S. Alkindi1, R.A. Elsadek2 and A.V. Pathare1.
1 Department of Haematology, College of Medicine & Health Sciences, Sultan Qaboos, Muscat, Oman.
2 Department of Medicine, Nizwa Hospital, Nizwa, Oman.
Correspondence to: Dr. Salam Alkindi, BA, MB, BCh, BAO, MSc, FRCP.
Professor in Haematology and Consultant Haematologist, Department of
Haematology, College of Medicine & Health Sciences, Sultan Qaboos
University, P. O. Box 35, Muscat 123, Sultanate of Oman. Tel:
+96824141182, Fax: +96824144887. E-mail:
sskindi@squ.edu.om alternate
sskindi@yahoo.com
Published: September 1, 2021
Received: July 17, 2021
Accepted: August 31, 2021
Mediterr J Hematol Infect Dis 2021, 13(1): e2021059 DOI
10.4084/MJHID.2021.059
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Vaccines
against acute respiratory syndrome Coronavirus 2(SARS-CoV2) are
critical weapons to control the spread of the deadly Coronavirus
2019(COVId-19) virus worldwide. Although these vaccines are generally
safe, their widespread use has produced reports of rare complications,
including vaccine-induced immune thrombotic thrombocytopenia (VIITT),
particularly in connection with ChAdOx1 nCov-19. We have identified
three cases of sickle cell disease (SCD) experiencing a severe
vaso-occlusive crisis (VOC) shortly after the vaccine. Despite being
stable for a long time, they had fever with tachycardia, along with a
significant rise in WBC, liver enzymes, particularly alkaline
phosphate, with a remarkable drop in hemoglobin, and platelets and one
of them had probably a fatal TTP like syndrome. Given these findings,
physicians and patients should exercise caution when taking this type
of vaccine and be aware of these safety concerns.
|
Introduction
Introduction
of vaccines against acute respiratory syndrome Coronavirus 2
(SARS-CoV2) is a critical weapon to control the spread of the deadly
Coronavirus 2019 (COVId-19) virus worldwide. More than 3.35 billion
vaccine doses have been administered worldwide, equal to 44 doses for
every 100 people, as of the beginning of July 2021.[1]
Vaccines approved by licensing authorities generally encode spike
protein of SARS-CoV-2, which encodes spike glycoprotein antigen
of SARS-CoV-2. Investigators used a recombinant adenoviral vector that
encodes spike glycoprotein, as produced by Astra-Zeneca (ChAdOx1
nCov-19) and AD26.COV2.S produced by Johnson & Johnson.
Additionally, platforms using m-RNA based technology are also in use
[(BNT162b2 of Pfizer-BioNTech), and (mRNA-1273 of Moderna)]. Although
these vaccines are generally safe, their widespread use worldwide has
produced reports of unusual complications. They include rare cases
associated with thrombocytopenia (thrombotic thrombocytopenic
purpura-like), particularly in connection with ChAdOx1 nCov-19.[2-3]
Sickle cell disease (SCD) is a systemic disease is characterized by
repetitive episodes of Vaso-occlusive crisis (VOC), with a
predisposition to infection and a higher risk of thromboembolic
disease.[4] We report here three cases of SCD
experiencing severe VOC and a fatal TTP-like syndrome with
thromboembolic complications following the ChAdOx1 nCov-19 vaccine.
Case 1
A
twenty-nine-year-old man with S/B0 thalassemia presented with shoulder
and back pain six days after receiving his COVID-19 vaccine
(AstraZeneca). He had no admissions over the past two years (21 months)
before this. At the time of entry, he had right shoulder tenderness,
with no other important findings. Past medical history was significant
for splenectomy, cholecystectomy, and bilateral avascular necrosis
(AVN) and received stem cell injection locally at the AVN site
previously. While in hospital, he developed fever, with no further
localizing signs. Blood work showed significant abnormal liver enzymes
and raised C-reactive protein (CRP) Table 1. He was given pain management and IV antibiotics, made a good recovery, and was discharged after eight days.
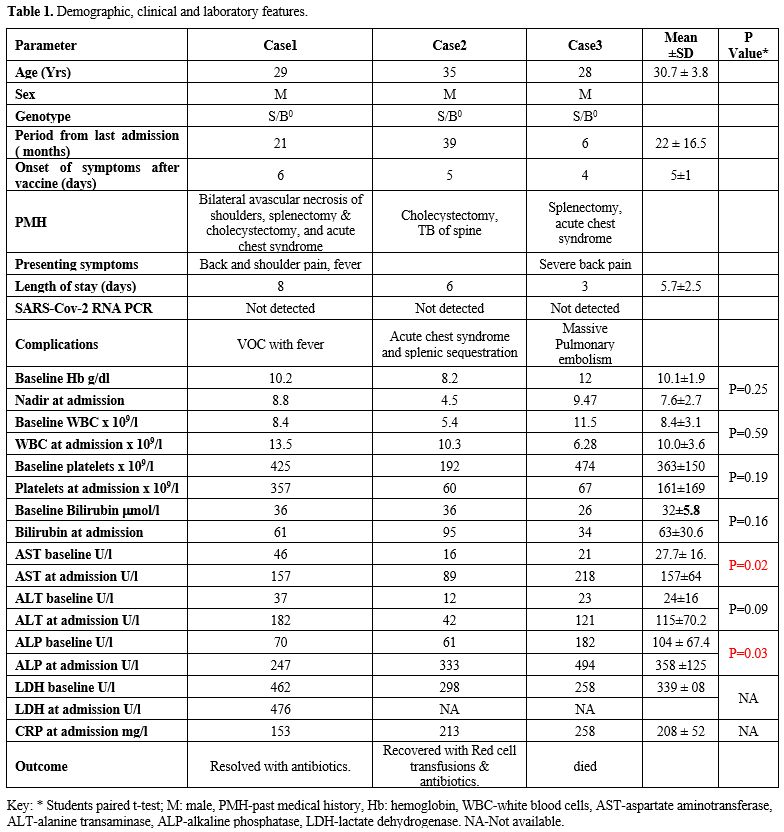 |
Table
1. Demographic, clinical and laboratory features.
|
Case 2
A thirty-four-year-old man with S/B0
thalassemia was admitted five days following the AstraZeneca vaccine
with lower back pain, chest pain, and shoulder pain. He was on
Hydroxyurea and had a history of splenic sequestration, tuberculosis of
the spine, and cholecystectomy. He had no recent admission for over
three years (39 months). While in hospital, he developed fever,
tachycardia, and dropped saturation, with right-sided
crepitations and chest X-rays showing right-sided
infiltrates. He was also noted to have significant anemia at 4.5 g/dl,
thrombocytopenia at 60 x 109/l, hyponatremia at 127, and a substantial rise in CRP, bilirubin, and other liver enzymes as outlined in Table 1.
SARS-COVID-19 testing by PCR was negative. He received blood
transfusions antibiotics and recovered gradually and was discharged
home six days later.
Case 3
A 28-year-old man with S/B0
thalassemia post-splenectomy and cholecystectomy presented with
significant back pain to the local hospital three days after receiving
the COVID-19 vaccine (AstraZeneca). He was started on pain analgesia,
noted to have tachycardia and tachypnea, and saturation dropped to 93%
on room air. Lung examination did not show any significant findings,
and Chest X-ray did not show any abnormalities. He was started on
Oxygen supplementation, and a chest CT scan with contrast confirmed
right-sided filling defects with mild bilateral pleural effusions.
Repeat blood tests showed a significant drop in hemoglobin to 9.47 g/dl
from baseline of 12 g/dl and platelets to 67 x 109/l from baseline of >400 x 109/l.
D-Dimers were elevated at >80mg/l (Normal 0.1-0.5), and he had a
high CRP at 258 (0-5). He was also noted to have raised liver enzymes,
in particular alkaline phosphatase, and other transaminases. He was
started on therapeutic low molecular weight heparin and exchange
transfusions. Although he was stable on oxygen via facemask, he
suddenly developed bradycardia, was resuscitated but became
hypotensive. It was then decided to proceed with thrombolysis; however,
he deteriorated, became severely hypotensive with bradycardia, and died
three days after admission.
Discussion
We
describe three young patients with SCD who presented with a significant
vaso-occlusive crisis after a long period of steady state. It was
characterized by severe back pain, a significant drop in hemoglobin,
platelets, and a statistically significant rise in liver enzymes, in
particular, ALP in all the three cases (P-value < 0.05), denoting
possibly significant bone infarction (most likely spinal, given the
persistent back pain). One patient had a massive pulmonary embolism.
Although we do not have the definitive laboratory confirmation, it
resembles the recently described vaccine-induced immune thrombotic
thrombocytopenia (VIITT) with a TTP-like syndrome. As all three
patients are young, it is plausible that the vaccine, through intensive
immune medicated antibody reaction or antibodies formation against
platelet PF4, led to platelets activation and consumption and
initiating thrombosis. As reported in the literature, the antibody
reaction could also have precipitated severe pain with a VOC crisis in
patients with sickle cell disease.[2-3] Subsequent
guidelines suggested avoiding heparin preparation for therapy, using
immunoglobulin (to block FC receptor of the binding antibodies), and
alternative anticoagulation agents for thrombosis such as rivaroxaban
and fondaparinux.[5] Recent data suggest that young patients are at increased risk of the Astra Zeneca COVID-19 vaccine.[6] It is also interesting to note that all three patients had S/B0
thalassemia, and it is not clear if this has contributed to the
development of these complications. In light of these findings, we have
asked our patients to exercise caution when taking this type of
vaccine. We have 12 patients who have taken two doses and nine who have
taken one dose of the BNT162b2 Pfizer-BioNTech vaccine without
significant complications. It is also important to note that although
mortality in patients with SCD who had COVID-19 was slightly higher
than that of the general population, our own experience with COVID-19
related illness in SCD was generally of a mild to moderate severity.[7]
References
- https://www.nytimes.com/interactive/2021/world/covid-vaccinations-tracker.html
- Greinacher
A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic
Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N Engl J 2021 Jun
3;384(22):2092-2101. https://doi.org/10.1056/NEJMoa2104840 PMid:33835769 PMCid:PMC8095372
- Nazy
I, Sachs UJ, Arnold DM, McKenzie SE, Choi P, Althaus K, Ahlen MT,
Sharma R, Grace RF, Bakchoul T. Recommendations for the clinical and
laboratory diagnosis of VITT against COVID-19: Communication from the
ISTH SSC Subcommittee on Platelet Immunology. J Thromb Haemost. 2021
Jun;19(6):1585-1588. https://doi.org/10.1111/jth.15341 PMid:34018298 PMCid:PMC8250233
- Naik
RP, Streiff MB, and Lanzkron S, Sickle cell disease and venous
thromboembolism: what the anticoagulation expert needs to know, J
Thromb Thrombolysis. 2013 Apr;35(3):352-8. https://doi.org/10.1007/s11239-013-0895-y PMid:23435703 PMCid:PMC4335704
- Schultz
NH, Sørvoll IH, Michelsen AE, Munthe LA, Lund-Johansen F, Ahlen MT,
Wiedmann M, Aamodt AH, Skattør TH, Tjønnfjord GE, Holme PA. Thrombosis
and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N Engl J Med.
2021 Jun 3;384(22):2124-2130. https://doi.org/10.1056/NEJMoa2104882 PMid:33835768 PMCid:PMC8112568
- Riad
A, Pokorná A, Mekhemar M, Conrad J, Klugarová J, Koščík M, Klugar M,
Attia S. Safety of ChAdOx1 nCoV-19 Vaccine: Independent Evidence from
Two EU States. Vaccines (Basel). 2021 Jun 18;9(6):673. https://doi.org/10.3390/vaccines9060673 PMid:34207369 PMCid:PMC8233751
- Alkindi
S, Elsadek RA, Al-Madhani A, Al-Musalhi M, AlKindi SY, Al-Khadouri G,
Al Rawahi B, Al-Ruqeishi S, Al-Yazeedi J, Wali YA, Al Shamakhi S, Al
Rawahi M, Pathare AV. Impact of COVID-19 on vasooclusive crisis in
patients with sickle cell anaemia. Int J Infect Dis. 2021
May;106:128-133. https://doi.org/10.1016/j.ijid.2021.03.044 PMid:33741487 PMCid:PMC7962915
[TOP]

