Daniel
Rivera1, Koichi Takahashi1,
Jean-Bernard Durand2 and Alessandra Ferrajoli1.
1
Department of Leukemia, The University of Texas MD Anderson Cancer
Center.
2 Department of Cardiology, The University of
Texas MD Anderson Cancer Center.
Correspondence to: Alessandra
Ferrajoli, BS, MD Department of Leukemia, The University of Texas MD
Anderson Cancer Center, 1515 Holcombe Blvd. Unit 0428. Houston, TX
77030. E-mail:
aferrajo@mdanderson.org
Published: July 1, 2021
Received: May 3, 2021
Accepted: June 6, 2021
Mediterr J Hematol Infect Dis 2021, 13(1): e2021044 DOI
10.4084/MJHID.2021.044
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Ibrutinib is a
well-tolerated and effective therapy
used for the treatment of chronic lymphocytic leukemia (CLL). However,
its use
has been associated with cardiovascular events such as atrial
fibrillation
(Afib), hypertension, and ventricular arrhythmias. Cardiac arrhythmias
represent a significant cause of morbidity and mortality. Implanted
loop
recorders have been integrated into our clinical practice and have been
considered a useful tool in guiding the management of patients with
cardiac
arrhythmias. We report a case that describes our experience on a
patient
diagnosed with CLL treated with ibrutinib.
|
Introduction. Learning objectives
•
The importance of Ibrutinib-related cardiovascular events.
• Differential diagnosis of patients with
a newly diagnosed tachyarrhythmia.
• The role of an implanted loop recorder
for the management of ibrutinib-associated arrhythmias.
History of presentation
The
patient is a 67 y.o. man, who in February 2017 presented to the
emergency center with a chief complaint of dizziness, lightheadedness,
and palpitations. He was found to be hypotensive, and his physical exam
was remarkable for tachycardia with an irregularly irregular heart
rhythm.
Past Medical History
The
patient had a history of hyperlipidemia treated with atorvastatin and
well-controlled essential hypertension treated with
losartan/hydrochlorothiazide. In 2015 he was diagnosed with CLL with
chromosome 11 deleted and IGHV mutated. In October 2016, the patient
required treatment because of progressive disease and started therapy
on a clinical trial with ibrutinib at the dose of 420 mg daily.
Differential
diagnosis
Laboratory
evaluation did not show electrolyte imbalances or thyroid dysfunction.
An echocardiogram did not detect valvular or structural abnormalities
and showed an LVEF of 61% with no regional wall motion abnormalities or
evidence of myocardial disorders. The patient was admitted with the
diagnosis of Afib with a rapid ventricular rate (RVR) and a CHADSVASc
of 2.
Investigations and Management
The
patient was initially treated with amiodarone without success;
electrical cardioversion was performed, achieving normal sinus rhythm
(NSR). The patient was discharged on amiodarone, 400 mg daily, apixaban
5 mg BID, and the ibrutinib dose was reduced to 140 mg daily. However,
fifteen days after the event, due to the concern of the interaction
between ibrutinib and amiodarone, which could reduce Ibrutinib
clearance and, consequently, increase the risk of bleeding due to its
interaction with apixaban, these two drugs were discontinued.
Ibrutinib
was increased to its total dose of 420 mg daily, and the patient was
started on metoprolol 12.5 mg daily and aspirin 81 mg daily. At this
time, the cardiology team implanted a subcutaneous insertable loop
recorder (Reveal) to optimize the monitoring of future arrhythmias. The
next phase of his treatment on clinical trial consisted of venetoclax
at the dose of 400 mg daily.
Over the next 2 months, the loop
recorder detected 2 episodes of Afib, which were asymptomatic and, on
one occasion, followed by a non-sustained monomorphic ventricular
tachycardia (NSVT) with no hemodynamic sequela. (Figure1 A and B).
The patient at this time was asymptomatic with a CHADSVASc of 2 and
HAS-BLED of 1. Due to the presence of recurrent arrhythmias, a
Pharmacological nuclear EKG stress test was done and showed normal
myocardial perfusion with no evidence of stress-induced ischemia and
normal left ventricular systolic function with a left ventricular
ejection fraction of 66%. His treatment was optimized with a change of
metoprolol 12.5 mg daily to metoprolol XL 50 mg daily.
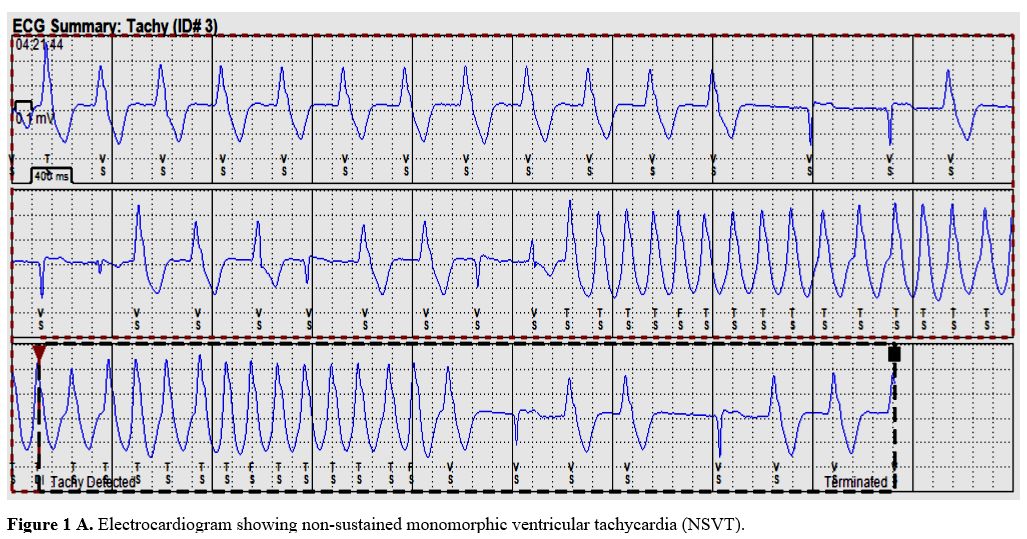 |
Figure
1A. Electrocardiogram showing non-sustained monomorphic ventricular
tachycardia (NSVT). |
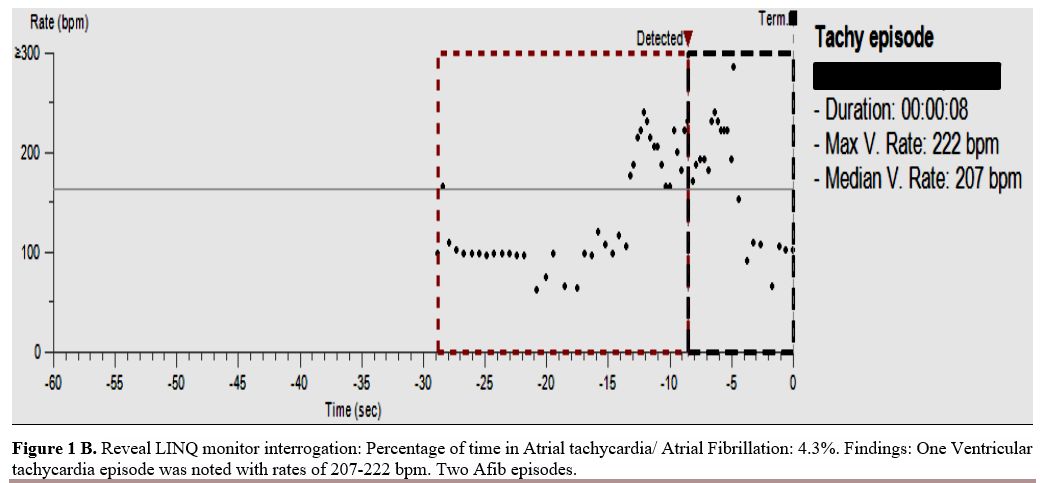 |
Figure
1B. Reveal LINQ monitor interrogation: Percentage of time in Atrial
tachycardia/ Atrial Fibrillation: 4.3%. Findings: One Ventricular
tachycardia episode was noted with rates of 207-222 bpm. Two Afib
episodes. |
Between May
2017 and
February 2018, the implantable loop recorded continued to detect Afib
with a burden that remained <6%, but no further episodes of VTs (Table 1). The
patient continued to experience borderline hypertension (Table 1).
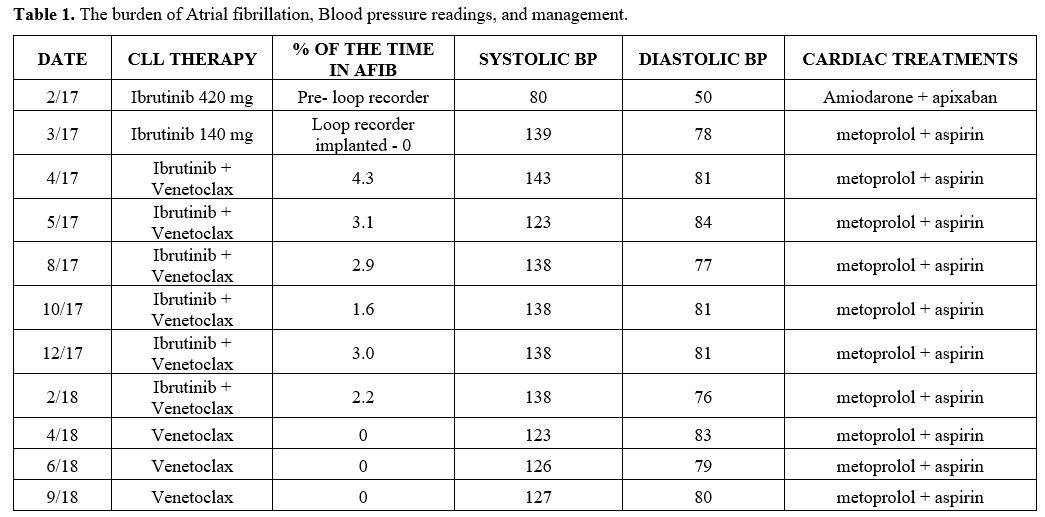 |
Table
1. The burden of Atrial fibrillation, Blood pressure readings, and
management.
|
In
February 2018, the patient developed an erythematous rash which was
attributed to ibrutinib. CLL re-staging was found to have achieved
complete remission with undetectable measurable residual disease on
bone marrow test. Ibrutinib was, therefore, discontinued in February
2018 and venetoclax monotherapy was continued for one additional year
until January 2019 when all treatments were discontinued.
Discussion
Ibrutinib
is a first-generation BTK inhibitor that has been effective for the
treatment of CLL. BTK inhibition reduces B-cell proliferation,
adhesion, and migration.[1,2]
treatment with ibrutinib
has been associated with cardiovascular toxicities. The proposed
mechanisms of the cardiovascular effect are not exactly known; however,
it has been thought they are related to off-target effects on kinases
different than BTK. Inhibition of cardiac PI3K-Akt signaling, which is
a critical regulator of cardiac protection under stress conditions,[3]
the binding of ibrutinib to ErbB2/HER2, ErbB4/HER4, and BMX receptors
in cardiomyocytes, and inhibition of C-terminal Src kinase have been
reported to be involved.[4]
In
the case reported here, shortly after starting ibrutinib, the patient
began experiencing cardiovascular events such as an increase in blood
pressure, recurrent episodes of Afibr, and one episode of VT, which
were attributed to ibrutinib therapy.
We
observed that our patient had a median systolic BP of 127 mmHg and a
diastolic BP of 81 mmHg while receiving treatment with ibrutinib. The
development of hypertension has been reported in up to 68% of the
patients undergoing treatment with ibrutinib, and worsening HTN is
common in patients with pre-existing HTN. The risk for HTN continues
for the entire duration of treatment with ibrutinib and can increase
the risk of major adverse cardiovascular events such as Afib, stroke,
or myocardial infarction.[5]
Our
patient experienced one symptomatic Afib episode four months after
initiating therapy with ibrutinib, requiring electrical cardioversion.
Antiarrhythmic treatment with amiodarone and anticoagulation with
apixaban was initially implemented but soon deemed not feasible due to
interactions with ibrutinib and increased risk for bleeding. This
highlights the complexity in managing these patients with risks for
drug interactions with strong or moderate CYP3A4 inhibitors/inducers.
Additionally, the concomitant administration of vitamin K antagonists
is prohibited in patients receiving ibrutinib, and insufficient data
are available on the risk of bleeding in patients receiving DOAC and
ibrutinib considering that the coadministration of DOAC and ibrutinib
could increase ibrutinib exposure via CYP3A4-mediated interaction.
Consequently,
our patient continued metoprolol for rate control. His loop recorder
showed a median percentage of time in Afib of 2.2% over 12 months. The
patient remained on ibrutinib since he remained asymptomatic with
successful medical management. Pooled analysis of 4 randomized trials
showed an incidence of ibrutinib related-atrial fibrillation of 3.3 per
100 person-year.[6] However, a
higher incidence has been reported in studies, with a longer follow-up
being up to 16% of the patients.[7]
The common etiologies for ventricular arrhythmias include untreated or
unrecognized ischemic heart disease; wherein ischemia can serve as a
substrate for ventricular tachycardia. Ventricular arrhythmias have
been associated with ibrutinib therapy. Avirup et al. reported a median
time-to-event of 16 months with an incidence rate of 617 per 100,000
person-year in the general population and patients without baseline
CAD, and heart failure; similar to our patient, the incidence rate was
lower at 596 per 100,000.[8]
Similarly, the REVEAL AF
study performed in the general population in 446 patients, reporting a
detection rate at 18 months of 29%, this proved to be of value in
detecting undiagnosed AF in patients with risk factors for Afib and
stroke.[9]
In
this case, Ibrutinib was not discontinued as this patient was
asymptomatic with no hemodynamic compromise. Early discontinuation of
Ibrutinib can impact the ability to control CLL and affect long-term
survival. The risks and benefits of discontinuing ibrutinib must be
discussed extensively with the oncology team, cardio-oncology
specialists, and patients.[10,11]
Follow-up
No
further episodes of Afib were observed after discontinuation of
ibrutinib. Since then, the patient continues to be without treatment
for his CLL, which remains in remission, with good quality of life and
no cardiovascular events to date.
Conclusions
The
management of ibrutinib-related cardiovascular toxicities remains a
challenge in daily practice, and their importance is going to increase
with the growing number of older patients being treated with this
agent. The presence of an implanted loop recorder helps monitor
patients, following the impact of treatment modifications, and trigger
additional testing to identify contributing factors or alternative
etiologies to the observed arrhythmias.
References
- Burger, JA, et al.,
Long-term efficacy and safety
of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of
follow-up from the phase 3 RESONATE-2 study. Leukemia, 2020. 34(3): p.
787-798. https://doi.org/10.1038/s41375-019-0602-x
- Munir,
T., et al., Final analysis from RESONATE: Up to six years of follow-up
on ibrutinib in patients with previously treated chronic lymphocytic
leukemia or small lymphocytic lymphoma. Am J Hematol, 2019. 94(12): p.
1353-1363. https://doi.org/10.1002/ajh.25638
- McMullen,
J.R., et al., Ibrutinib increases the risk of atrial fibrillation,
potentially through inhibition of cardiac PI3K-Akt signaling. Blood,
2014. 124(25): p. 3829-30. https://doi.org/10.1182/blood-2014-10-604272
- Xiao,
L., et al., Ibrutinib-Mediated Atrial Fibrillation Attributable to
Inhibition of C-Terminal Src Kinase. Circulation, 2020. 142(25): p.
2443-2455. https://doi.org/10.1161/CIRCULATIONAHA.120.049210
- Dickerson,
T., et al., hypertension and incident cardiovascular events following
ibrutinib initiation. Blood, 2019. 134(22): p. 1919-1928. https://doi.org/10.1182/blood.2019000840
- Leong,
D.P., et al., The risk of atrial fibrillation with ibrutinib use: a
systematic review and meta-analysis. Blood, 2016. 128(1): p. 138-40. https://doi.org/10.1182/blood-2016-05-712828
- Thompson,
P.A., et al., Fludarabine, cyclophosphamide, and rituximab treatment
achieves long-term disease-free survival in IGHV-mutated chronic
lymphocytic leukemia. Blood, 2016. 127(3): p. 303-9. https://doi.org/10.1182/blood-2015-09-667675
- Guha,
A., et al., Ventricular Arrhythmias Following Ibrutinib Initiation for
Lymphoid Malignancies. J Am Coll Cardiol, 2018. 72(6): p. 697-698. https://doi.org/10.1016/j.jacc.2018.06.002
- Reiffel,
J.A., et al., Incidence of Previously Undiagnosed Atrial Fibrillation
Using Insertable Cardiac Monitors in a High-Risk Population: The REVEAL
AF Study. JAMA Cardiol, 2017. 2(10): p. 1120-1127. https://doi.org/10.1001/jamacardio.2017.3180
- Maddocks,
K.J., et al., Etiology of Ibrutinib Therapy Discontinuation and
Outcomes in Patients With Chronic Lymphocytic Leukemia. JAMA Oncol,
2015. 1(1): p. 80-7. https://doi.org/10.1001/jamaoncol.2014.218
- Falchi
L., Baron J.M., Orlikowski C.A., Ferrajoli A.BC Signaling Inhibitors:
an Overview of Toxicities Associated with Ibrutinib and Idelalisib in
Patients with Chronic Lymphocytic Leukemia. Mediterr J Hematol Infect
Dis 2016, 8(1): e2016011, https://doi.org/10.4084/mjhid.2016.01
[TOP]


