Nicola Semeraro and Mario Colucci.
| This is an Open Access article distributed
under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by-nc/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
|
Abstract Severe
coronavirus disease-2019 (COVID-19) is frequently associated with
microvascular thrombosis, especially in the lung, or macrovascular
thrombosis, mainly venous thromboembolism, which significantly
contributes to the disease mortality burden. COVID-19 patients also
exhibit distinctive laboratory abnormalities that are compatible with a
prothrombotic state. The key event underlying COVID-19-associated
thrombotic complications is an excessive host inflammatory response to
severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) infection
generating multiple inflammatory mediators, mainly cytokines and
complement activation products. The latter, along with the virus
itself, the increased levels of angiotensin II and hypoxia, drive the
major cellular changes promoting thrombosis, which include: (1)
aberrant expression of tissue factor by activated alveolar epithelial
cells, monocytes-macrophages and neutrophils, and production of other
prothrombotic factors by activated endothelial cells (ECs) and
platelets; (2) reduced expression of physiological anticoagulants by
dysfunctional ECs, and (3) suppression of fibrinolysis by the
endothelial overproduction of plasminogen activator inhibitor-1 and,
likely, by heightened thrombin-mediated activation of
thrombin-activatable fibrinolysis inhibitor. Moreover, upon activation
or death, neutrophils and other cells release nuclear materials that
are endowed with potent prothrombotic properties. The ensuing
thrombosis significantly contributes to lung injury and, in most severe
COVID-19 patients, to multiple organ dysfunction. Insights into the
pathogenesis of COVID-19-associated thrombosis may have implications
for the development of new diagnostic and therapeutic tools. |
Introduction
Laboratory Haemostatic Abnormalities
Pathophysiology
Upregulation of Cellular Procoagulant Pathways: the Role of Tissue Factor
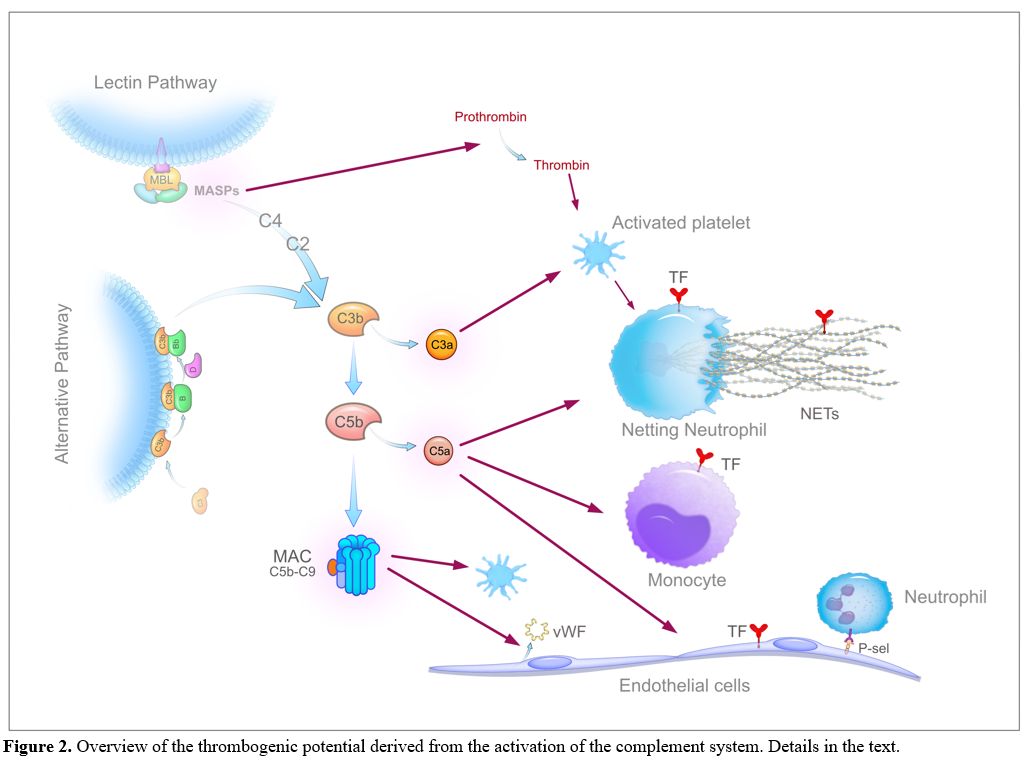 |
Figure
2. Overview of the thrombogenic potential derived from the activation
of the complement system. Details in the text. |
Changes in Physiological Anticoagulant Mechanisms
Changes in Fibrinolysis
Conclusion and Perspectives
References
[TOP]