Maja Ružić1, Natalija Rajić1, Milotka Fabri2, Ivana Urošević3, Marina Dragičević Jojkić3, Tomislav Preveden1, Maria Pete1 and Nebojša Rajić3.
1Faculty of Medicine, University of Novi Sad, Clinical Centre of Vojvodina, Clinic for Infectious Diseases.
2 Faculty of Pharmacy of Novi Sad, Business Academy University in Novi Sad.
3 Faculty of Medicine, University of Novi Sad, Clinical Centre of Vojvodina, Clinic for Hematology.
Correspondence to: Maja
Ružić, MD, PhD, Professor of Infectious Diseases, Faculty of Medicine,
Clinical Centre of Vojvodina, University of Novi Sad, Serbia. Tel:
+381641646026. E-mail:
maja.ruzic@mf.uns.ac.rs
Published: September 1, 2021
Received: April 23, 2021
Accepted: August 8, 2021
Mediterr J Hematol Infect Dis 2021, 13(1): e2021058 DOI
10.4084/MJHID.2021.058
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Treating HCV in people with hemophilia prevents the development of
end-stage liver disease (ESLD) and hepatocellular carcinoma (HCC) and
greatly increases the quality of life for people living with
hemophilia. There are many obstacles in reaching the WHO goal of
globally eradicating HCV by 2030, mainly its scale, complexity, and
implementation. That is why many countries have implemented a
micro-elimination strategy: a pragmatic elimination approach in
populations with the most efficacy. The aim of this publication is to
present the morbidity and mortality rates, the clinical course and
treatment outcomes of chronic HCV infection in people with hemophilia
(PwH), as well as to show an example of a successfully conducted HCV
micro-elimination strategy among people with hemophilia in the Province
of Vojvodina.
Methods: A
retrospective, single-center study, performed using medical
documentation of all registered PwH in the Clinical Center of Vojvodina
from 1994. until 2020. It included 74 hemophilia patients, out of which
32 were patients with hemophilia and chronic HCV infection.
Results:
The mean age of HCV-positive positive people with hemophilia (PwH) was
42.3 years, with the duration of infection of 30-35 years. Co-infection
with HIV was observed in 6.25% of cases. Furthermore, 18.75% of
patients had spontaneous HCV elimination, and 75% were treated with
antiviral protocols. Cirrhosis developed in 21.87% with an incidence
rate of 0.6 per 100 patient-years. After treatment with Pegylated IFN
and ribavirin (RBV), 58.3% achieved SVR. Side effects of IFN-based
therapy regimens were recorded in 20.8% of treated (PwH). In 37.5% PWH,
DAA protocols were administered, and these patients achieved SVR. HCV-
PwH have a statistically higher mortality rate than non-infected people
with hemophilia. Among the HCV-positive PwH, hemophilia-related deaths
were 6.25%, and HCV-related deaths were 9.37%. Currently, in the
Registry of PwH in Vojvodina, there are no patients with active HCV
infection.
Conclusion: The
micro-elimination strategy in the subpopulation of PwH was successfully
implemented in Vojvodina by hematologists and infectious diseases
specialists in close collaboration.
|
Introduction
The prevalence of hepatitis C virus (HCV) positive cases ranks Serbia in the range of mid-endemic European countries.[1]
Chronic HCV infection is the leading cause of a rising prevalence in
end-stage liver disease (ESLD): cirrhosis and hepatocellular carcinoma
(HCC).[2] Currently, the world is facing an epidemic
of HCV-related complications in patients who received transfusions of
blood and blood derivatives until the 1990s, making people with
hemophilia (PwH) one of the most vulnerable populations.[3]
Even though hemophilia has become the prime example for successful
prevention in chronic illnesses using coagulation factors, the
blood-borne transmission of HCV was nearly inevitable until the 1990s
because of the technological processes involved in the development of
blood derivatives, making this infection endemic in PwH.[4,5]
Until
the discovery of direct-acting antivirals (DAA), chronic HCV infection
was incurable for the 30 to 50% of patients treated with
Interferon-based therapy regimens. PwH were frequently put in the group
of non-responders, or worse, and doctors were unwilling to treat them
with this regiments due to its side effects. DAA treatments improve the
SVR rate up to 98%, and because of this, the world is on the verge of
HCV eradication.[6] However, this goal may be prolonged due to the socio-economic factors in low-income countries.[2]
Consequently, many authorities have supported a "step by step" micro
elimination strategy for HCV: a pragmatic approach to identification
and treatment of populations where it would have the highest efficacy.
Thanks to the enormous prosperity of the preventive measures,
mortality, and morbidity of PwH are greatly improved.[7]
Unfortunately, HCV is the leading cause of death in PwH, and this group
should be considered a priority according to the micro-elimination
strategy.[8]
The aim of this publication is to
present the morbidity and mortality rates, clinical course, and
treatment outcomes of chronic HCV infection in PwH, as well as to show
an example of a successfully conducted HCV micro-elimination strategy
among this endangered subpopulation in the Province of Vojvodina,
bringing us the one step closer to the World Health Organizations'
(WHO) global goal of eradicating HCV by the year 2030.[9]
Material and Methods
This
single-center study is retrospective and includes 74 hemophilia A and B
patients who were followed in the tertiary healthcare system in the
northern region of Serbia (the Province of Vojvodina) from 1994 to
2020.
Monitoring and bleeding prevention and treatment of PwH
have been taking place in our institution since 1994 when the Registry
of people with hemophilia was formed. The severity of hemophilia was
classified as mild (5–40% of normal factor level), moderate (1–5% of
normal factor level), or severe (<1% of normal factor levels).[4] According
to the WFH recommendations for testing PWH for blood-borne diseases,
all registered patients were tested for the presence of anti-HCV
antibodies.[7]
Between 1994 and 2020, 32 PwH were
identified as anti-HCV antibody positive. They were diagnosed and
treated using the EASL recommendations for the Management of HCV
infection.[9] Up to 2015, patients were treated with
IFN based therapy regiment with partial success. In 2016, with the
introduction of DAA therapy, the last 9 PwH with chronic hepatitis C
were proclaimed priority in our medical center and successfully cured
using the newer DAA regiment by December 2020 (Figure 1).
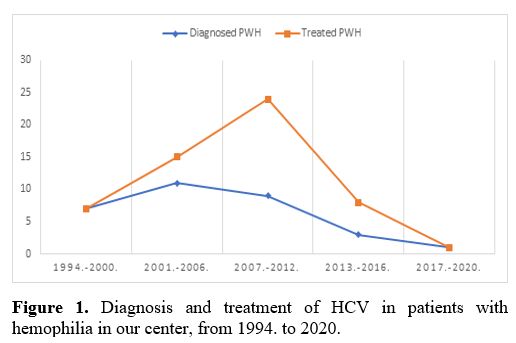 |
Figure 1. Diagnosis and treatment of HCV in patients with hemophilia in our center, from 1994. to 2020. |
To
present a successfully conducted HCV micro-elimination strategy among
PwH in the northern region of Serbia, we examined in this study
multiple factors: patient age, how HCV infection was detected,
infection duration (time passed from the first exposure to blood
products), co-infections (HIV, HbsAg, anti-HBc antibodies), number of
HBV vaccinated PwH, HCV viral copies, HCV genotype, aminotransferase
activity levels, presence of liver cirrhosis and HCC (confirmed by
imaging diagnosis), HCV treatment options and outcomes (Sustained
Virological Response – SVR), patient mortality. The study was approved
by the local medical ethics committee.
Statistical analysis was
performed using the student test (t-test) with the software program
SPSS version 23. g. Categorical variables were presented as frequencies
and percentages, and continuous variables as means and standard
deviation (SD) or medians and interquartile ranges (IQR) for variables
with skewed distributions. Statistical significance was set at p-value
<0.05.
Results
Patient Population.
There were 74 hemophilia A and B patients in our institution, of whom
32 (43.25%) were anti-HCV antibody positive, and this subgroup of
patients will be further analyzed (Table 1).
The prevalence of anti-HCV positivity in our population of persons with
hemophilia was 0.43%. All anti-HCV antibody positive PwH were male,
with a mean patient age of 42.4 years (42.43 ±3.84, n=32).
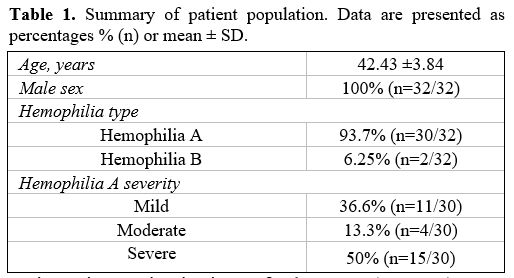 |
Table 1. Summary of patient population. Data are presented as percentages % (n) or mean ± SD. |
Following
WHF recommendations for testing PwH for blood-borne diseases, we
discovered 28/32 (87.5%) of anti-HCV antibody-positive patients, while
4/32 (12.5%) were found within ESLD etiology examinations. In addition,
from first blood products exposure, patients were observed through a
median follow-up time of 30 years (IQR 30–35).
Co-infection with
HIV was observed in 2/32 (6.25%) PwH. Hepatitis B (HBV) viral infection
markers were studied in 14/32 (43.75%): total HBc was present in 4/14
(28.6%) patients, while 3/14 (21.4%) were HbsAg positive. All seven
patients were PCR HBV DNA negative. In total, 5/32 (15.6%) anti-HCV
antibody positive PwH were HBV vaccinated.
Spontaneous HCV Elimination.
Spontaneous HCV elimination was observed in 6/32 (18.75%) PwH, and it
is determined using two consecutive negative PCR HCV RNA tests over six
months.
Characteristics of Chronic HCV Infection. The biochemical and virologic parameters of 26/32 (81.25%) PwH with chronic HCV infection are shown in the table below (Table 2).
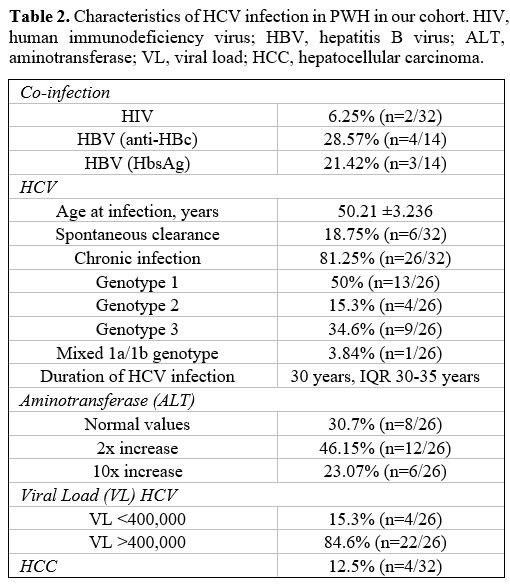 |
Table 2. Characteristics
of HCV infection in PWH in our cohort. HIV, human immunodeficiency
virus; HBV, hepatitis B virus; ALT, aminotransferase; VL, viral load;
HCC, hepatocellular carcinoma. |
Cirrhosis
developed in 7/26 (26.9%) of patients, and an incidence rate of 0.6 per
100 patient-years. Out of seven patients with liver cirrhosis, three
are currently alive with compensated cirrhosis (Child-Pugh Score A) and
have achieved SVR. HCC was present in 4/26 (15.4%) HCV-positive PwH in
the Registry, and three patients with cirrhosis have died due to HCC (Table 2).
Treatment. From 1994. until today, our institution has treated 24/26 (92.3%) PwH with chronic HCV infection with antivirals (Figure 1).
The mean age of PwH treated for HCV infection was 50.21 years (50.21
±3.24, n=24). The average time PwH waited for antiviral treatment was
4.96 years (4.96 ±1.92, n=24).
IFN based therapy was conducted
in 20/24 patients (83.3%) – SVR was achieved in 14/20 (70%), while in
6/20 (30%) PWH, the response to this line of treatment was not
satisfactory. Side effects of IFN-based therapy regiment were recorded
in 5/20 (25%) of treated PwH. DAA treatment regimens were conducted in
9/24 (37.5%) – 5 of them were non-responders or had relapsed on IFN
based therapy, and 4 of them were naïve (Figure 2).
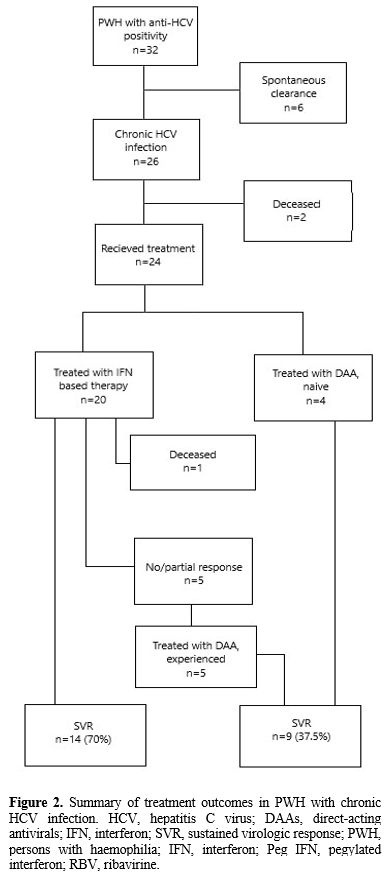 |
Figure 2. Summary of
treatment outcomes in PWH with chronic HCV infection. HCV, hepatitis C
virus; DAAs, direct-acting antivirals; IFN, interferon; SVR, sustained
virologic response; PWH, persons with haemophilia; IFN, interferon; Peg
IFN, pegylated interferon; RBV, ribavirine. |
Mortality.
The cumulative mortality rate for people with hemophilia A and B in our
center is 13.51%, and the yearly mortality rate is 0.09% per year. From
1994. until 2020, 9/32 (28.1%) of anti-HCV antibody positive PWH died,
with an annual mortality rate of 1.07% per year. For non-infected PwH,
the yearly mortality rate is 0.09%, and in total, 1/42 (2.3%) of
non-infected PWH died. Thus, HCV-positive PwH have a statistically
higher mortality rate than non-infected people with hemophilia
(Fisher's exact test, p=0.0016, p<0.05, n=74) (Table 3).
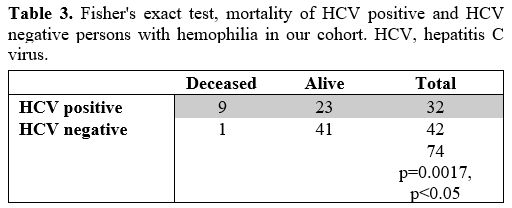 |
Table 3. Fisher's exact
test, mortality of HCV positive and HCV negative persons with
hemophilia in our cohort. HCV, hepatitis C virus. |
At the time of death in anti-HCV antibody positive PwH, the average age is 53.7 years (53.7 ±6.74, n=9).
Among
the deceased anti-HCV antibody positive PwH, hemophilia-related deaths
were 2/32 (6.25%), and HCV-related deaths were 3/32 (9.37%), and the
remaining 4/32 (12.5%) died from other causes.
Ending in December
2020, after 35 years of treating HCV-positive patients with hemophilia
in our institution, we can conclude that in the northern region of
Serbia, there are no active HCV infections in this population.
Discussion
The
prevalence of HCV infection in persons with hemophilia in Serbia is
thought to be around 0.37%, but until now, we did not have a definite
number.[10] We can confirm that every patient with
hemophilia A and B in the northern region of Serbia (the Province of
Vojvodina) has been tested for anti-HCV antibodies and that the
prevalence is 0.43% (43.25%). The majority of patients (87.5%) were
screened for anti-HCV by the hematologist at the moment of registration
in our institutions' Hemophilia Registry, unrelated to the severity of
hemophilia. However, 12.5% of PwH were discovered late while diagnosing
ESLD, and until then, they have never been included in the Registry or
examined by a hematologist. In the rest of Serbia, only 57.3% of PwH
have been tested for anti-HCV antibodies, and 37.5% were positive.[10]
However, the exact prevalence of HCV infection (past or active) in PWH
in Serbia is unknown. This "gap" in the prevalence of HCV among PwH in
the different regions in Serbia supports the position of The European
Hemophilia Consortium against centralized hemophilia supervision.[11]
The recorded prevalence of anti-HCV antibody positive PwH in Vojvodina
does not differ significantly from other European countries with a
similar socio-economic status (Hungary, Slovenia, Croatia) during the
'60s, '70s, and '80s but is expectedly lower than the most developed
countries of the world (the USA 90%, Austria 80%, Italy 83%, Denmark
51% prevalence).[12] This is a paradox caused by
poorly guided policies of donating blood (donating blood for profit,
unprecise epidemiological surveys for donors etc.) and inadequate
response of doctors and regulatory bodies at the beginning of the HCV
epidemic.[13] Namely, in highly developed countries,
coagulation factor concentrates were made out of a pool of 20-30 000
voluntary blood donors. By fault of inadequate triage, most of them
belonged to the high-risk population for blood-borne diseases (mostly
in the USA). Apart from that, not adhering to screening tests and viral
inactivation processes resulted in a high risk of transmission, 5% per
ordinated unit of factor concentrate until 1991. At the same time, the
use of factor concentrates was far more flexible widespread in these
countries.8 In underdeveloped countries such as Serbia, hemophilia
treatment was administered with restrictive protocols,
cryoprecipitates, or fresh-frozen plasma, made from a much smaller pool
of blood donors who were part of the local community.[14]
Spontaneous
HCV clearance (seroconversion) is confirmed using two consecutive HCV
RNA tests in the span of 6 months. Patients with spontaneous HCV
clearance were defined as PwH with positive anti-HCV antibodies and
negative HCV RNA (HCV Ab+/RNA-) without prior antiviral therapy.
Spontaneous HCV elimination was confirmed in 18.75% of PwH, a lower
rate than in most studies, where the rates range from 20% to 40%.[15]
A good prognosis of HCV infection is determined by a complex set of
interactions between virus and host that is only partly understood.
Male sex and genotype 1 are probably linked to a clearance rate that is
lower than average.[16]
The prevalence of HIV/HCV co-infection of 6.2% (2/32) relates to neighboring countries (Slovenia 7%, rest of Europe 11%).[12]
Paradoxically, even in this age of highly potent anti-retroviral
treatment, PwH with HIV/HCV co-infection still have a high rate of
progression into ESLD if the HCV infection goes untreated, mainly
because of superimposed hepatotoxic effect and evolves metabolic
syndrome.[17] Both patients in this review were
cured, one in the PegIFB+RBV era and the others with DAA treatment.
Testing voluntary blood donors for HBsAg was implemented in 1972;
therefore, the prevalence of acute and chronic HBV infections is low in
this population and ranges from 3%-11%.[18] Nonetheless, the prevalence of "occult" HBV is caused by nosocomial transmission.[19]
Only 43.7% of patients in our institution are tested for HBV infection
markers, even in the scenario of acutely aware hematologists and
infectious disease experts to the consequences of blood-borne diseases.
It has been proven that in the event of HCV/HBV co-infection, liver
cirrhosis and HCC develop more frequently. Moreover, there is a
possibility of reactivation of HBV during IFN or DAA treatment
protocols.[20] Those facts implicate the need for all
PwH to be tested for markers of HBV infection. The reach of vaccination
against HBV is extremely low - 15.6% in our cohort, emphasizing the
necessity of promoting vaccination in this group of patients.[21]
In
the studied cohort, the distribution of HCV genotypes matches the
distribution in the general population of Vojvodina. Mixed genotype
(1a/1b) was found in 1/26 (3.8%) PwH. Most studies reported a greater
frequency of mixed genotypes of HCV in infected PwH due to recombined
HCV genomes in the event of long-lasting infections.[22,23]
This phenomenon could affect the rate of resistance-associated
substitutions and the genotype 1a resistance to DAA treatment
protocols.[24] We have to indicate that this low
percentage of mixed genotypes in our cohort could primarily result from
unavailable molecular detection methods.
Using indirect
diagnostic methods (serum markers such as Fib4, APRI score, ultrasound
methods such as FibroScan, doppler ultrasound of the hepatic vein,
etc.), liver cirrhosis was verified in 21.8% of patients. Until the
advent of ultrasound elastography, the "golden standard" of diagnosing
liver fibrosis and cirrhosis was a biopsy. In people with hemophilia,
liver biopsy is almost always contraindicated from a cost-benefit
assessment standpoint, which is why none of the patients in this study
had undergone this invasive diagnostic procedure.[22,25]
Even
though the HCV infections started in early childhood, liver cirrhosis
was observed in 21.8% of patients. With the IQR 30–35 years, we
observed that the duration of infection is the most important factor in
the development of ESLD, in conjunction with co-infection (HIV/HBV),
male sex, diabetes, obesity, and alcohol abuse.[26]
The rate of liver cirrhosis is 0.6 per 100 patient-years in our study
group. Sadly, in more than half of PwH suffering from liver cirrhosis,
the diagnosis was made only after liver decompensation. The late
diagnosis emphasizes the need for HCV testing for people at risk of
infection who have received blood products before 1994 and multiple
blood transfusions, especially people with hemophilia.[27]
Even though chronic hepatitis C is the most important cause of ESLD
today, it is mostly undiagnosed in the general population.[28]
In
this cohort, we observed HCC in 12.5% of patients. Thus, the risk of
HCC development in PwH is the same as in the general population with
HCV. According to The Liver Disease patient registry (HEREPA) 48% of
all HCC diagnosed in Serbia is caused by HCV infection (unpublished
data).
The irony of PwH living long and productive lives thanks to
improvements in the production of coagulation factor concentrates, but
dying from a curable disease like HCV is frustrating. According to the
National Inpatients Sample database USA (NIS), only 40-50% of PwH are
treated for HCV infection.[8] The first HCV-positive
Hemophiliac with CCV was treated with monotherapy of IFN alfa. During
the following 25 years, treatment of HCV in PwH was conducted according
to EASL protocols: PegIFN+RBV, and as a last resort DAA. As shown in Figure 1,
in our center, HCV diagnosis and treatment rates were consistently
equal throughout the years, which demonstrates the willingness of PwH
to accept antiviral treatment protocols. A high rate of SVR was
achieved in 73.6% of PwH treated with PegIFN+RBV in our cohort, in
conjunction with an expected side-effects rate of 20.8%, which
disproves healthcare providers' biases that PwH are difficult to treat
with INF-based protocols.[4]
With the
registration of highly efficient and safe DAA treatment by the Food and
Drug Administration in 2013. and the European Medical Association in
2015, the goal of eradicating HCV by 2030. was set by the WHO.[29]
However, the biggest obstacle in setting national strategies for
eradication is financial - the price of DAA treatments is still
unreachable for a large number of low-income countries.[30]
In 2016, the European Directorate for Quality of Medicines (EDQM)
stated that PwH should be given priority in DAA treatment protocols in
national health budgets because of its benefit for PwH in reducing HCV
morbidity and mortality, which is its leading cause. Also, ESLD and its
sequelae greatly increase the cost of treatment: ESLD increases the
risk of bleeding and the needing for invasive diagnostic and treatment
procedures such as EGDS and paracentesis.[11] In our
study, 37.5% of infected PwH were treated with DAA's, and all achieved
SVR. Treating HCV in PwH not only prevents the development of ESLD and
HCC, but it also greatly increases the quality of life for people
living with hemophilia, which is generally lower in PwH and depends on
hemophilia severity, age, the use of orthopedic aids, and other
comorbidities at first HCV infection.[30]
The
mortality rate is unsurprisingly significantly higher in PwH with HCV,
1.07% per year, instead of 0.09% for HCV negative PwH, incidence of
liver cirrhosis, and HCC in Vojvodina is not different from other
regions in the world.[30]
After more than 35
years, the northern region of Serbia (Province of Vojvodina) has
reached the WHO goal of micro-eliminating HCV well before 2030. There
are many obstacles in gaining the WHO goal of globally eradicating HCV
until 2030, mainly its scale, complexity, and implementation. That is
why many countries have implemented a micro-elimination strategy: a
pragmatic elimination approach in populations where it would have the
highest efficacy. For the time being, this micro-elimination concept
has proven realistic in the population of patients with hemophilia in
northern Serbia, a well-defined subpopulation of HCV infected, under
constant medical supervision.[6]
References
- Mitrović N, Delić D, Marković Denić L, Nikolić N,
Bojović K, Simonović Babić J et al. The prevalence and the risk factors
for hepatitis C virus infection in Serbia. J Infect Dev Ctries
2018;12:171-177. https://doi.org/10.3855/jidc.10172
- Sepanlou
SG, Safiri S, Bisignano C, Ikuta KS, Merat S, Saberifiroozi M et al.
The global, regional, and national burden of cirrhosis by cause in 195
countries and territories, 1990-2017: a systematic analysis for the
Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol
2020;5(3):245-266. https://doi.org/10.1016/S2468-1253(19)30349-8
- Lanini
S, Easterbrook PJ, Zumla A, Ippolito G. Hepatitis C: global
epidemiology and strategies for control. Clin Microbiol Infect
2016;22(10):833-838. https://doi.org/10.1016/j.cmi.2016.07.035
- Guedes
TP, Garrido M, Magalhães RK, Moreira T, Rocha M, Maia L et al.
Long-Term Follow-Up of a Portuguese Single-Centre Cohort of Persons
with Haemophilia and Hepatitis C Virus Infection. GE Port J
Gastroenterol 2021;28(2):79-86.
- Orman ES,
Fried MW. Hepatitis C viral infection in patients with hemophilia and
hemolytic disorders. Clin Liver Dis 2012;1(3):95. https://doi.org/10.1002/cld.42
- Lazarus
JV, Safreed-Harmon K, Thursz MR, Dillon JF, El-Sayed MH, Elsharkawy AM
et al. The Micro-Elimination Approach to Eliminating Hepatitis C:
Strategic and Operational Considerations. Semin Liver Dis
2018;38(3):181-192. https://doi.org/10.1055/s-0038-1666841
- Srivastava
A, Santagostino E, Dougall A, Kitchen S, Sutherland M, Pipe SW et al.
WFH Guidelines for the Management of Hemophilia. Haemophilia.
2020;26:1-58. https://doi.org/10.1111/hae.14046
- Angelotta
C, McKoy JM, Fisher MJ, Buffie CG, Barfi K, Ramsey G et al. Legal,
financial, and public health consequences of transfusion-transmitted
hepatitis C virus in persons with haemophilia. Vox Sang
2007;93(2):159-165. https://doi.org/10.1111/j.1423-0410.2007.00941.x
- Pawlotsky
JM, Negro F, Aghemo A, Berenguer M, Dalgard O, Dusheiko G et al.
European Association for the Study of the Liver. EASL recommendations
on treatment of hepatitis C: Final update of the series. J Hepatol
2020;73(5):1170-1218. https://doi.org/10.1016/j.jhep.2020.08.018
- Bojović
K, Simonović Babić J, Mijailović Ž, Milošević I, Jovanović M, Ružić M
et al. Micro-elimination of HCV as a possible therapeutic strategy: our
experience and a review of literature. J Infect Dev Ctries
2020;14(2):117-124. https://doi.org/10.3855/jidc.11785
- Savini
L, Kaczmarek R, Noone D, Giangrande P, Dusheiko G, O'Mahony B.
Hepatitis C and bleeding disorders in Europe. J Haem Pract
2018;5(1):50-65. https://doi.org/10.17225/jhp00112
- O'Mahony
B, Noone D, Giangrande PLF, Prihodova, L. Haemophilia care in Europe -
a survey of 35 countries. Haemophilia 2013;19:239-247. https://doi.org/10.1111/hae.12125
- Evatt BL. The tragic history of AIDS in the hemophilia population, 1982-1984. J Thromb Haemost 2006;4(11):2295-2301. https://doi.org/10.1111/j.1538-7836.2006.02213.x
- Kostić
V, Đorđević M, Popović L, Kostić E, Đorđević J, Govedarević N. Efficacy
of antiviral therapy in patients with hemophilia and hepatitis C virus
infection. Med Pregl 2009;3-4:129-132. https://doi.org/10.2298/MPNS0904129K
- Thalappillil
A, Ragni MV, Comer DM, Yabes JG. Incidence and risk factors for
hepatocellular cancer in individuals with haemophilia: A National
Inpatient Sample Study. Haemophilia 2019;25(2):221-228. https://doi.org/10.1111/hae.13668
- Grebely
J, Prins M, Hellard M, Cox AL, Osburn WO, Lauer G, Page K, Lloyd AR,
Dore GJ. Hepatitis C virus clearance, reinfection, and persistence,
with insights from studies of injecting drug users: towards a vaccine.
The Lancet infectious diseases. 2012;12(5):408-414. https://doi.org/10.1016/S1473-3099(12)70010-5
- Phusanti
S, Manosudprasit K, Sungkanuparph S. Long-Term Liver Diseases after
Initiation of Antiretroviral Therapy in HIV-Infected Patients with and
without HBV or HCV Coinfection. J Int Assoc Provid AIDS Care
2017;16(2):194-200. https://doi.org/10.1177/2325957416686838
- Hanley
JP, Dolan G, Day S, Skidmore SJ, Irving WL. Interaction of hepatitis B
and hepatitis C infection in haemophilia. Br J Haematol
1993;85(3):611-612. https://doi.org/10.1111/j.1365-2141.1993.tb03356.x
- Lanini
S, Puro V, Lauria FN, Fusco FM, Nisii C, Ippolito G. Patient to patient
transmission of hepatitis B virus: a systematic review of reports on
outbreaks between 1992 and 2007. BMC Med 2009;8:7-15. https://doi.org/10.1186/1741-7015-7-15
- Mavilia
MG, Wu GY. HBV-HCV Coinfection: Viral Interactions, Management, and
Viral Reactivation. J Clin Transl Hepatol 2018;6:296-305. https://doi.org/10.14218/JCTH.2018.00016
- Schillie
S, Vellozzi C, Reingold A, Harris A, Haber P, Ward JW et al. Prevention
of Hepatitis B Virus Infection in the United States: Recommendations of
the Advisory Committee on Immunization Practices. MMWR Recomm Rep
2018;67(1):1-31. https://doi.org/10.15585/mmwr.rr6701a1
- Preveden
T, Vereš B, Ružić M, Pete M, Luzza F, Pellicano R. Noninvasive
assessment of liver fibrosis in chronic hepatitis C virus patients
compared to liver biopsy: the experience of tertiary level hospital in
Serbia. Minerva Med 2020;111(3):197-202. https://doi.org/10.23736/S0026-4806.19.06109-3
- Moreno
P, Alvarez M, López L, Moratorio G, Casane D, Castells M et al.
Evidence of recombination in Hepatitis C Virus populations infecting a
hemophiliac patient. Virol J 2009;6:203. https://doi.org/10.1186/1743-422X-6-203
- Sharafi
H, Alavian SM. Hepatitis C resistance to NS5A inhibitors: Is it going
to be a problem? World J Hepatol 2018;10(9):543-548. https://doi.org/10.4254/wjh.v10.i9.543
- Lai
M, Afdhal N. Liver biopsy in hepatitis C patinets with easy-to-treat
characteristics: should we bother or just do biomarkers? In: Foster GR,
Reddy RK. Clinical Dilemmas in Viral Liver Disease. Blackwell
Publishing 2010; 6-8. https://doi.org/10.1002/9781444319590.ch2
- Posthouwer
D, Makris M, Yee TT, Fischer K, Van Veen JJ, Griffioen A et al.
Progression to end-stage liver disease in patients with inherited
bleeding disorders and hepatitis C: an international, multicenter
cohort study. Blood 2007;109(9):3667-3671. https://doi.org/10.1182/blood-2006-08-038349
- Alkindi
S., AL-Umairi N., Jaju S., Pathare A.Prevalence of Hepatitis B,
Hepatitis C, and HIV in multiply transfused Sickle Cell disease
patients from Oman. Mediterr J Hematol Infect Dis 2019;11(1): e2019058.
https://doi.org/10.4084/mjhid.2019.058
- The
Polaris Observatory HCV Collaborators. Global prevalence and genotype
distribution of hepatitis C virus infection in 2015: a modelling study.
Lancet Gastroenterol Hepatol 2017;2:161-176.
- Papadopoulos
N, Argiana V, Deutsch M. Hepatitis C infection in patients with
hereditary bleeding disorders: epidemiology, natural history, and
management. Ann Gastroenterol 2018;31(1):35-41.
- Matičič
M, Lombardi A, Mondelli MU, Colombo M; ESCMID Study Group for Viral
Hepatitis (ESGVH). Elimination of hepatitis C in Europe: can WHO
targets be achieved? Clin Microbiol Infect 2020;26(7):818-823. https://doi.org/10.1016/j.cmi.2020.01.014
[TOP]




