Gabriele Magliano1, Annarosa Cuccaro3, Francesco d’Alo’1,2, Elena Maiolo2, Silvia Bellesi2, Stefan Hohaus1,2, Andrea Bacigalupo1,2, Livio Pagano1,2.
1Sezione di Ematologia, Dipartimento di Scienze Radiologiche ed Ematologiche, Università Cattolica del Sacro Cuore, Roma
2
Dipartimento di Diagnostica per Immagini, Radioterapia Oncologica ed
Ematologia, Fondazione Policlinico Universitario A. Gemelli IRCCS, Roma;
3 UOC Ematologia Aziendale, Azienda Toscana Nord-Ovest, Livorno, Italy.
Correspondence to:
Gabriele Magliano. Sezione di Ematologia, Dipartimento di Scienze
Radiologiche ed Ematologiche, Università Cattolica del Sacro Cuore,
Roma.
Published: September 1, 2021
Received: May 26, 2021
Accepted: August 7, 2021
Mediterr J Hematol Infect Dis 2021, 13(1): e2021054 DOI
10.4084/MJHID.2021.054
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
To the editor
We
discuss the case of a 74-year old male patient with mantle cell
lymphoma, who faced severe cytomegalovirus (CMV) infection after the
fifth cycle of first-line chemo-immunotherapy with a dose-reduced
bendamustine and rituximab regimen.
The patient came to our
attention in May 2017. He reported weight loss of 10% and night
sweats in the previous two months; his performance was reduced (ECOG
3). His past medical history was unremarkable.
Bone marrow
biopsy revealed a pleomorphic variant of mantle cell lymphoma. The
stage was IVB (superior and inferior nodal site involvement, B
symptoms). MIPI score was 9.4 (high risk): age 74 years, LDH 3116 UI/L,
WBCs 3.01 x10^9/L, ECOG 3, Ki67 85% on histology.
At
diagnosis, CD4 count was 0.24x10^9/L (0.63-1.4), with inversion of
CD4/CD8 ratio. There were no other detectable causes of immune
suppression.
Considering age and performance status, we started chemo-immunotherapy with rituximab (375 mg/m2 on day 2)-bendamustine (70 mg/m2 on day 1-2) every 28 days. As a common clinical practice, we did not perform antiviral prophylaxis.
Two
weeks after the fifth cycle, the patient was admitted to our hospital
with fever (38.5 °C), dyspnea, and diarrhea. Chest X-ray revealed
interstitial pneumonitis with bilateral basal thickening and left
pleural effusion. Thoracic CT scan showed pulmonary edema with diffuse
ground-glass opacities, bilateral pleural effusions, and small
pericardial effusion of 8 mm (Figure 1).
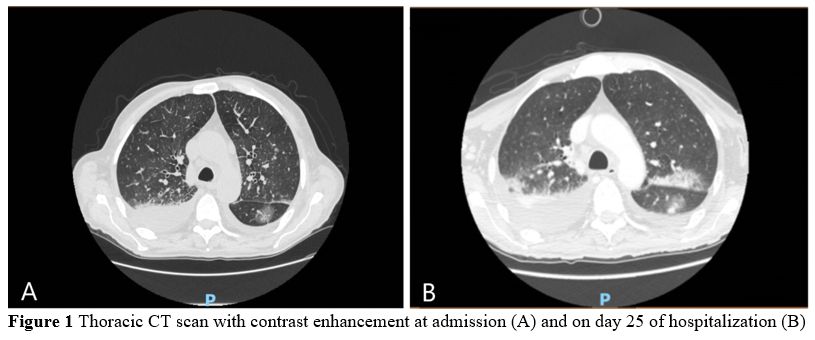 |
Figure 1. Thoracic CT scan with contrast enhancement at admission (A) and on day 25 of hospitalization (B). |
Hemoglobin
levels were 8.1 g/dL, WBCs were 2.37x10^9/L, plts were 55x10^9/L.
Intravenous antibiotic therapy with piperacillin-tazobactam and
levofloxacin and oxygen support was started. Unfortunately, fever
persisted with no clinical improvement (Table 1).
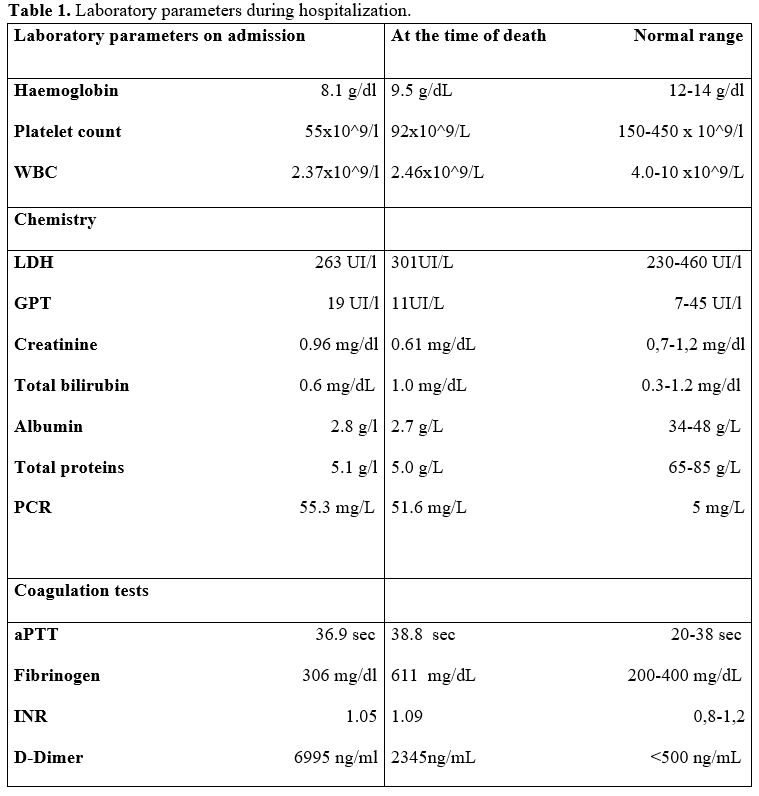 |
Table 1. Laboratory parameters during hospitalization. |
On
the sixth day of hospitalization blood PCR test for CMV yielded
1,400,000 copies/mL, and intravenous antiviral therapy with ganciclovir
5 mg/Kg bid was started (Figure 2).
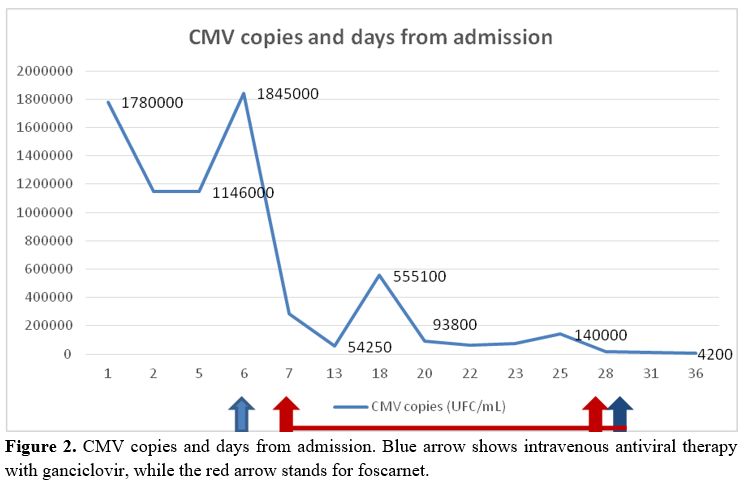 |
Figure
2. CMV copies and days from admission. Blue arrow shows intravenous
antiviral therapy with ganciclovir, while the red arrow stands for
foscarnet. |
After
one day of antiviral therapy, the patient developed neurological
symptoms with paresthesia and tremor. For the suspect of an adverse
drug effect to ganciclovir, antiviral treatment was modified to
foscarnet 20 mg/Kg.
Despite antiviral therapy, clinical conditions
kept worsening, as the patient required increased oxygen support and
remained febrile. CT scan of the thorax performed on day 25 revealed
new ground-glass opacities in the superior pulmonary lobes. In
addition, pericardial effusion increased to 13 mm (Figure 1).
Microbiological
examination of bronchoalveolar lavage yielded 54,250 copies of
CMV-DNA/ml bronchoalveolar fluid, 462 pg/ml of Candida antigen, and 0.7
pg/mL Aspergillus spp. In addition, intravenous antifungal therapy with
voriconazole was added.
CMV copy number in peripheral blood
remained high (140,000/mmc), and antiviral therapy was changed to
ganciclovir on day 29 of hospital admission. Lymphocyte counts
decreased: CD4+ cells, 0.042 x 10^9/L (0.63-1.40), CD8, 0.12 x 10^9/L
(0.35-0.81), CD56+, 0.093 x 10^9/L (0.14-0.42), and CD19 cells were
undetectable. After another six days, the patient developed psychomotor
agitation. Antiviral therapy was stopped. The patient died after two
other days of hospitalization.
Discussion
Mantle cell lymphoma (MCL) primarily occurs among elderly patients with a median age greater than 60 years.[1]
Half of these patients are not eligible for standard therapy that
includes autologous blood stem cell transplantation due to the presence
of comorbidities and to the general performance status.
Bendamustine-rituximab
(BR) is currently becoming the treatment of choice in older patients
with indolent non-Hodgkin lymphomas (NHL) and MCL,[2,3] having a favorable toxicity profile.[2]
However,
the BR combination has been shown to cause myelosuppression, including
combined T and B cell lymphopenia, with more profound T cell depletion.[4]
In addition, Saito and colleagues observed that the median lymphocyte
and CD4+ T-cell count decreased significantly after the first
administration of bendamustine in patients with relapsed or refractory
indolent B-NHL.[5]
Infection rates in patients
receiving bendamustine in randomized, controlled clinical trials range
from 6% to 55%, with 1% to 35% of them being grade 3 to 4.[4]
CD4+ recovery after BR is often delayed and consequently correlated
with the risk of any type of infection. Time to recovery to
pre-treatment values ranges from 7-9 months[5] to more
than two years after the last administration. In addition, low
end-of-treatment absolute lymphocyte count (ALC) and a total dose of
bendamustine higher than 1080 mg/m2 have been reported to predict delayed CD4+ recovery.[6]
Receiving
bendamustine as part of later lines of therapy (third-line and above
treatment) has been identified as another risk factor for infections.[7,8]
This
impairment in T cell immunity has been shown to trigger, in particular,
CMV reactivation. For example, in the study by Saito, CMV antigenemia
was detected in 15 of 56 patients (27%) and CMV colitis in 1 patient.
All these events occurred within nine months after completion of
treatment.[5,8,9]
In a recent
real-world study on 167 NHL, age ≥ 60 was a risk factor for CMV
reactivation in patients treated with first-line bendamustine.[10]
Another
report by Cona and colleagues described a severe, disseminated form of
CMV reactivation in a 75-year-old lymphoplasmacytic lymphoma patient,
who faced a profound imbalance in phenotype and function of B-and
T-cell subsets after BR.[11]
Our case highlights
how severe CMV reactivation can occur in the elderly MCL patient
treated with bendamustine, even when administered at a lower dose (our
patient had received a total of 700 mg) and as part of
first-line therapy.
Our patient presented with low CD4+ cell
counts (0.24x10^9/L) at diagnosis, before starting treatment, without
having other detectable causes of immune suppression (e.g.,
co-infections).
Age-related changes in the immune system,
collectively called immunosenescence, in addition to immune suppression
correlated with the underlying disease, might have contributed to
CMV reactivation after BR.
Despite treatment with ganciclovir
and foscarnet, CMV pneumonitis did not resolve, and immune suppression
was persistent, as CD4+ cells were 0.042x10^9/L on day 22.
We
could not assess if CMV pneumonia was the single main cause of death,
as an autopsy could not be performed. However, considering the
significant worsening of the clinical conditions (together with the
radiological picture), we believe it contributed to the dismal outcome.
Lymphocyte
profiling and monitoring ALC and CD4+ count before, during, and
after the end of treatment could help to identify patients who might be
at particular risk of delayed CD4+ recovery and consequently of viral
and opportunistic infectious complications.[6]
A
low CD4+ count could be a trigger to monitor also CMV DNA. However,
studies are needed to assess the effectiveness of a pre-emptive
antiviral therapy in this context, as recommended in 2017 by the UK
Medicines and Healthcare products Regulatory Agency (MHRA).[12]
A
recent study by Fung and colleagues estimated that antiviral
prophylaxis could prevent one CMV case every 269
bendamustine-treated patients with NHL.[7]
In our
case, bronchoalveolar lavage detected a concomitant fungal infection,
although CT of the thorax was suggestive for CMV pneumonia. Neutropenia
was an additional risk factor for fungal infection.
In an Israeli
retrospective analysis, the frequency of fungal infections in 183
patients affected by lymphoproliferative neoplasms and treated with a
bendamustine-containing regimen was 3.8%.[13]
In the study by Fung, bendamustine treatment was associated with a higher incidence of neutropenia and candida infection.[7]
Conclusions
Our
case is an alert for clinicians that BR can be associated with severe
infections in elderly patients even in first-line therapy at a reduced
dose. However, as BR is otherwise well tolerated, the infectious risk
should not be underestimated in the presence of an overall low burden
of side effects.
Randomized prospective studies are required to
assess the efficacy of monitoring, prophylaxis, and pre-emptive therapy
to mitigate the risk of infections, hospital admission, and mortality
in bendamustine-treated patients with NHL. These studies should
consider characteristics of the patient, such as age and immune status,
the type of NHL, the line of treatment, and the addition of other
drugs, including rituximab.
References
- Jares P, Campo E. Advances in the understanding of mantlecell lymphoma. Br J Haematol. 2008 Jun;142(2):149-65
- Doorduijn JK, Kluin-Nelemans HC. Management of mantle cell lymphoma in the elderly patient. Clin IntervAging. 2013;8:1229-36
- Flinn
IW, van der Jagt R, Kahl BS, Wood P, Hawkins TE, Macdonald D, Hertzberg
M, Kwan YL, Simpson D, Craig M, Kolibaba K, Issa S, Clementi R, Hallman
DM, Munteanu M, Chen L, Burke JM. Randomized trial of
bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of
indolent NHL or MCL: the BRIGHT study. Blood. 2014 May 8;123(19):2944-52
- Gafter-Gvili
A, Ribakovsky E, Mizrahi N, Avigdor A, Aviv A, Vidal L, Ram R, Perry C,
Avivi I, Kedmi M, Nagler A, Raanani P, Gurion R. Infections associated
with bendamustine containing regimens in hematological patients: a
retrospective multi-center study. Leuk Lymphoma. 2016;57(1):63-9. https://doi.org/10.3109/10428194.2015.1046862
- Saito
H, Maruyama D, Maeshima AM, Makita S, Kitahara H, Miyamoto K, Fukuhara
S, Munakata W, Suzuki T, Kobayashi Y, Taniguchi H, Tobinai K. Prolonged
lymphocytopenia after bendamustine therapy in patients with relapsed or
refractory indolent B-cell and mantle cell lymphoma. Blood Cancer J.
2015 Oct 23;5(10):e362.
- Martínez-Calle N,
Hartley S, Ahearne M, Kasenda B, Beech A, Knight H, Balotis C, Kennedy
B, Wagner S, Dyer MJS, Smith D, McMillan AK, Miall F, Bishton M, Fox
CP. Kinetics of T-cell subset reconstitution following treatment with
bendamustine and rituximab for low-grade lymphoproliferative disease: a
population-based analysis. Br J Haematol. 2019 Mar;184(6):957-968
- Fung
M, Jacobsen E, Freedman A, Prestes D, Farmakiotis D, Gu X, Nguyen PL,
Koo S. Increased Risk of Infectious Complications in Older Patients
With Indolent Non-Hodgkin Lymphoma Exposed to Bendamustine. Clin Infect
Dis. 2019 Jan 7;68(2):247-255
- Hasegawa T,
Aisa Y, Shimazaki K, Nakazato T. Cytomegalovirus reactivation with
bendamustine in patients with low-grade B-cell lymphoma. Ann Hematol.
2015 Mar;94(3):515-7
- Hosoda T, Yokoyama
A, Yoneda M et al. bendamustine can severely impair T-cell immunity
against cytomegalovirus. Leuk Lymphoma 2013; 54: 1327-1328. https://doi.org/10.3109/10428194.2012.739285
- Pezzullo
L, Giudice V, Serio B, Fontana R, Guariglia R, Martorelli MC, Ferrara
I, Mettivier L, Bruno A, Bianco R, Vaccaro E, Pagliano P, Montuori N,
Filippelli A, Selleri C. Real-world evidence of cytomegalovirus
reactivation in non-Hodgkin lymphomas treated with
bendamustine-containing regi-mens. Open Med (Wars). 2021 Apr
21;16(1):672-682.
- Cona A, Tesoro D,
Chiamenti M, Merlini E, Ferrari D, Marti A, Codecà C, Ancona G, Tincati
C, d'Arminio Monforte A, Marchetti G. Disseminated cytomegalovirus
disease after bendamustine: a case report and analysis of circulating
B- and T-cell subsets. BMC Infect Dis. 2019 Oct 22;19(1):881
- García
Muñoz R, Izquierdo-Gil A, Muñoz A, Roldan-Galiacho V, Rabasa P, Panizo
C. Lymphocyte recovery is impaired in patients with chronic lymphocytic
leukemia and indolent non-Hodgkin lymphomas treated with bendamustine
plus rituximab. Ann Hematol. 2014 Nov;93(11):1879-87
- Gafter-Gvili
A, Ribakovsky E, Mizrahi N, Avigdor A, Aviv A, Vidal L, Ram R, Perry C,
Avivi I, Kedmi M, Nagler A, Raanani P, Gurion R. Infections associated
with bendamustine containing regimens in hematological patients: a
retrospective multi-center study. Leuk Lymphoma. 2016;57(1):63-9. https://doi.org/10.3109/10428194.2015.1046862
[TOP]


