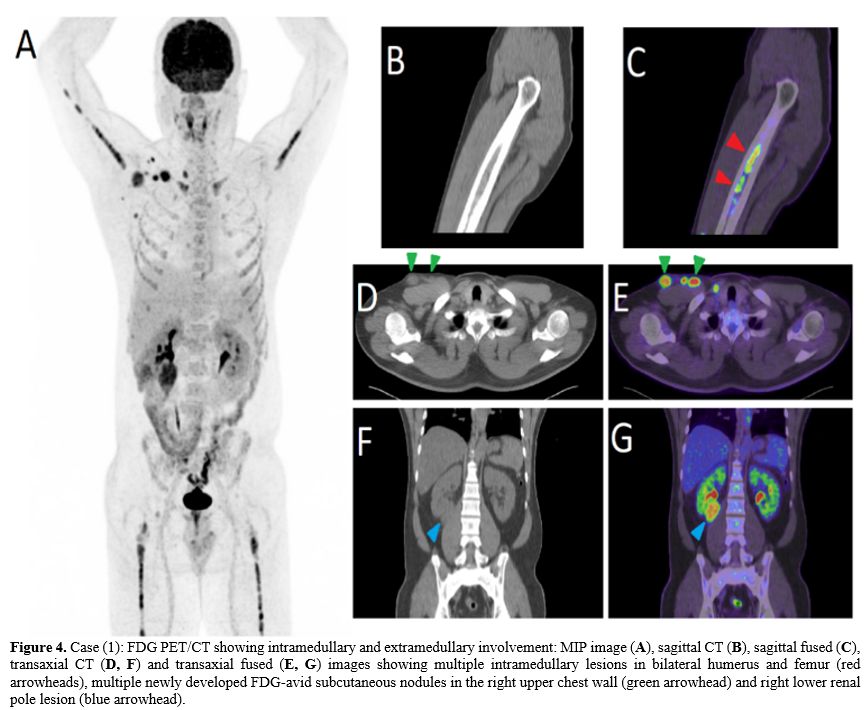 |
Figure 4. Case (1): FDG PET/CT showing intramedullary and extramedullary involvement: MIP image (A), sagittal CT (B), sagittal fused (C), transaxial CT (D, F) and transaxial fused (E, G)
images showing multiple intramedullary lesions in bilateral humerus and
femur (red arrowheads), multiple newly developed FDG-avid subcutaneous
nodules in the right upper chest wall (green arrowhead) and right lower
renal pole lesion (blue arrowhead).
|
Case 2.
A 56-year-old male presented with lower limb numbness with spinal
plasmacytoma (at T4 level) and 22% clonal plasma cells in the BM,
confirming PCM diagnosis.
The patient underwent decompressive
laminectomy and local radiotherapy followed by Lenalidomide (25 mg for
21 days every 28 days cycle) with dexamethasone for 12 cycles, and he
achieved complete remission.
Four years later, he relapsed and
retreated with Lenalidomide-dexamethasone for 7 cycles with partial
response, then he was started on VCD (Bortezomib -
Cyclophosphamide-Dexamethasone), followed by ASCT and achieved a second
complete remission, but no maintenance was given.
Sixteen months
later, he relapsed and was started on Carfilzomib-Dexamethasone, but he
progressed while on therapy with multifocal spinal lesions, right chest
wall, and axillary masses when he was treated with Pomalidomide and
Daratumumab. However, he progressed with a newly developed scalp mass
lesion.
At his latest presentation, SPE and immunofixation
showed IgG kappa monoclonal band (20.9 g/L), Kappa FLC was markedly
increased at (1227 mg/L), with a high K/L ratio at 204.5.
The BM
was infiltrated by many myeloma cells with plasmablastic morphology.
FCM on BM showed monotypic PCs (53%), with variable forward and side
light scattering, expressing CD45 and with a heterogeneous expression
of CD138 and CD38 with cytoplasmic kappa light chain restriction and
aberrant expression of CD56 and CD4. In addition, there was a partial
expression of CD10 and CD79b and aberrant partial expression of CD117
and CD33. This monotypic population is negative for CD19 and CD20.
BM
biopsy was hypercellular (90-95%) with extensive and diffuse
infiltration by sheets of abnormal kappa-restricted monotypic PCs,
which by immunostains were positive for CD138, MUM1, CD56, BCL2, c-Myc,
P53 with markedly suppressed residual hematopoiesis and increased
marrow fibrosis (MF 2). Karyotype was complex: 58~59, XY,
+der(1)t(1;17)(p12;q11.1), del(1)(p11p13), +3, +5, +6, +7, +7, +9, +11,
+15, -17, +18, +19, +21, +2mar[cp20]/46, XY.[14]
Overall findings concluded a diagnosis of plasmablastic transformation
of PCM. A new line of therapy, including
Elotuzumab–Pomalidomide–dexamethasone, was started, and unfortunately,
the patient shortly succumbed.
Case 3.
A-63-year-old male with a medical background of diabetes presented in
April 2017 with bone pain; imaging revealed multiple bony lytic lesions
and pleural-based soft mass (3x1.2cm).
Histopathologic examination
of CT-guided biopsy revealed KLC plasmacytoma, BM aspiration showed 3%
plasma cells, and the diagnosis of MM was concluded. At presentation,
the myeloma cells mostly were mature-looking, and they did not show
evidence of aberrant expression of any T-cell associated markers.
Back
then, the patient was treated with Bortezomib, Cyclophosphamide, and
Dexamethasone chemotherapy combination and achieved complete remission,
he underwent stem cell mobilization and harvesting, but he refused stem
cell infusion. Thereafter, the patient was kept on lenalidomide
maintenance and maintained complete remission. Two years later, the
patient presented with biochemical progression with increasing free
KLC, and was started on Daratumumab and dexamethasone. A PET/CT scan
after 3 months (Figure 5)
revealed disease progression with supraclavicular and axillary
lymphadenopathy and increased uptake within the muscles. Lymph node
(LN) biopsy (Figure 6) revealed
an effaced LNs architecture by diffuse sheets of PCs with many
plasmablasts, scattered anaplastic forms, and significantly increased
mitotic figures. By IHC stains, the neoplastic PCs are positive for
CD138, BCL-2, CD10 (weak), CD3 (weak), CD7 (weak), and c-MYC with Kappa
restriction.
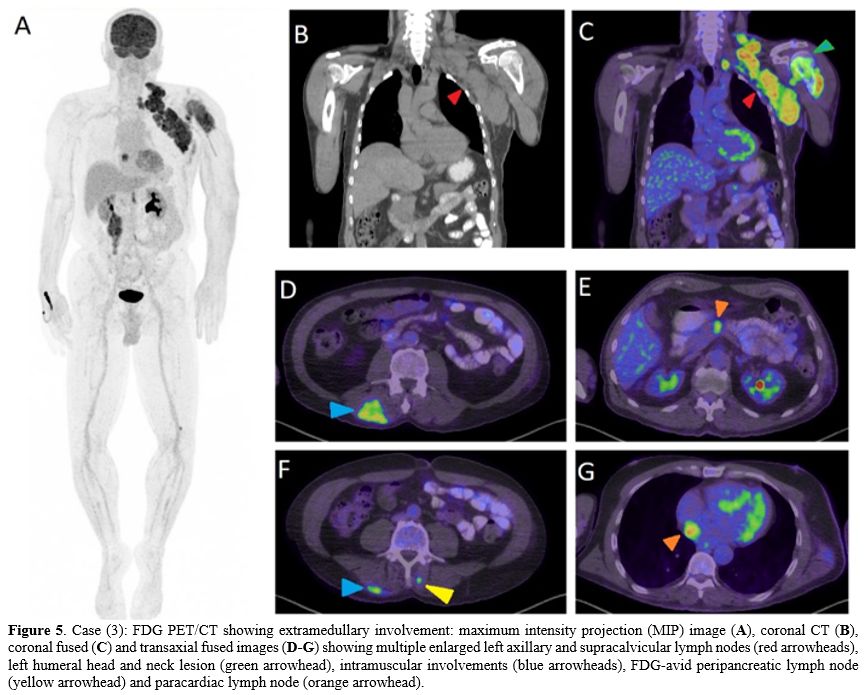 |
Figure 5. Case (3): FDG PET/CT showing extramedullary involvement: maximum intensity projection (MIP) image (A), coronal CT (B), coronal fused (C) and transaxial fused images (D-G)
showing multiple enlarged left axillary and supracalvicular lymph nodes
(red arrowheads), left humeral head and neck lesion (green arrowhead),
intramuscular involvements (blue arrowheads), FDG-avid peripancreatic
lymph node (yellow arrowhead) and paracardiac lymph node (orange
arrowhead).
|
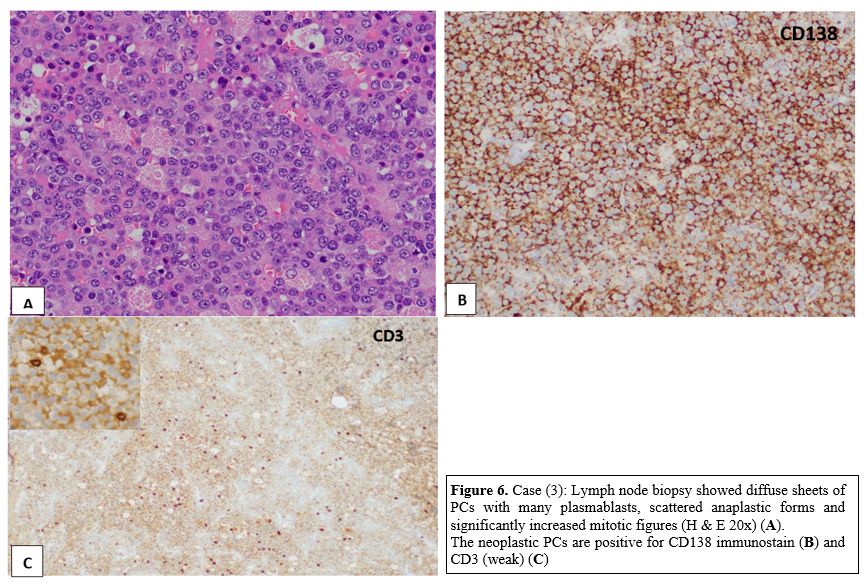 |
Figure 6. Case (3): Lymph
node biopsy showed diffuse sheets of PCs with many plasmablasts,
scattered anaplastic forms and significantly increased mitotic figures
(H & E 20x) (A). The neoplastic PCs are positive for CD138 immunostain (B) and CD3 (weak) (C) |
They are negative for CD45, CD20, PAX 5, CD30, CD4, CD8, CD56, BCL6, cyclin D1, HHV8, CD43, ALK–1 and EBER.
FISH
studies on tissue biopsy revealed negativity for BCL-2, BCL-6, and cMYC
rearrangements; karyotype was not performed. Carfilzomib was added to
Daratumumab and dexamethasone; however, the patient experienced
clinical and biochemical progression. Therefore, a new line of
chemotherapy [cisplatin, doxorubicin, etoposide, cyclophosphamide]
combined with pomalidomide and Carfilzomib was started for two cycles
the patient planned for ASCT.
Results of similar cases (Literature review) (Table 1, cases 4-22).
Upon an extensive review of English literature, a total of 22 cases of
PCNs (including the three cases reported here) showed aberrant
co-expression of T-cell associated markers. In addition, 12 out of 17
cases were relapsed PCN while five patients showed an aberrant
expression of T-cell associated antigens at their initial presentation.
Among PCN that showed aberrant expression of T-cell associated
antigens, 14/16 were male patients and two female patients. The median
age of the patients is 57.5 years (range 37-69).
The majority of
these patients (10 out of 16 cases) showed evidence of extramedullary
involvement either at presentation or in association with aberrant
T-cells markers acquisition; with cutaneous/soft tissue involvement
being the most frequently involved extramedullary sites (in five
patients) and lymph node involvement in four patients, while four
patients had solitary extramedullary plasmacytomas.
Anaplastic/plasmablastic
morphology was reported in 11/12. 6/6 cases (including all three cases
reported in our center) had a high proliferation index reflected by
high KI-67 >60-95% with frequent mitotic figures. EBER/EBV was
negative in 12 out of 13 cases
Upon review of the prevalence of
T-cell markers expressed on the neoplastic PCs, in the majority of
cases (15/22), the clonal PCs showed aberrant expression of CD3
(surface or cytoplasmic at different intensities), 11/ 22 (50%) showed
aberrant expression of CD4. Other T-cell associated markers were rarely
reported; CD7 in 3/22 cases, CD2 in 2/22, and CD8 in 1/22 cases. 10/12
cases had poor outcome with very short survival.
Discussion
Aberrant
expression of differentiation markers of a different cell lineage on
the malignant hematopoietic cells is well documented in the literature.
In addition, lineage ambiguity has been recognized in various
hematopoietic neoplasms, particularly in those originating from early
precursors.[2]
B-cell neoplasms co-expressing
T-cell-associated antigens (other than CD5 or CD43) have been only
rarely reported. In addition, aberrant expression of T-cell related
markers like CD2, CD3, CD4, CD5, CD7 or CD8 has been rarely documented
in chronic lymphocytic leukemia, diffuse large B cell lymphoma,
ALK-positive large B cell lymphoma, plasmablastic lymphoma, and
Waldenstrom's Macroglobulinemia (WM).[3-8]
Most
of the reported B-cell neoplasms with aberrant expression of T-cell
associated markers were EBV-associated malignancies, including
immunodeficiency-associated lymphomas and PBL.[9]
Upregulation
of T-cell markers (by gene expression profiling) was detected in a
subset of B-cells in patients with WM, suggesting that this small
population of cells may have de-differentiated during tumor
development.[8]
Comparable to other types of
hematologic neoplasms, PCN can show aberrant expression of different
lineages' associated antigens with co-expression of B & or myeloid
lineage associated antigens being the most commonly reported.[10] Aberrant expression of CD10, CD13, or CD33 on myeloma cells has been associated with poor prognosis.[11] CD43 and cytokeratin expression on myeloma cells had been very rarely reported.[12]
Gorczyca and colleagues showed that an aberrant immunophenotype is
detected mainly in poorly differentiated or anaplastic myelomas
associated with poor prognosis.[13]
Nevertheless,
PCNs co-expressing T-cell associated markers are extremely rare,
specifically the co-expression of more than one T-cell differentiation
marker.
Like PCL, IgD MM often afflicts younger patients than IgG
or IgA MM, and often presents as an aggressive disease, with clinical
and analytical features associated with bad prognosis (renal failure,
thrombocytopenia, elevated LDH, and beta2-microglobulin, abnormal
cytogenetics).[10] Lambda-chain preference is also correlated with IgD disease.[10-12]
Among
the myeloma cases (151 cases) diagnosed in our center (NCCCR); from
2010 to 2020; we detected three cases of relapsed PCN with aggressive
morphologic features (plasmablastic /anaplastic) with extramedullary
involvement, high proliferative index ki67 >90%, and all of them
showed an aberrant acquisition of T-cell markers with aggressive
clinical course. Expression of T-cell associated markers on clonal PCs
was confirmed not only by mean of FCM immunophenotyping but also by
IHC, with different antibody clones used in each technique (monoclonal
antibody UCHT1 mouse monoclonal antibody in FCM) and polyclonal Rabbit
Anti-Human CD3 (Dako Omnis) antibody used by IHC. The T-cell markers
were not detected at the initial diagnosis.
The reported cases
were detected on a prospective basis, and there was not a comprehensive
retrospective screen; therefore, a reliable estimate of prevalence was
not postulated.
In the majority of reviewed cases (12/17), the
acquisition of T-cell markers occurs mostly during the relapsed status
when limited immunophenotypic markers (not usually including T-cell
markers) are performed. Hence, the accurate prevalence of aberrant
expression of T-cell associated markers in PCN might be underestimated.
The
incidence of extramedullary involvement in Multiple Myeloma (MM) was
reported between 7-18% at diagnosis and 20% at relapse or progression.[14]
In
a large series analysis including 1965 patients, Usmani et Al. found
that the frequency at diagnosis of extramedullary plasmacytoma (EMP)
was 3.4%, with skin and subcutaneous nodules being the most frequently
involved sites.[15]
The association between
immature plasma cell morphology (plasmablastic) and adverse myeloma
outcome has been well documented in the literature. Hao et al. recently
demonstrated that morphologic features, including plasmablastic
morphology, and high mitotic index, significantly correlate with high
risk disease.[16]
Moreover, anaplastic myeloma
variant, in which the malignant PCs are highly pleomorphic, has been
reported more common in younger patients with a predisposition for the
extramedullary site and poor prognosis.[14,17] This morphologic variant may present initially at diagnosis[16] or as a feature of disease progression.[18]
Aberrant
expression of T-cell antigens has also been rarely described in cases
with the plasmablastic transformation of PCM, and it has been
postulated that EBV may stimulate T-cell antigen expression in
B-lineage neoplasms. However, the majority of cases described in this
report were having previously well-established diagnosis of PCN. In
addition, the lack of expression of CD30 and EBER/EBV (except in case
11[19] would argue against the plasmablastic transformation of PCM.
The majority of PCN typically have a low proliferation rate with Ki67 < 10%.[20]
However, morphologically aggressive PCM with extramedullary involvement
has shown a very high Ki67 proliferative index up to 55% to 96% with a
strong association with 13q deletion and abnormalities of chromosome 1.[21]
While Spier et Al.[7]
reported no difference in the presenting clinical features, histology,
and plasma cell morphology from MM patients who did not express the
T-cell antigen; however, the same group reported a very short survival
from the demonstration of T-antigens (2-7+ months), with 5/6 (80%)
patients died ≤5 months after the study. Similarly, Oliveira et Al.[22]
suggested that CD3 expression is associated with disease progression
and poor prognosis of PCN. Two of three cases detected in our center
died within three months of the latest relapse.
Conclusions
Here
we discuss an interesting yet rare finding of aberrant acquisition of
T-cell associated markers on PCM. This review emphasizes the importance
of recognizing atypical and rare immunophenotypic aberrancies in PCN
that could lead to diagnostic pitfalls (particularly with
extramedullary involvement) and provide important prognostic
information.
We conclude that there is an evident association
between aberrant expression of T-cell associated markers on PCM and
aggressive disease, including plasmablastic morphology, high KI-67,
extramedullary involvement, and adverse outcome with short survival.
References
- S. H. Swerdlow, E.
Campo, N. L. Harris et al., 2016
revision of the World Health Organization classification of lymphoid
neoplasms, Blood, vol. 127, no. 20, pp. 2375-2390,2016. https://doi.org/10.1182/blood-2016-01-643569
- Lau
LG, Tan LK, Koay ES, Ee MH, Tan SH, Liu TC. Acute lymphoblastic
leukemia with the phenotype of a putative B-cell/T-cell bipotential
precursor. Am J Hematol. 2004;77(2):156-60. https://doi.org/10.1002/ajh.20163
- Suzuki
Y, Yoshida T, Wang G, Aoki T, Katayama T, Miyamoto S, et al. Incidence
and clinical significance of aberrant T-cell marker expression on
diffuse large B-cell lymphoma cells. Acta Haematol 2013;130(4):230-7 https://doi.org/10.1159/000348550
- Tsuyama
N, Ennishi D, Yokoyama M, Baba S, Asaka R, Mishima Y, et al. Clinical
and prognostic significance of aberrant T-cell marker expression in 225
cases of de novo diffuse large B-cell lymphoma and 276 cases of other
B-cell lymphomas. Oncotarget 2017;8(20):33487-500 https://doi.org/10.18632/oncotarget.16532
- Samah
Kohla, Feryal A. Ibrahima; Deena Mudawi; Susanna Akiki, Dina Soliman,
et al. High-Grade Epstein-Barr Virus-Negative Biphenotypic Lymphoma
with Expression of B- and T-Cell Markers and Leukemia Presentation:
Case Report and Literature Review. Case Rep Oncol 2020;13:1215-1226 https://doi.org/10.1159/000510403
- Pan
Z, Hu S, Li M, Zhou Y, Kim YS, Reddy V, et al. ALK-positive Large
B-cell Lymphoma: A Clinicopathologic Study of 26 Cases With Review of
Additional 108 Cases in the Literature. Am J Surg Pathol
2017;41(1):25-38 https://doi.org/10.1097/PAS.0000000000000753
- Spier CM, Grogan TM,
Durie BG, et al. T-cell antigen-positive multiple myeloma. Mod Pathol
1990; 3: 302-7. https://doi.org/10.1182/blood.V73.3.763.bloodjournal733763
- Hao
M, Barlogie B, Tricot G, Liu L, Qiu L, et al. Gene Expression Profiling
Reveals Aberrant T-cell Marker Expression on Tumor Cells of
Waldenström's Macroglobulinemia. Clin Cancer Res; 25(1) January 1,
2019. https://doi.org/10.1158/1078-0432.CCR-18-1435
- Petitjean
B, Jardin F, Joly B, et al. Pyothorax-associated lymphoma: a peculiar
clinicopathologic entity derived from B cells at late stage of
differentiation and with occasional aberrant dual B- and T-cell
phenotype. Am J Surg Pathol. 2002;26:724-732. https://doi.org/10.1097/00000478-200206000-00005
- Epstein
J, Xiao HQ, He XY. Markers of multiple hematopoietic-cell lineages in
multiple myeloma. N Engl J Med. 1990 Mar 8;322(10):664-8. https://doi.org/10.1056/NEJM199003083221005
- Yagci
M, Sucak GT, Akyol G, Haznedar R. Hepatic failure due to CD3+ plasma
cell infiltration of the liver in multiple myeloma. Acta Haematol 2002;
107: 38-42 https://doi.org/10.1159/000046628
- Shin
JS, Stopyra GA, Warhol MJ, Multhaupt HA. Plasmacytoma with aberrant
expression of myeloid markers, T-cell markers, and cytokeratin. J
Histochem Cytochem. 2001 Jun;49(6):791-2. https://doi.org/10.1177/002215540104900613
- Gorczyca W. Atlas of
differential diagnosis in neoplastic hematopathology. 3rd ed. Boca
Raton: CRC Press, 2014; 359-81. https://doi.org/10.1201/b16685-16
- Bladé
J, Lust JA, Kyle RA. Immunoglobulin D multiple myeloma: presenting
features, response to therapy, and survival in a series of 53 cases. J
Clin Oncol. 1994;12:2398-404 https://doi.org/10.1200/JCO.1994.12.11.2398
- Usmani
SZ, Heuck C, Mitchell A, et al. Extramedullary disease portends poor
prognosis in multiple myeloma and is over-represented in high-risk
disease even in the era of novel agents. Haematologica. 2012;97:1761-7.
https://doi.org/10.3324/haematol.2012.065698
- Hao
Y, Khaykin D, Machado L, et al: Bone marrow morphologic feature, MyPRS
and gene mutation correlations in plasma cell myeloma. Mod Pathol, 2019
https://doi.org/10.1038/s41379-019-0333-6
- Elsabah
H, Soliman DS, Ibrahim F. et al.: Plasma Cell Myeloma with an
Aggressive Clinical Course and Anaplastic Morphology in a 22-Year-Old
Patient: A Case Report and Review of Literature. Am J Case Rep, 2020;
21: e920489 https://doi.org/10.12659/AJCR.920489
- Akihito
F, Yasuhiro N, Naofumi Y, Yuji K: Morphological transformation of
myeloma cells into multilobated plasma cell nuclei within 7 Days in a
case of secondary plasma cell leukemia that finally transformed as
anaplastic myeloma. Case Rep Hematol, 2017; 2017: 5758368 https://doi.org/10.1155/2017/5758368
- Jai-Hyang
Go. Aberrant CD3 Expression in a Relapsed Plasma Cell Neoplasm. Journal
of Pathology and Translational Medicine 2018; 52: 202-205 https://doi.org/10.4132/jptm.2017.09.05
- Marković
O, Marisavljević D, Cemerikić V et al: Proliferative activity of
myeloma cells determined by Ki-67 antibody: Biological and clinical
significance. Vojnosanit Pregl, 2005; 62(1): 33-38 https://doi.org/10.2298/VSP0501033M
- Juskevicius
R, Murthy H, Dangott B: Plasma cell myeloma with very high Ki67
proliferation rate: comparison of visual estimation and computational
image analysis with description of clinical and pathologic features. Am
J Clin Pathol, 2015; 144(2): A132 https://doi.org/10.1093/ajcp/144.suppl2.132
- Jennifer
L. Oliveira, Karen L. Grogg, William R. Macon, et al. Clinicopathologic
Features of B-Cell Lineage Neoplasms with aberrant expression of CD3. A
Study of 21 Cases. Am J Surg Patho. 2012 Sep;36(9):1364-70. https://doi.org/10.1097/PAS.0b013e31825e63a9
- Mishra
P, Kakri S, Gujral S. Plasmablastic transformation of plasma cell
myeloma with heterotropic expression of CD3 and CD4: a case report.
Acta Clin Belg 2017;72(4):250-3 https://doi.org/10.1080/17843286.2016.1201629
- Habermehl
GK, Chesser JD, Theil KS, Rogers HJ. Very unusual expression of
multiple aberrant T-cell markers in plasmablastic plasma cell myeloma.
Int J Lab Hematol. 2019 Aug;41(4):e89-e91. Epub 2019 Feb 11. PMID:
30742364. https://doi.org/10.1111/ijlh.12985
- Smita
P. & Bachir A. Aberrant T-cell antigen expression in a plasma
cell
neoplasm. Blood works. Images in hematology ASH image Bank DOI https://doi.org/10.1182/blood-2018-04-843730
- Sorigue
M, Junc_a J, Gassiot S, Mill_a F, Mate J-L, and Navarro JT. A Case of
CD138-/CD19+/CD4+ IgD Plasma Cell Leukemia. Cytometry Part B 2015; 88B:
69-73. https://doi.org/10.1002/cyto.b.21173
- Tang
YL, Chau CY, Yap WM, Chuah KL. CD3 expression in plasma cell neoplasm
(multiple myeloma): a diagnostic pitfall. Pathology 2012; 44: 668-70. https://doi.org/10.1097/PAT.0b013e328359fba6
- Luo
X, Kuklani R, Bains A. Dual CD3 and CD4 positive plasma cell neoplasm
with indistinct morphology: a diagnostic pitfall. Pathology 2016; 48:
378-80. https://doi.org/10.1016/j.pathol.2016.02.019
- Varricchio
S, Pagliuca F, Travaglino A, Gallo L, Villa MR, Mascolo M. Cutaneous
localization of plasmablastic multiple myeloma with heterotopic
expression of CD3 and CD4: Skin involvement revealing systemic disease.
J Cutan Pathol. 2019;46:619-622. https://doi.org/10.1111/cup.13486
[TOP]






