F.V. Segala1,2, G. Micheli2, C. Seguiti1,2, A. Pierantozzi3, R. Lukwiya4, B. Odong6, F. Aloi5, E. Ochola7, R. Cauda1,2, K. De Gaetano Donati1,2, and A. Cingolani1,2.
1Fondazione Policlinico A. Gemelli, IRCCS, Infectious Diseases, 00168, Roma, Italy.
2 Catholic University of the Sacred Heart, Infectious Diseases Unit, 00168, Roma, Italy.
3 AIFA-Italian Medicine Agency, 00187, Roma, Italy.
4 Comboni Samaritans of Gulu Health Center, 701102, Gulu, Uganda.
5 Università Cattolica S. Cuore, Special Medical Pathology, 00168, Roma, Italy.
6 Medical Teams International, Kitgum, Uganda.
7 Lacor Hospital, Department of HIV, Epidemiology and Documentation, Gulu, Uganda.
Correspondence to:
Francesco Vladimiro Segala, MD, Division of Infectious Diseases,
Catholic University of the Sacred Heart, 00168, Rome, Italy, +39
0630159480. E-mail:
fvsegala@gmail.com
Published: September 1, 2021
Received: May 27, 2021
Accepted: August 8, 2021
Mediterr J Hematol Infect Dis 2021, 13(1): e2021055 DOI
10.4084/MJHID.2021.055
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background and Objectives:
HIV infection among vulnerable women (VW) has been attributed to
unfavourable power relations and limited access to sexual and
reproductive health information and services. This work aims to report
sexually-transmitted infections (STI) prevalence and assess the impact
of HIV awareness, demographic and socio-behavioural factors on HIV
status in a rural area of northern Uganda.
Methods:
Pe Atye Kena is a longitudinal cohort intervention study enrolling
young women aged 18-49 years old living in the municipality of Gulu,
Uganda. HIV, HBV, syphilis serologic tests, and a comprehensive
electronic questionnaire on sexual high-risk behaviours were
administered before intervention. In this work, we report baseline
characteristics of the population along with factors associated with
HIV status. Statistical analysis was performed by uni- and
multivariable regression models.
Results:
461 VW were enrolled (mean age: 29 (SD7.7)). 40 (8.7%) were found to be
positive for HIV, 42 (9.1%) for syphilis and 29 (6.3%) for HBV. Older
age (> 34 years vs. < 24 years; OR 4.95, 95% CI: 1.7 to 14);
having done the last HIV test > 12m before the interview (OR 5.21,
95% CI: 2.3 to 11); suspecting the male sexual partner to be HIV+ (OR
2.2; 95% CI: 1.1 to 4.3); not having used condom at first sexual
intercourse (OR 2.6; 95% CI 1.3 to 5.15) were all factors associated
with an incident HIV diagnosis.
Conclusions:
In this cohort, HIV prevalence is high, and sexual high-risk behaviours
are multifaced; future interventions will be aimed to reduce HIV/STIs
misconceptions and to promote a sense of community, self-determination
and female empowerment.
|
Introduction
Despite
substantial reduction in sub-Saharan Africa HIV incidence, young women
remain at the epicentre of the epidemic, representing one of the
populations with the highest number of new cases per year globally.[1,2]
According to the 2020 UNAIDS report, adolescent girls and women account
for 25% of new infections in Western and Southern Africa.[3]
In fact, girls between 14-24 years old are four times more likely to be
infected with HIV than their male peers, and HIV prevalence among women
below 35 years old in Uganda is 7.5% versus 4.3% among males of the
same age.[4] In addition, gender-based violence and inequalities are key drivers of the epidemic.[5]
On the one hand, population-level evidence produced in other African settings[6,7]
show that up to 35% of women below 25 years old are involved in
age-disparate sex (defined as being at least five years younger than
their male partners), identifying this phenomenon as a key risk factor
for an earlier acquisition of HIV.[2,8,9]
On the other hand, several studies have shown that an older male
partner is not only more likely to be HIV positive and have
unsuppressed viral loads,[10] but also that
age-disparate partnerships are frequently associated with other high
risk sexual behaviours (HRSB), such as alcohol consumption,
inconsistent condom use, concurrent sexual partnering and engaging in
transactional sex.[11-15] Furthermore, 10.8% of females reported having sex before the age of 15.
In
addition, existing evidence suggests that socio-economic factors may
exacerbate the risk. For example, being out of school has been
identified as a significant risk factor for HIV acquisition in
Sub-Saharan Africa[16,17] and, in Uganda, women are
consistently more at risk to report a lower level of education or
no-education at all (10.9% versus 3.8%) compared with their male peers.[4]
In addition, according to the 2017 Uganda population-based HIV impact
assessment, recent intimate partner violence (IPV) was reported by
15.5% of women under the age of 24, and exposure to IPV has been linked
both to increased risk of HIV acquisition and reduced ability to
negotiate forms of safe sex.[18] Altogether, these
factors contribute to reducing women's personal agency and their
ability to define and act upon their healthcare choices.[19]
At present, although female empowerment is recognized as a powerful
tool in increasing HIV prevention, only limited evidence is available
on the impact of community interventions on STI incidence and sexual
wellbeing.[20] The present study aims to describe the
state of HIV awareness, risky sexual behaviours, STI epidemiology and
assess the impact of these factors on HIV status in a population of
young women living in a rural area near Gulu, Northern Uganda.
Methods
Study Design and Objectives.
Pe Atye Kena is a longitudinal cohort intervention study enrolling
vulnerable young women (VW) aged 18-49 years old living in a rural area
in the municipality of Gulu, Uganda. However, in this work, we assess
sexually transmitted infections (STI) epidemiology and the impact of
HIV awareness, demographic and socio-behavioural factors on HIV
prevalence before the intended intervention occurs. Therefore, since it
reports data at one precise point in time, the present article is
designed to be a cross-sectional study.
The cohort follow-up has a planned duration of 3 years, from 2019 to 2022 (Figure 1).
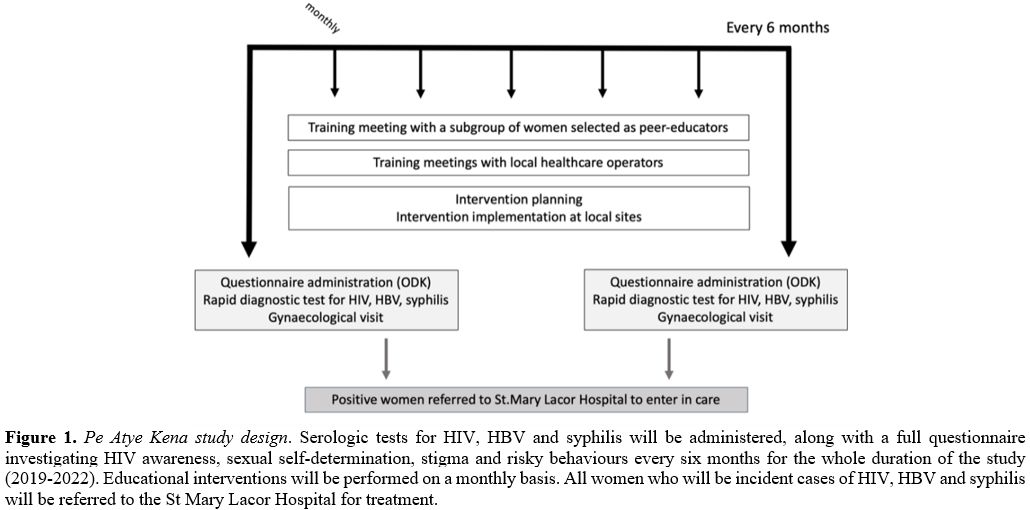 |
Figure 1. Pe Atye Kena study design.
Serologic tests for HIV, HBV and syphilis will be administered, along
with a full questionnaire investigating HIV awareness, sexual
self-determination, stigma and risky behaviours every six months for
the whole duration of the study (2019-2022). Educational interventions
will be performed on a monthly basis. All women who will be incident
cases of HIV, HBV and syphilis will be referred to the St Mary Lacor
Hospital for treatment. |
We
expect to identify population-specific critical issues to develop a
permanent, community-based educational service aimed to encourage
sexual self-determination, female personal agency, and improve HIV
prevention. A full electronic questionnaire about HIV awareness and
sexual high-risk behaviours, clinical visits, and laboratory tests
(which included screening for HIV, syphilis, and HBV infection) was
administered before intervention and will be administered every six
months. Behavioural/educational interventions will be delivered monthly
by selected peer-women and CSHC health personnel. Planned interventions
are structured as educational meetings, focus groups,
sharing-experiences groups, and special events involving other
community members (e.g., male partners, friends, family members).
Results from the baseline evaluation were disseminated to the recruited
population during the questionnaire administration and the following
educational meetings.
Recruitment and Cohort Description. Recruitment was conducted by selected peer women through community outreach[21] from the 15th to 30th
April, 2019. A total of eight peer outreach workers were actively
involved in the recruitment phase, who were then selected as peer
educators as well. Enrolled participants were then encouraged to
recruit other women from their community.
Women were eligible if
they were aged between 18 and 49 years old, were living in the area
referring to the Comboni Samaritans Health Center (CSHC) and consented
to data and specimen collection. Women susceptible to contract HIV
infection were defined as "vulnerable" and, therefore, exclusion
criteria included knowing to be positive for HIV at the time of the
interview or participation in other HIV intervention studies. However,
to reinforce the sense of community and prevent stigmatization, women
who were already aware of their HIV status were kept in the follow-up
surveys and into the intervention plan, but they were excluded from the
analysis.
All participants provided written informed consent in
accordance with the ethical standards of the Helsinki Declaration. The
study was approved by the Lacor Hospital Institutional Research and
Ethics Committee.
Data Collection.
At study entry, participants received a medical history and full
physical examination. The questionnaire was collected through Open Data
Kit Collect (ODK Collect software), hosted on portable Android devices.
Structured interviews were performed by peer educators and CSHC
personnel. The questionnaire was built upon the Uganda AIDS Indicator
Survey variables,[4] and it included a total of 115
questions investigating socio-demographical aspects (23 questions),
sexual activity and life (34 questions), HIV awareness (41 questions),
stigma (10 questions) and sexual self-determination (7 questions). Age
disparate sex was defined when the male partner was five years older or
more.[22]
HIV status was determined through the
4th Generation Alere HIV1/2 rapid Test. In addition, HBV infection was
screened by detection of Hepatitis B surface antigen (HBsAg), an
enzyme-linked immunosorbent assay (ELISA), while syphilis was screened
using VDRL rapid test-kit. Results were communicated by trained local
health personnel the same day the ODK questionnaire was administered.
During this phase, health operators provided post-test counselling to
all participants. Women who resulted positive for HIV, HBV or Syphilis
were then referred to St. Mary's Lacor Hospital for treatment and
follow-up. Results of serologic tests and responses to the
questionnaire were used as variables for the statistical analysis.
Statistical Analysis.
An initial descriptive analysis was applied to the studied population
for the major variables. Absolute numbers and percentages are shown in
the total sample and subgroups: no known STI; HIV positive; STI
positive. The same indicators have been calculated to evaluate the
prevalence of HIV, HBV, and syphilis, as well as co-infections (Table 1).
Using
the HIV status to split the population into two groups, positive and
negative, we applied the Chi-Square test to evaluate the difference of
all the major variables of Table 1. Continuous variables were handled as discrete using the categories reported in Table 1.
When we found a statistical difference, logistic regressions were
performed to assess the contribution of each variable in predicting HIV
status in terms of the odds ratio. The crude odds ratio and p-value are
shown in Table 2. The significant variables of table 2 have been tested in a Multivariable Multinomial Logistic Regression, forward method (Table 3). A p-value < 0.05 was considered statistically significant.
All the analyses were carried out using the SAS (v 9.4) software.
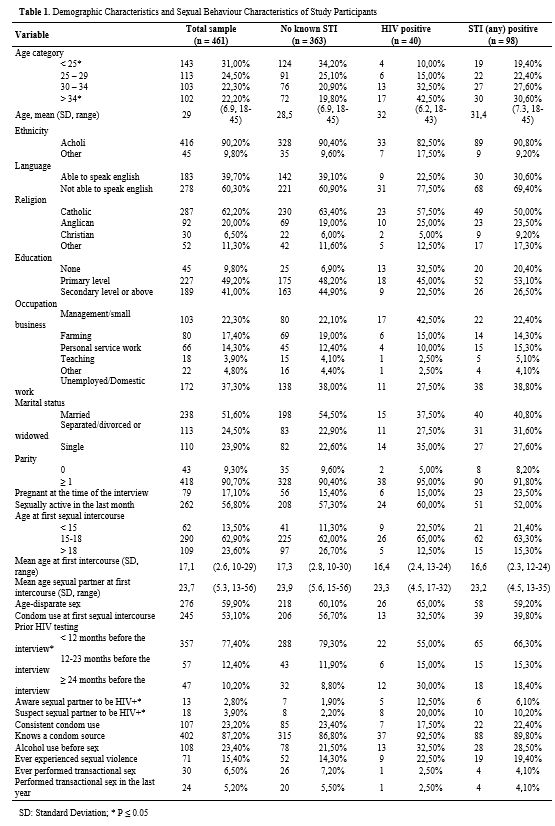 |
Table 1. Demographic Characteristics and Sexual Behaviour Characteristics of Study Participants |
 |
Table 2. Unadjusted multinomial logistic regression analysis of factors associated with HIV status (n = 461). |
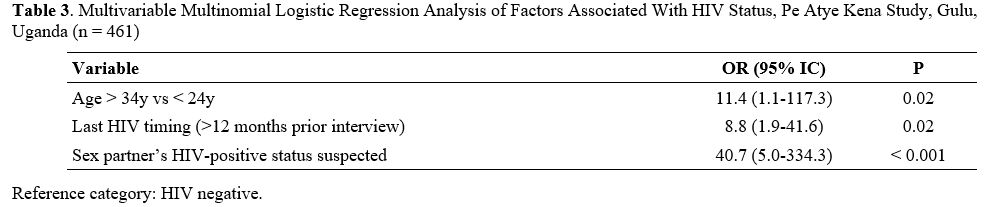 |
Table 3. Unadjusted multinomial logistic regression analysis of factors associated with HIV status (n = 461). |
Results
Study Participants.
Over the recruitment period (April 15-30, 2019), 523 women were
screened, and 62 were ineligible. Among those, 53 women were already
aware of being HIV-infected, and five were excluded because either
questionnaire responses or biological data were not available for data
analysis. Four women were enrolled twice. Thus, 461 young women were
included in the analysis (Figure 2).
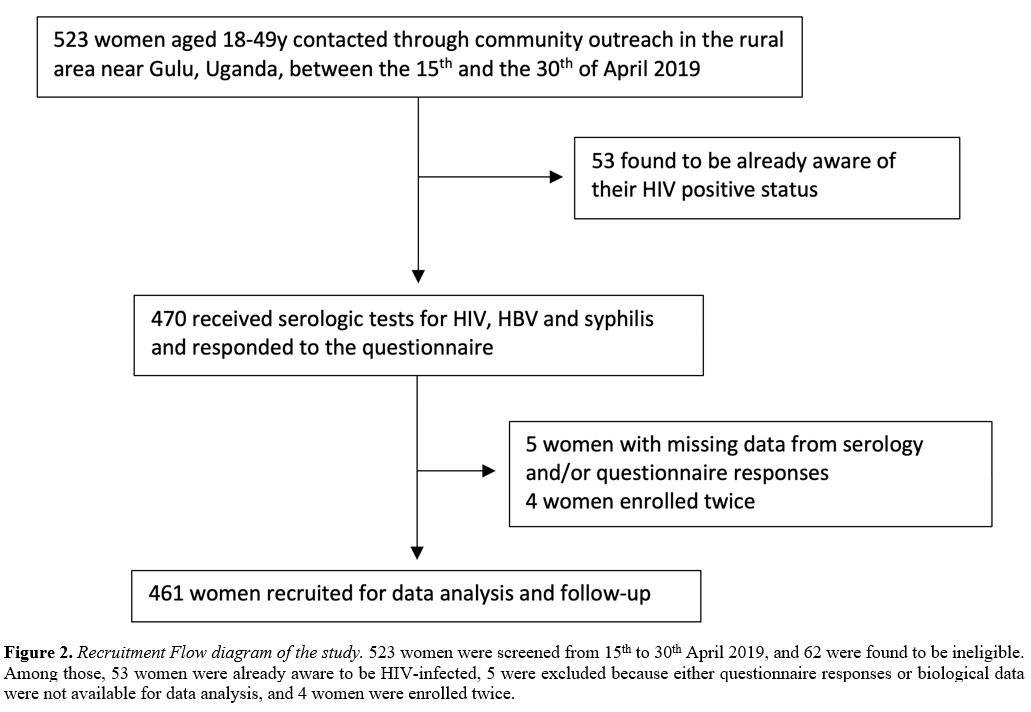 |
Figure 2. Recruitment Flow
diagram of the study. 523 women were screened from 15th to 30th April
2019, and 62 were found to be ineligible. Among those, 53 women were
already aware to be HIV-infected, 5 were excluded because either
questionnaire responses or biological data were not available for data
analysis, and 4 women were enrolled twice. |
Overall,
the median age (interquartile range; IQR) was 28 years (23 - 34). Two
hundred twenty-seven VW (49.2%) attended only primary school, and 183
(39.7%) were able to understand English, and 37.3% (n = 172) declared
to be unemployed at the moment of the interview. Most women (90.7%; n =
418) had given birth at some point in their lifetime, with 79 VW
(17.1%) reporting to be pregnant when the questionnaire was
administered. Demographic Characteristics of study participants are
shown in Table 1.
Out of
461 enrolled women, 40 (8.7%) were found to be HIV positive. Women who
tested positive for syphilis and HBV were, respectively, 42 (9.1%) and
29 (6.3%). Eight HIV-infected women (20%) were also found to be
infected with syphilis, and two women resulted positive to HIV, HBV,
and VDRL serologic tests.
Sexual Activity and Life.
Three-hundred fifty-two women (76.4%;) reported having had their first
sex by the age of 18, 29.1% (n = 136) by the age of 15 and, at their
first sexual intercourse, 53.1% (n = 245) of all women reported not
having used condom. One-hundred sixty-one (34.9%) women reported having
never been tested for HIV. Also, for 276 women (59.9%), their first
male partner was at least five years older than them (i.e.,
age-disparate sex), with a median age difference of 6 years (IQR: 3-9).
Among women who were sexually active in the previous 12 months (87%; n
= 401), only 26.7% (n = 107) consistently used condoms, while 26.9% (n
= 108) reported having consumed alcohol before or after sex at least
once. In addition, 59 women (12.8%) didn't know any place where they
could find condoms, and 15.4% (n = 71) of all women reported having
experienced sexual assault.
13 VW (2.8%) were aware that their
sex partner was HIV-infected, while 18 women (3.8%) suspected their
partner of being HIV+. In addition, up to 5% (n = 24) of recruited
women reported to have performed transactional sex at least once in the
last 12 months, and 6.4% (n = 30) at least once in their lifetime.
Factors Associated with HIV Status.
Older age (> 34 years vs < 24 years; OR 4.95, 95% CI: 1.7 to 14,
p < 0.01), having done the last HIV test more than 12 months before
the interview (OR 5.21, 95% CI: 2.3 to 11, p < 0.01), knowing (OR
13.7; 95% CI: 3.7 to 45, p < 0.01) or suspecting (OR 18.1; 95% CI:
6.1 to 54, p < 0.01) their respective male sex partner to be HIV
positive, not having used condom at the first sexual intercourse (OR
2.6; 95% CI 1.3 to 5.15, p < 0.01), and having performed
transactional sex at least once (OR 2.7; 95% CI: 1 to 7.2, p = 0.45)
were all associated with HIV status in unadjusted multinomial logistic
regression analysis (Table 2).
The multivariable regression model (Table 3)
confirmed a statistically significant effect of older age (> 34
years vs < 24 years; OR 11.4; 95% CI: 1.1 to 117.4, p = 0.02),
infrequent serologic testing (OR 8.8; 95% CI 1.9 to 41.6, p = 0.02),
and of suspecting one’s sexual partner to be HIV+ (OR 40.7; 95% CI 5 to
334.3, p < 0.001).
Discussion
To
our knowledge, with its 461 recruited women, Pe Atye Kena study
represents one of the largest perspective monocentric cohorts
investigating the impact of an intervention in HIV prevention among
women living in rural areas of Sub-Saharan Africa. Our study found
that, in a post-conflict rural area in Northern Uganda, women presented
a high prevalence of HIV, HBV, syphilis and infrequent HIV testing,
older age, and lack of trust in one partner HIV status are all
predictors of being infected with HIV.
With the persistent high
HIV prevalence described in this population, there is an urgent need to
detect community-specific risk factors for HIV acquisition and, more
importantly, to identify strategies effective in averting this
epidemiological trend.[23,24] Therefore, the
development of population-specific interventions aimed to reduce STI
vulnerability among girls and young women is included in 2017/2018
National HIV and AIDS Action Plan.[25]
Our population showed an HIV prevalence similar to the one reported by the 2018 Uganda Population-based HIV Assessment.[4] Also, consonant with other studies in Sub-Saharan Africa,[10]
an important portion of women reported age-disparate sex, few years of
schooling, inconsistent condom use, early sexual debut, and experience
of sexual-related violence.[8,26-28] HIV testing and old age are well-recognized factors associated with HIV-positive status.
Similar
to UPHIA assessment was also the rate of self-reported HIV-status
awareness, defined as having done the last HIV test within 12 months
before the interview. During the screening procedures, 53 women did not
meet the inclusion criteria because they already knew to be
HIV-infected, for HIV, and they were almost entirely already taking ARV
(52/53). Hence, among a total of 93 HIV-positive women who participated
in Pe Atye Kena screening activities, 43% was unaware of their HIV
status, which is almost twice the rate expected for women living in the
region.[4] Since our study targeted women at risk for
contracting STIs, this data is likely altered by a selection bias.
However, lack of HIV-status awareness represents a critical challenge
to achieve UNAIDS 90-90-90 targets.[3] Thus, our study may highlight the need to increase outreach and screening efforts among women living in the Gulu region.
In
our view, it is important to emphasize that the data presented here
refer to a population that is largely underrepresented in other large,
sub-Saharan cohort studies: to our knowledge, the DREAMS partnership is
the only study targeting, to some extent, a population of women living
in rural areas, where AIDS/HIV education and epidemiological data are
most needed.[29] Furthermore, unlike other large
cohort studies targeting similar populations, the present study is
entirely conducted by a second-level health center (village-based
health centre with a target population of 5000 people).[30] Thus, it may be used as a model for further community-based interventions.
Among
women enrolled in Pe Atye Kena study, both the prevalence of ever
having had syphilis and prevalence of current HBV infection (acute or
chronic, defined as being positive to hepatitis B surface antigen
assay) was higher than the one described for women living in the same
region by 2018 UPHIA report but lower than the one reported in other
studies.[31,32] In our study, 5% (2/40) of
HIV-infected women were co-infected with HBV, and 20% (8/40) of
HIV-infected women screened positive for syphilis. Interestingly, the
two women who resulted positive both to HBV and HIV also had syphilis.
Our study did not intend to assess the overall prevalence of sexually
transmitted diseases in the population. Therefore, the results are not
generalizable to all Gulu female population, but, noteworthy, the
burden of co-infections between HIV and other STIs in Sub-saharan
Africa remain controversial.[33,34]
Our study
has several limitations. First, as mentioned above, the reported
prevalence of HIV and other sexually transmitted diseases is not
generalizable to the overall population due to both outreach targets
and small sample size. Second, we did not include girls below 18 years
old, knowing that the risk of HIV acquisition substantially increases
from the age of 15.[13] Third, although standardized
and administered by trained healthcare personnel, interviews were
performed face-to-face, and some interviewer bias, response bias, or
misreporting of sexual behaviors is possible. Finally, longitudinal
follow-up is expected to clarify risk behaviours and retention in the
study and assess the efficacy of a peer-conducted educational
intervention in sexual behaviours and HIV/STIs acquisition.
Conclusions
Among
the 461 women included in this analysis, the prevalence of HIV and
other STIs was high, and the proportion of women who were unaware of
their serological status was much higher than the one expected for the
region. Also, despite substantial efforts in promoting HIV and sexual
prevention, high-risk sexual behaviours were a persistent challenge,
also considering that experiences of sexual assault, intimate partner
violence, and transactional sex were, most likely, consistently
underreported. In response to these findings, we plan to implement
targeted interventions to reduce HIV/STIs misconceptions and promote a
sense of community, self-determination, and female empowerment. If we
would be able to engage and retain women in our project successfully,
we expect to reduce the incidence of risky behaviours through the
construction of a stable, community-based educational subsidy. Women
play an essential role in the socio-economic development of sub-Saharan
Africa and, as we proceed to analyse whether our intervention is going
to impact sexual health among our population, we recognize the need for
future research to focus on the importance of insufficient female
personal agency and unequal power relations in the fight for HIV and
STI prevention.
Acknowledgements
The
authors thank the women of Pe Atye Kena study. Special recognition is
due to the "peer educators" whose contribution was essential in
recruiting and retention in study. Pe Atye Kena peer educators are:
Ajok Gloria, Lamara Barbara, Akello Kevine, Aineligio Irene, Akello
Hellen, Aol Nighty, Akello Susan and Lamunu Grace.
Ethics Committee and Consent
The
present study was approved by St Mary's Hospital Lacor Ethics Committee
(LHIREC, contact n° 0772561783), by written consent (n° 096/05/19). All
participants provided written informed consent for data and specimen
collection.
References
- Dellar RC, Dlamini S, Karim QA. Adolescent girls
and young women: key populations for HIV epidemic control. J Int AIDS
Soc. 2015;18(suppl 1):19408. https://doi.org/10.7448/IAS.18.2.19408
- Shisana
O, Rehle T, Simbayi L, et al. South African National HIV Prevalence,
Incidence and Behaviour Survey, 2012. Cape Town, South Africa: Human
Sciences Research Council Press; 2014. http://hdl.handle.net/20.500.11910/2490
- UNAIDS. 2020 Global AIDS update. https://www.unaids.org/en/resources/documents/2020/global-aids-report [accessed 2020 Nov 17].
- Ministry
of Health, Uganda. Uganda Population-based HIV Impact Assessment
(UPHIA) 2016-2017: Final Report. Kampala: Ministry of Health; July,
2019. https://phia.icap.columbia.edu/wp-content/uploads/2019/07/UPHIA_Final_Report_Revise_07.11.2019_Final_for-web.pdf
- Robinson
JL, Narasimhan M, Amin A, et al. Interventions to address unequal
gender and power relations and improve self-efficacy and empowerment
for sexual and reproductive health decision-making for women living
with HIV: A systematic review. PLoS One. 2017 Aug 24;12(8):e0180699. https://doi.org/10.1371/journal.pone.0180699
- Mabaso
M, Sokhela Z, Mohlabane N, et al. Determinants of HIV infection among
adolescent girls and young women aged 15-24 years in South Africa: a
2012 population- based national household survey. BMC Public Health.
2018;18(1):183. https://doi.org/10.1186/s12889-018-5051-3
- Evans
M, Risher K, Zungu N, et al. Age-disparate sex and HIV risk for young
women from 2002 to 2012 in South Africa. J Int AIDS Soc.
2016;19(1):21310. https://doi.org/10.7448/IAS.19.1.21310
- Topazian
HM, Stoner MCD, Edwards JK, Kahn K, et al. Variations in HIV risk by
young women's age and partner age-disparity in rural South Africa (HPTN
068). JAIDS. 2020; 83(4):350-356. https://doi.org/10.1097/QAI.0000000000002270
- Maughan-Brown
B, Kenyon C, Lurie MN. Partner age differences and concurrency in South
Africa: Implications for HIV-infection risk among young women. AIDS
Behav. 2014;18(12):2469-2476. https://doi.org/10.1007/s10461-014-0828-6
- Maughan-Brown
B, George G, Beckett S, Evans M, et al. HIV Risk Among Adolescent Girls
and Young Women in Age-Disparate Partnerships: Evidence From
KwaZulu-Natal, South Africa. JAIDS. 2018; 78(2):155-162 HTTPS://DOI.ORG/10.1097/QAI.0000000000001656
- Santelli
JS, Edelstein ZR, Mathur S, Wei Y, Zhang W, Orr MG, et al. Behavioral,
biological, and demo- graphic risk and protective factors for new HIV
infections among youth in Rakai, Uganda. JAIDS. 2013; 63(3):393–400. https://doi.org/10.1097/QAI.0b013e3182926795
- Balkus
JE, Brown E, Palanee T, Nair G, Gafoor Z, Zhang J, et al. An Empiric
HIV Risk Scoring Tool to Predict HIV-1 Acquisition in African Women.
JAIDS. 2016; 72(3):333–43. https://doi.org/10.1097/QAI.0000000000000974
- Saul
J, Bachman G, Allen S, Toiv NF, Cooney C, Beamon T. The DREAMS core
package of interventions: A comprehensive approach to preventing HIV
among adolescent girls and young women. PLoS ONE. 2018;13(12):
e0208167. https://doi.org/10.1371/journal.pone.0208167
- Gaffoor
Z, Wand H, Daniels B, Ramjee G. High risk sexual behaviors are
associated with sexual violence among a cohort of women in Durban,
South Africa. BMC Research Notes. 2013, 6:532 https://doi.org/10.1186/1756-0500-6-532
- Maughan-Brown
B, Evans M, George G. Sexual behaviour of men and women within
age-disparate partnerships in South Africa: implications for young
Women's HIV risk. PLoS One. 2016;11:e0159162. https://doi.org/10.1371/journal.pone.0159162
- De
Neve JW, Fink G, Subramanian SV, Moyo S, Bor J. Length of secondary
schooling and risk of HIV infection in Botswana: evidence from a
natural experiment. Lancet Glob Health. 2015; 3(8):e470–e7. https://doi.org/10.1016/S2214-109X(15)00087-X
- Behrman
JA. The effect of increased primary schooling on adult women's HIV
status in Malawi and Uganda: Universal Primary Education as a natural
experiment. Soc Sci Med. 2015; 127:108–15. https://doi.org/10.1016/j.socscimed.2014.06.034
- Maman
S, Campbell J, Sweat MD, Gielen AC. The intersections of HIV and
violence: Directions for future research and interventions. Soc Sci
Med. 2000 Feb;50(4):459-78.
- Donald A, Koolwal G, Annan J, Falb K, Goldstein M. Measuring Women's Agency. World Bank Group. 2017. https://elibrary.worldbank.org/doi/abs/10.1596/1813-9450-8148
- UNAIDS. Empowering women is critical to ending the AIDS epidemic. UNAIDS Press Statement. Available at: https://www.unaids.org/en/resources/presscentre/pressreleaseandstatementarchive/2015/march/20150308_IWD
- Dancy NC, Dutcher GA. HIV/AIDS information outreach: a community-based approach. J Med Libr Assoc. 2007;95(3):323-329. https://doi.org/10.3163/1536-5050.95.3.323
- UNAIDS. UNAIDS Terminology Guidelines. Geneva, Switzerland: UNAIDS; 2015. https://www.unaids.org/sites/default/files/media_asset/2015_terminology_guidelines_en.pdf
- Pettifor
A, Bekker LG, Hosek S, DiClemente R, Rosenberg M, Bull SS, et al.
Preventing HIV among young people: research priorities for the future.
JAIDS. 2013; 63 Suppl 2:S155– 60. https://doi.org/10.1097/qai.0b013e31829871fb
- UNAIDS.
HIV prevention among adolescent girls and young women: Putting HIV
prevention among adolescent girls and young women on the Fast-Track and
engaging men and boys. UNAIDS Guidance. 2016. https://www.unaids.org/sites/default/files/media_asset/UNAIDS_HIV_prevention_among_adolescent_girls_and_young_women.pdf
- UAC
(2015) National HIV and AIDS Strategic Plan 2015/2016- 2019/2020, An
AIDS free Uganda, My responsibility! Uganda AIDS Commission
Secretariat. https://uac.go.ug/sites/default/files/National%20HIV%20and%20AIDS%20Strategic%20Plan%202015-2020.pdf
- Pettifor
AE, Rees HV, Kleinschmidt I, Steffenson AE, MacPhail C, Hlongwa-
Madikizela L, et al. Young people's sexual health in South Africa: HIV
prevalence and sexual behaviors from a nationally representative
household survey. AIDS. 2005;19:1525-34. https://doi.org/10.1097/01.aids.0000183129.16830.06
- Hallman
K. Gendered socio-economic conditions and HIV risk behaviours among
young people in South Africa. Afr J AIDS Res. 2005;4:37-50. https://doi.org/10.2989/16085900509490340
- Joint
United Nations Programme on HIV/AIDS (UNAIDS), Interagency Task Team on
HIV and Young People. Guidance brief: HIV interventions for most
at-risk young people. New York: UNFPA; 2008. https://www.unfpa.org/sites/default/files/pub-pdf/mostatrisk.pdf
- Birdthistle
I, Schaffnit SB, Kwaro D, Shahmanesh M, Ziraba A, et al. Evaluating the
impact of the DREAMS partnership to reduce HIV incidence among
adolescent girls and young women in four settings: a study protocol BMC
Public Health. 2018; 18:912. https://doi.org/10.1186/s12889-018-5789-7
- Ministry
of Health, The Republic of Uganda. Guidelines for Designation,
Establishment and Upgrading of Health Units. The Health lnfrastructure
Working Group, 2011. Available at: https://health.go.ug/docs/guidelines.pdf
- Ocama
P, Seremba E, Apica B, Opio K. Hepatitis B and HIV co-infection is
still treated using lamivudine-only antiretroviral therapy combination
in Uganda. African Health Sciences. 2015; (15) 2: 328-333. https://doi.org/10.4314/ahs.v15i2.4
- Sun
HY, Sheng WH, Tsai MS, Lee KY, Chang SY, Hung CC. Hepatitis B virus
co-infection in human immunodeficiency virus-infected patients: A
review. World J Gastroenterol. 2014;20:14598-614 https://doi.org/10.3748/wjg.v20.i40.14598
- Barth
RE, Huijgen Q, Taljaard J, Hoepelman AIM. Hepatitis B/C and HIV in
sub-Saharan Africa: an association between highly prevalent infectious
diseases. A systematic review and meta-analysis. International Journal
of Infectious Diseases. 2010. 14(12):e1024-e1031. https://doi.org/10.1016/j.ijid.2010.06.013
- Chiesa
A, Ochola E, Oreni L, Vassalini P, Rizzardini G, Galli M. Hepatitis B
and HIV co-infection in Northern Uganda: Is a decline in HBV prevalence
on the horizon? PLoS ONE 2020. 15(11): e0242278. https://doi.org/10.1371/journal.pone.0242278
[TOP]




