Sirapat Rungwittayatiwat1,
Paisarn Boonsakan2, Pichika Chantrathammachart1, Teeraya Puavilai1,
Sulada Pukiat1, Sithakom Phusanti1,3, Kochawan Boonyawat1, Pathawut
Wacharapornin1, Pantep Angchaisuksiri1, Artit Ungkanont1,3, Suporn
Chuncharunee1 and Pimjai Niparuck1.
1 Division of Hematology, Department of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand.
2 Department of Pathology, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand.
3 Department of Medicine, Chakri Naruebodindra Medical Institute, Mahidol University, Bangkok, Thailand.
Correspondence to:
Pimjai
Niparuck, Division of Hematology, Department of Medicine, Ramathibodi
Hospital, Mahidol University, Thailand. Tel: +662-201-1392 Fax:
+662-201-1392. E-mail:
niparuckblue@gmail.com
Published: November 1, 2021
Received: July 18, 2021
Accepted: October 15, 2021
Mediterr J Hematol Infect Dis 2021, 13(1): e2021063 DOI
10.4084/MJHID.2021.063
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Double-expressor lymphoma (DEL) was found to account for 20- 30% of
DLBCL. We conducted this study to analyze the survival, the clinical
presentation, and the factors associated with treatment outcomes in
DEL-DLBCL.
Methods: A retrospective study of 291 patients diagnosed with DLBCL during January 2015 - December 2018 was conducted.
Results:
Of the 291 patients, the median age was 63 years, germinal center B
cell-like DLBCL (GCB) and non-GCB subtypes were found in 32% and 68%,
respectively. DEL was found in 46% of 264 patients with available
immunohistochemistry staining for MYC protein. Patients with DEL was
significantly more common in elderly patients (p= 0.017) and non-GCB subtype (p=
0.006). High serum lactate dehydrogenase (LDH) levels and high Ki-67
index were significantly found in DEL patients than non-DEL patients (p= 0.024 and p= 0.04, respectively). The 3y-OS rate was shorter in the DEL group than in the non-DEL group, 58.7% versus 78.9% (p= 0.026), whereas no significant difference in 3y-DFS was identified between these groups (58.4% versus 67.7%, p=
0.343). Independent factors affecting OS and DFS in DEL patients were
ECOG 3-4, high LDH levels, extranodal involvement > 1 site, high
IPI, and stage III-IV in univariate analysis.
Conclusions:
High incidence of DEL was observed in this study, especially in
patients aged 60 years or older and non-GCB subtype. Patients with DEL
showed dismal DFS and OS.
|
Introduction
Diffuse
large B-cell lymphoma (DLBCL) is the most common aggressive B-cell
non-Hodgkin lymphoma (NHL), accounting for 65% of NHL in Thailand.[1]
It is a heterogeneous disease classified as germinal center-like B-cell
(GCB) and non-germinal B-cell subtypes that arise from different cells
of origin (COO). Hans algorithm including CD10, BCL6, and MUM1 protein
expressions are used for the classification of COO of DLBCL, and the
common methods for determining the COO are immunohistochemistry (IHC)
and gene expression profiling (GEP).[2] MYC and BCL2 protein expressions are found in 30-50% and 20- 35% DLBCL, respectively.[3]
Translocations of MYC and BCL2 and/or BCL6 are called triple and
double-hit lymphomas (TH/DHL), whereas the coexpression of MYC and BCL2
proteins without MYC/BCL2 and/or BCL6 rearrangement is described as
double-expressor lymphoma (DEL).[4] The progression-free survival (PFS)
and overall survival (OS) were dismal in DEL patients receiving R-CHOP
therapy. Rituximab plus CHOP (cyclophosphamide, doxorubicin,
vincristine, and prednisone) exhibited a favorable outcome for
DLBCL-GCB (2y-PFS of 64% and 2y-OS of 74%), compared with those in
non-GCB subtypes (2y-PFS of 28% and 2y-OS of 46%).[5]
The OS in patients with DEL and non-DEL were 20 and 36 months,
respectively; DEL patients receiving R-CHOP had a higher relapse rate
than treatment with R-EPOCH (80% versus 18%).[5] The
previous study of Italian patients with DEL illustrated that R-DA-EPOCH
every three weeks had 2y-OS longer than that in DEL patients treated
with R-CHOP, 90%, and 67%, respectively, whereas 2y-PFS in DEL patients
receiving R-DA-EPOCH and R-CHOP were 57% and 51%, respectively.[6]
Although the previous studies have demonstrated worse outcomes in
patients with DEL, the survival and the prognostic factors affecting
outcome in this subtype of DLBCL in the Asian population are not well
known. Hence, we conducted this study to analyze the survival, clinical
presentation, and factors associated with treatment outcomes in DEL.
Materials and Methods
Patients.
Patients with newly diagnosed DLBCL receiving chemotherapy plus
rituximab or chemotherapy alone at Ramathibodi Hospital between January
2015 and December 2018 were recruited and reviewed. All patients were
18 years of age and older. The diagnosis and subtypes of DLBCL were
reviewed and classified according to the 2016 revision of WHO
classification by an experienced hemato-pathologist.[4,7,8]
DLBCL with the cut-off level of 40% for MYC positivity and 50% for BCL2
protein coexpression was classified as double expressor (DE)-DLBCL,
whereas this subtype with MYC and BCL2 and/or BCL6 rearrangement was
classified as THL/DHL.[7] In this study, fluorescence
in situ hybridization (FISH) testing for MYC, BCL2, and BCL6
rearrangement was performed in DLBCL patients with MYC protein
expression > 40%.
Demographic
characteristics of patients including age, serum lactate dehydrogenase
(LDH), ECOG, site of lesion (extranodal/nodal), number of extranodal
involvement, bulky lesion, International prognostic index (IPI) score,
chemotherapy regimen, treatment with and without surgery or radiation
therapy were recorded. We excluded primary CNS lymphoma, primary
mediastinal B cell lymphoma, and indolent lymphoma with large cell
transformation. Patients receiving prior chemotherapy and/or radiation
therapy were also excluded. Six to eight cycles of intrathecal
methotrexate administration at a dose of 15 mg were performed for all
DLBCL patients with a high (4-6 points) CNS-IPI score[9] and/or testicular, adrenal/kidney or breast involvement.
Statistical analysis.
The primary endpoints were to analyze the rates of overall survival
(OS) and disease-free survival (DFS) in patients with double expressor
lymphoma (DEL), and secondary endpoints were to evaluate the response
and the complete remission (CR) rates between DEL and non-DEL and
identify factors affecting survival in DEL and non-DEL patients. The
response rate (RR) was defined as the percentage of patients who
achieved at least partial remission (reduction in tumor size> 50%
after treatment) and CR (no evidence of tumor after treatment).
Kaplan-Meier
analysis and log-rank test were used to evaluate and compare DFS and OS
between patients with DEL and non-DEL. The Cox regression model was
applied for multivariate survival analysis and identify independent
prognostic factors for survival. A Chi-square test was used to compare
the clinical factors and treatment outcomes between DEL and non-DEL
groups. Finally, all statistical analysis was performed using SPSS
version 18, and a P value less than 0.05 was considered statistically
significant.
This retrospective study was approved by the Local
Ethics Committee on Human Rights related to research involving human
subjects at Ramathibodi Hospital, Mahidol University.
Results
Patient characteristics.
The study included 291 DLBCL patients with a median age of 63 years
(19- 92 years), 157 of whom were female, and 184 patients were older
than 60 years. The tissue diagnosis was taken from lymph nodes (51%),
bone marrow (0.3%), and other organs (51%). Extranodal involvement was
found in 169 patients (58%), which the common sites of extranodal
involvement were the gastrointestine (22%), bone marrow (17%), and
nasal cavity (11%). GCB and non-GCB subtypes were found in 92 (32%) and
199 patients (68%), respectively. In the GCB group, 75 patients had
CD10+, and 17 patients were BCL6+/MUM1-. DEL was seen in 121 out of 264
patients with available IHC staining for MYC protein (45.8%), and it
was detected in non-GCB subtype (77%) greater than GCB-DLBCL (23%).
BCL6+ and MUM1+ were found in 82.6% and 84.3% of DEL patients,
respectively.
Of
121 DEL patients, the median age was 67 years (28- 90 years). Patients
aged> 60 years, stage III-IV, extranodal involvement and ECOG
performance 3-4 were observed in 70%, 59%, 56.2%, and 13% of DEL
patients, respectively, whereas high LDH levels, high IPI, and bulky
lesion (maximum tumor diameter> 7.5 cm) were found in 72%, 18% and
52% of DEL patients, respectively. In the group of DE-DLBCL patients,
extranodal involvement was found in 68 patients (56%), the common sites
of lymphoma involvements were BM (14%), nasal cavity (12%), stomach,
small and large bowel (12%), lung and pleura (10%). Central nervous
system involvement was found in only 3% of DEL patients. FISH for MYC,
BCL2, and BCL6 gene rearrangement was done in 87.6% of 121 DEL patients
(only available tissue samples), and DHL was detected in three
patients, including two patients with GCB and one patient with non-GCB.
BCL6+ and MUM1+ were found in 82.6% and 84.3% of DEL patients,
respectively. In the non-DEL group (143 patients), MYC+/BCL2- DLBCL was
detected by IHC in 17 patients which FISH for MYC/BCL2/ BCL6 gene
rearrangement was performed in 82% of 17 patients.
Patients
aged> 60 years, high LDH levels, Ki-67 >80%, non-GCB subtype, and
MUM1+ DLBCL were found significantly in DEL patients compared to those
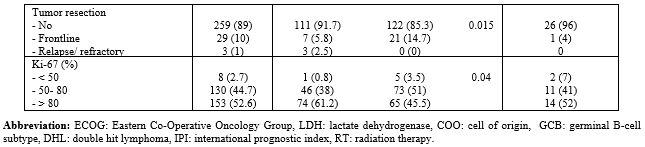
in non-DEL DLBCL. Patients' characteristics are shown in Table 1.
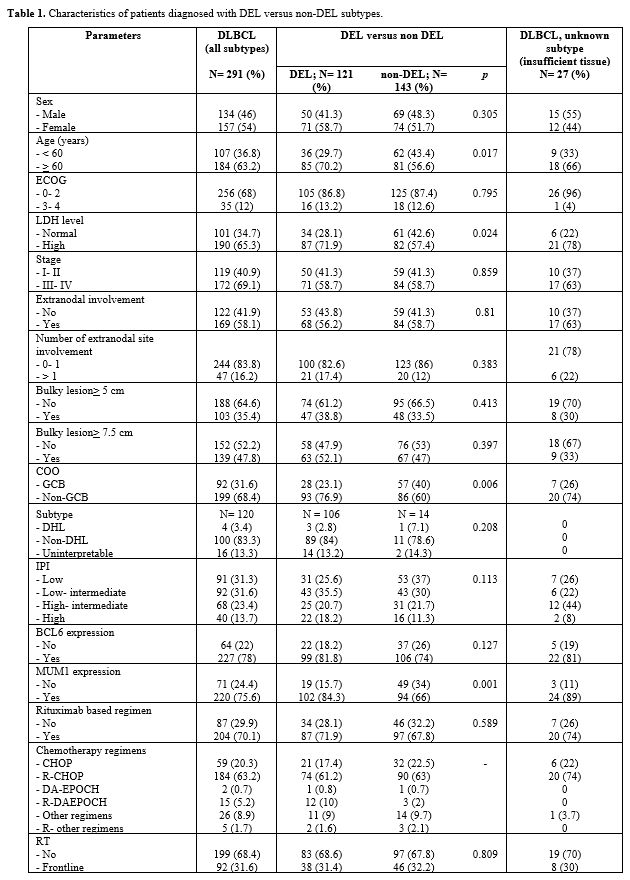 |
Table
1. Characteristics of patients diagnosed with DEL versus non-DEL subtypes. |
 |
|
Treatment outcomes.
During the entire study period, Thai patients with DLBCL treated under
the civil servant medical benefit scheme and health insurance could
access treatment with rituximab-based chemotherapy. In contrast,
patients with DLBCL who were treated under the universal coverage and
social security schemes could not claim rituximab therapy
reimbursement. Therefore, only 204 patients (70%) received rituximab
based chemotherapy, 184 (63%), 59 (20%), 15 (5%), 2 (1%) and 31
patients (11%) were treated with R-CHOP, CHOP, R-DA-EPOCH, DA-EPOCH,
and other chemotherapy regimens, respectively. In addition, DA-EPOCH
was given depending on the personalized chemotherapy selection for
patients with DLBCL who were younger than 60 years and suitable for
DA-EPOCH therapy; however, the current frontline standard of treatment
DLBCL (non-THL/DHL) remains CHOP regimen.
The CR rate and
survival analysis were performed only in patients with DEL (87
patients) and non-DEL DLBCL (97 patients) treated with rituximab-based
chemotherapy. CR rates were seen in 87% and 93% of DEL and non-DEL
patients, respectively. In addition, 91% of non-GCB patients with DEL
and 76% of GCB patients with DEL achieved CR. ECOG 0-2, normal LDH
levels, stage I-II, extranodal involvement< 1 site, and low or
intermediate IPI were significantly associated with higher CR rates in
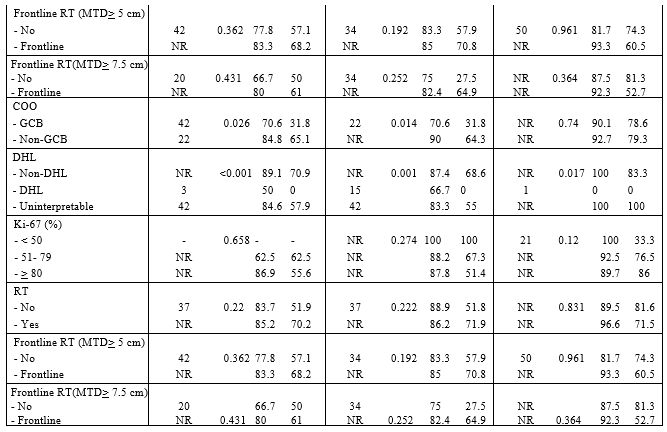
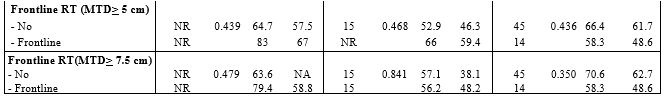
both DEL and non-DEL subtypes (Table 2).
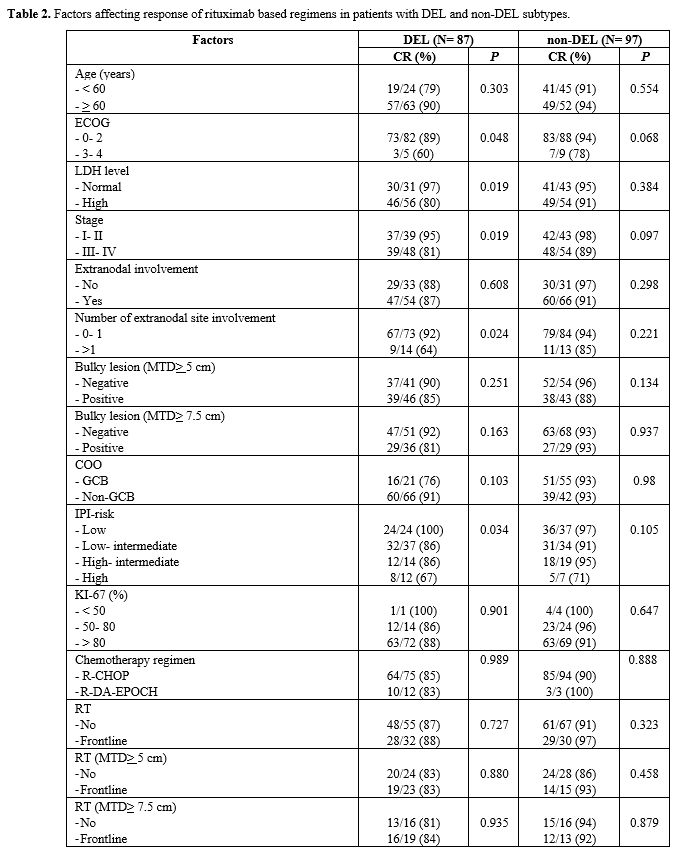 |
Table
2. Factors affecting response of rituximab based regimens in patients with DEL and non-DEL subtypes. |
In
the entire study population, 1y-OS, 3y-OS, 1y-DFS and 3y-DFS were
79.5%, 62.9% 68.5% and 58.4%, respectively. The survival analysis was
restricted to DEL and non-DEL patients who received rituximab-based
chemotherapy (R-chemo). After a median follow-up of 26.5 months, 1y-OS,
3y-OS, 1y-DFS and 3y-DFS rates in DEL patients were 86.7%, 58.7%,
69.7%, 58.4%, respectively. The 3y-OS rate was significantly shorter in
the DEL group than in the non-DEL group who were treated with R-chemo
(58.7% vs. 78.9%, p = 0.026), whereas there was no significant
difference in 3y-DFS was identified between these groups (58.4% vs.
67.7%, p = 0.343). The survival curves are shown in Figure 1.
After a median follow-up duration of 25 months, the 1y-OS rates in
patients with DEL and non-DEL who received R-CHOP were 86.7% and 94.3%,
respectively, whereas the 3y-OS rates in these groups were 58.7% and
82.6%, respectively (p = 0.004). In addition, the 1y-DFS rates in the
DEL and non-DEL patients treated with R-CHOP were 68.4% and 84.9%,
respectively, whereas the 3y-DFS rates in these groups were 50.2% and
70.5%, respectively (p = 0.19). Figure 2
Patients with refractory or relapsed (R/R) DEL and non-DEL after
R-chemo therapy were treated with salvage chemotherapy regimens such as
ifosfamide, carboplatin, and etoposide (ICE); cisplatin, cytarabine,
and dexamethasone (DHAP); etoposide, methylprednisolone, cytarabine,
and platinum (ESHAP); ifosfamide, methotrexate, and etoposide
(IMVP-16); rituximab and bendamustine (RB); or PD-1 inhibitors. Among
33 patients with DEL, 52% received more than one salvage chemotherapy
regimen, versus 56% of patients in the non-DEL group (27 patients). In
total, 12% and 11% of patients with R/R DEL and non-DEL, respectively,
had CNS involvement. CR was achieved after salvage chemotherapy for 6%
and 22% of patients in the R/R DEL and non-DEL groups, respectively. In
the group of patients with R/R DE-DLBCL, 94% did not respond to salvage
chemotherapy and died from progressive disease (PD), whereas 22% of
non-DEL patients with R/R disease achieved CR after salvage therapy and
were still alive at the end of the study.
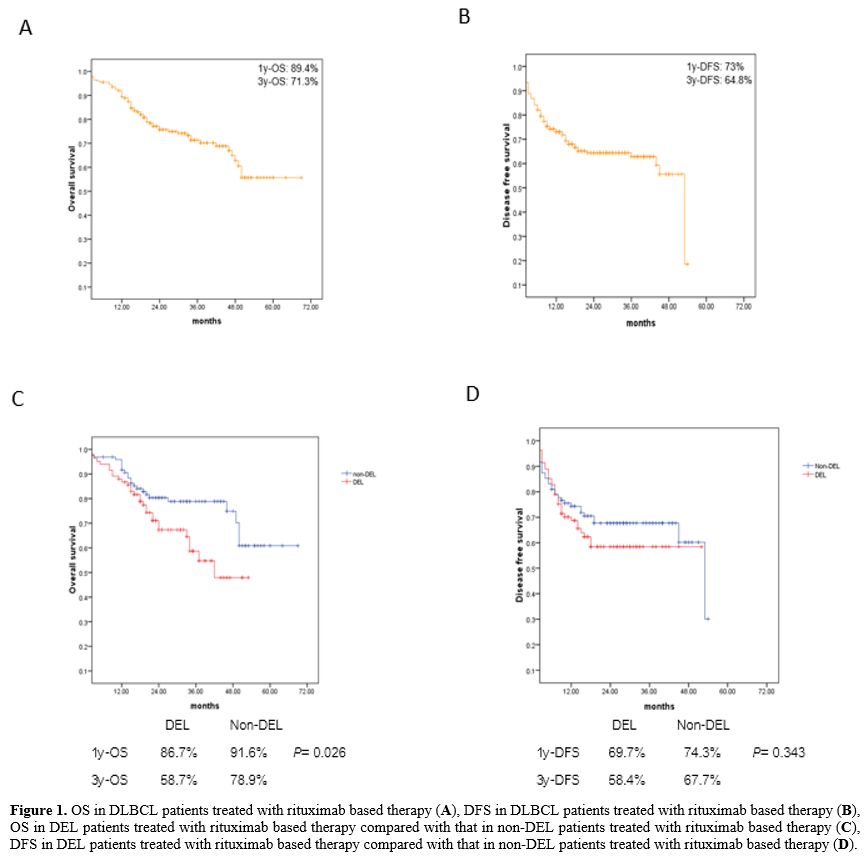 |
Figure
1. OS in DLBCL patients treated with rituximab based therapy (A), DFS
in DLBCL patients treated with rituximab based therapy (B), OS in DEL
patients treated with rituximab based therapy compared with that in
non-DEL patients treated with rituximab based therapy (C), DFS in DEL
patients treated with rituximab based therapy compared with that in
non-DEL patients treated with rituximab based therapy (D). |
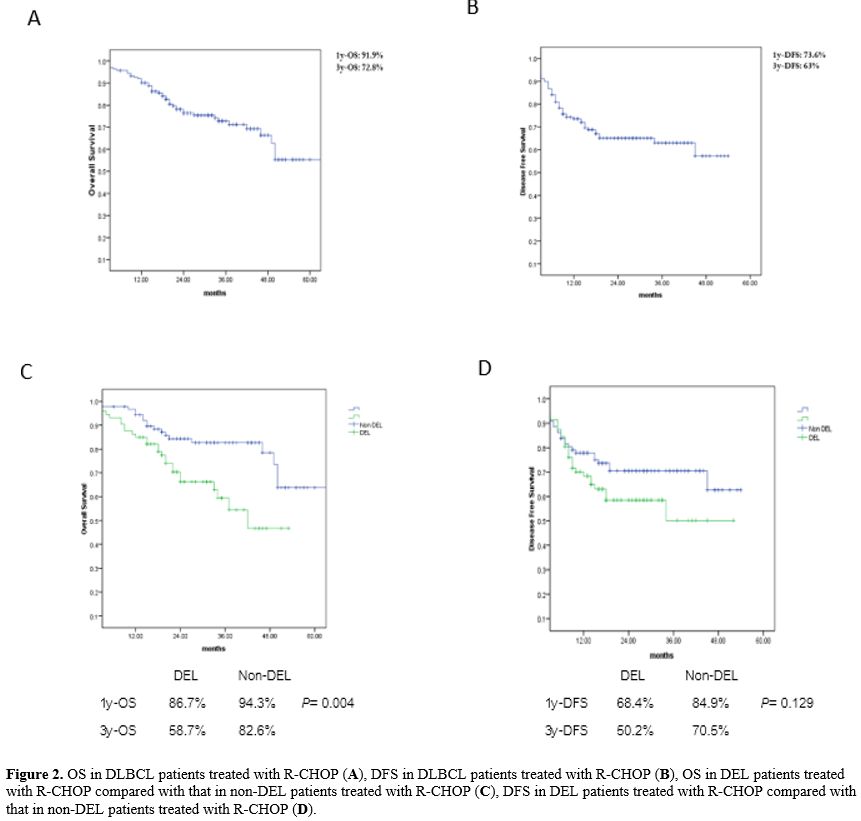 |
Figure 2. OS in
DLBCL patients treated with R-CHOP (A), DFS in DLBCL patients treated
with R-CHOP (B), OS in DEL patients treated with R-CHOP compared with
that in non-DEL patients treated with R-CHOP (C), DFS in DEL patients
treated with R-CHOP compared with that in non-DEL patients treated with
R-CHOP (D).
|
In univariate
analysis, parameters significantly associated with poorer OS in both
DEL and non-DEL patients were ECOG 3-4 and high IPI. In contrast, high
LDH level, stage III-IV, extranodal involvement> 1 site, GCB
subtype, and high-intermediate or high IPI were independent factors
affecting OS only in DEL patients (Table 3).
Only high LDH levels and stage III-IV were significantly associated
with dismal OS in DEL patients who were treated with both R-chemo and
R-CHOP in multivariate Cox regression analysis, p= 0.005 (R-chemo)
versus p= 0.01 (R-CHOP) for high LDH level group and p= 0.034 (R-chemo)
versus p= 0.031 (R-CHOP) for stage III-IV group. The results of the
multivariate Cox regression analysis are shown in Table 4.
DEL patients with high LDH levels and stage III-IV treated with R-CHOP
had 3y-OS of 41.8% and 37.6%, respectively. In the non-DEL group, ECOG
3–4 was significantly associated with poorer OS in multivariate Cox
analysis, p< 0.001.
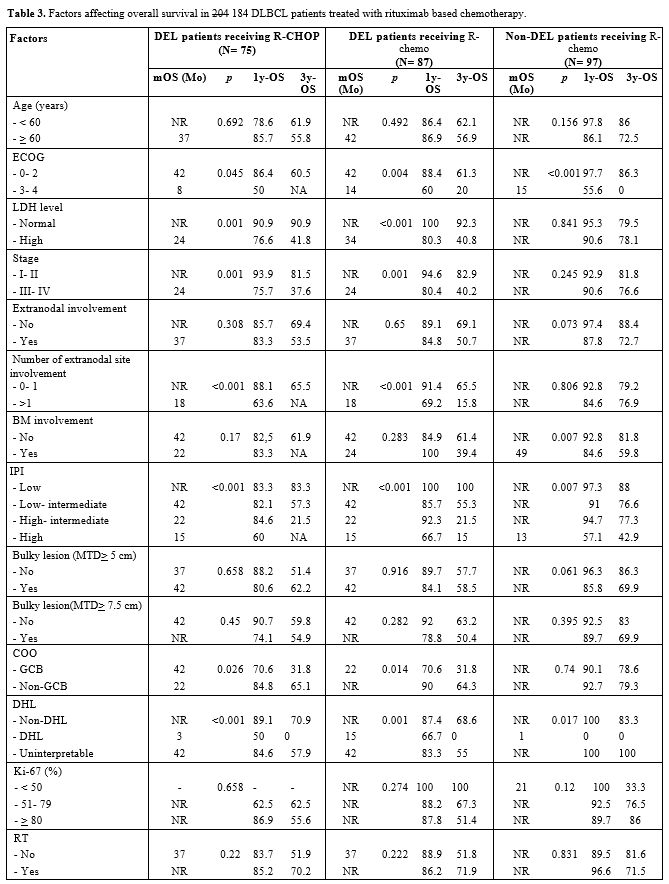 |
Table 3. Factors affecting overall survival in 204 184 DLBCL patients treated with rituximab based chemotherapy. |
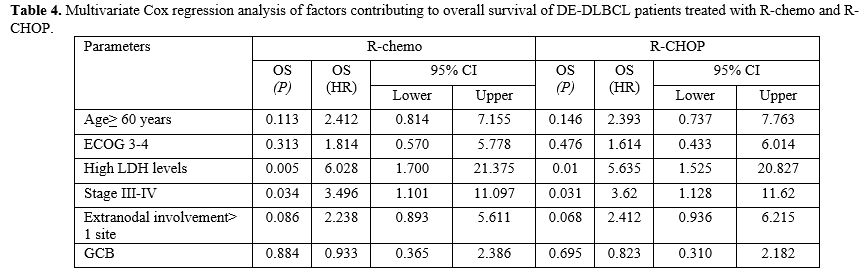 |
Table 4. Multivariate Cox
regression analysis of factors contributing to overall survival of
DE-DLBCL patients treated with R-chemo and R-CHOP.
|
In
addition, the univariate analysis showed that the parameters
significantly affecting DFS in both DEL and non-DEL patients were ECOG
3-4, stage III-IV, and high IPI. Whereas high LDH levels, extranodal
involvement >1, maximum tumor diameter (MTD) >5 or 7.5 cm, and BM
involvement were independent factors for poorer DFS in DEL patients. (Table 5)
Nevertheless, in multivariate analysis, only high LDH levels (p= 0.011)
and stage III-IV (p= 0.035) were the independent factors affecting DFS
in DEL patients receiving R-chemo. Stage III-IV (p= 0.028) was also
associated with shorter DFS in DEL patients treated with R-CHOP in
multivariate analysis. (Table 6)
DEL patients with high LDH levels and stage III-IV treated with R-CHOP
had 3y-DFS of 45.3% and 37.5%, respectively. Factors affecting DFS in
non-DEL patients receiving R-chemo were ECOG3-4 (p< 0.001), stage
III-IV (p= 0.017) and MTD) >5 (p= 0.001) in multivariate
analysis.
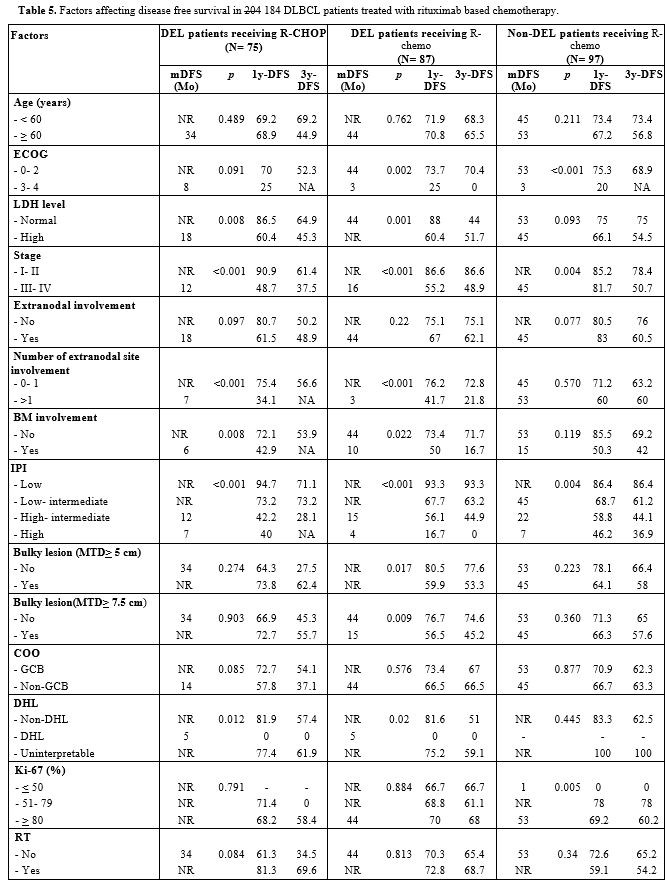 |
Table 5. Factors affecting disease free survival in 204 184 DLBCL patients treated with rituximab based chemotherapy. |
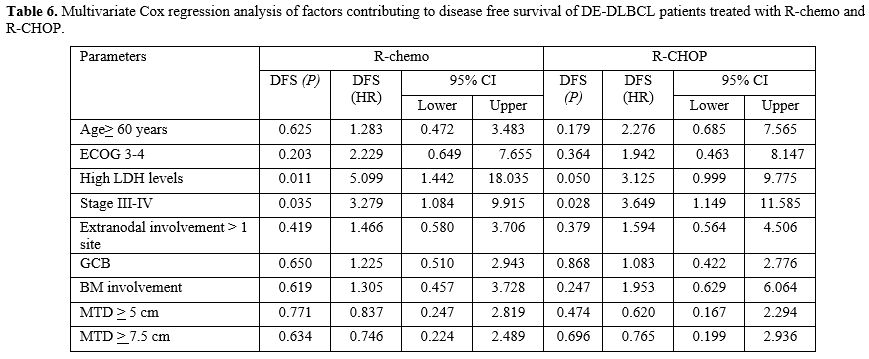 |
Table 6. Multivariate Cox
regression analysis of factors contributing to disease free survival of
DE-DLBCL patients treated with R-chemo and R-CHOP.
|
DEL
with BCL6 expression had no significant difference in 3y-OS and 3y-DFS
compared with those in DEL with BCL6 negative DLBCL (71.3% versus
68.8%, p= 0.729 and 60.7% versus 62.5%, p= 1.00, respectively).
Patients receiving R-DA-EPOCH had 1y-OS of 91.67% and 3y-OS of 64.3%,
whereas 1y-OS and 3y-OS in patients receiving R-CHOP were 86.7% and
58.7%, respectively (p= 0.497). The 1y-DFS of 75% and 3y-DFS of 60%
following R-DA-EPOCH therapy, and 1y-DFS of 68.4% and 3y-DFS of 50.2%
following R-CHOP therapy, (p= 0.959).
Discussion
In
this study, the frequency of DEL was 46% of DLBCL patients, and 77% of
DE-DLBCL was non-GCB subtype, and the prevalence of both DEL and
non-GCB with DE was higher than those reported in the previous studies.[10-13] Therefore, DEL is commonly found in non-GCB compared to GCB subtype.[11-13]
Nevertheless, non-DEL DLBCL was also often observed in non-GCB in our
study (60%) which was in contrast to the previous report that non-DEL
was commonly found in GCB patients.[11] In addition,
DHL had an extremely low prevalence in our cohort (3.4%), and the
prevalence of DEL and DHL differed from that in previous studies, which
might be attributable to the fact that our study was a single-center
retrospective study conducted at an academic tertiary referral hospital
and we only recruited DLBCL patients undergoing DLBCL treatment at our
center. Patients with DEL had significantly older age, high LDH levels
and high Ki-67 proliferation than those with non-DEL, in line with the
clinical manifestations in patients with DEL in previous reports.
However, only small population of our DEL patients had poor performance
status, high IPI or multiple extranodal sites of involvement.[11]
GCB with DEL subtype had lower CR rate than that in non-GCB with DE
patients which might be associated with the small number of GCB with DE
patients receiving R-chemo (21 patients).
Among patients who
received R-CHOP therapy, our study demonstrated that the DFS rate in
non-DEL patients was higher than that in DEL patients with a 16.5%
difference in DFS at 1 year (84.9% versus 68.4%) and 20% difference in
DFS at three years (70.5% versus 50.2%), even though the result was not
statistically significant between these groups. This result is
consistent with the fact that DE-DLBCL is more aggressive than the
non-DEL subtype.[3,6,10-16]
Conversely, the OS rate was significantly lower in the DEL group than
in the non-DEL group. We found that 94% of R/R DE-DLBCL patients did
not respond to salvage chemotherapy and died from progressive disease
(PD). Meanwhile, 22% of patients with R/R non-DEL achieved CR after
salvage therapy and remained alive at the end of the study. Similar
results were observed in patients who received R-chemo, and a lower
3-year OS rate was observed in patients with DEL than in patients with
non-DEL (58.7% vs. 78.9%), and the cause of significantly shorter OS in
DEL patients was PD after salvage therapy.
Conversely, there was
no difference in DFS between the DEL and non-DEL arms among patients
treated with receiving R-chemo at 1 (69.7% versus 74.3%) and three
years (58.4% versus 66.7%). The possible cause of slightly higher DFS
rates at 1 and 3 years in the non-DEL patients than in the DEL group
might be the higher rate of treatment with R-DAEPOCH in the DEL group.
Furthermore, our data also illustrated that both OS and DFS were
markedly decreased in patients with DEL within two years after
diagnosis, confirming that DEL is an aggressive lymphoma and did not
respond to salvage therapy. In previous studies, the 2-year OS and PFS
rates in patients with DEL treated with R-CHOP were approximately
50%-70% and 50%-54%, respectively,[6,11] and the 5-year OS and PFS rates were 30%–36% and 27%-32%, respectively.[9,10]
Similarly, the 2-year OS and DFS rates among patients with DEL treated
with R-CHOP in this study were 66.3% and 58.5%, respectively (Figure 2).
However, the study's median duration of follow-up time was only two
years, and we also lacked data on molecular features in our DLBCL
patients. Therefore a long-term follow-up (5 years) and further study
on the molecular biology in our DLBCL patients are needed.
Factors
affecting OS and DFS in DE-DLBCL patients were ECOG 3-4, high LDH
levels, extranodal involvement >1 site, stage III-IV and
high-intermediate/ high IPI. Nevertheless, only high LDH levels and
stage III-IV were independent factors for OS in the DEL patients
treated with both R-chemo and R-CHOP in multivariate analysis, in line
with previous studies.[11,12,14]
High LDH levels and stage III-IV were the independent factors affecting
DFS in DEL patients receiving R-chemo, whereas stage III-IV was
associated with shorter DFS in DEL patients treated with R-CHOP in
multivariate analysis. ECOG 3-4, high LDH levels, extranodal
involvement >1 site, stage III-IV and high-intermediate/ high IPI
were also significantly associated with lower CR rate in DEL patients.
There was no significant difference in OS and DFS rate between DEL
patients who received R-CHOP (75 patients) and R-DA-EPOCH (12
patients), as previously reported in a retrospective study from MD
Anderson;[15] however, the limitation of our survival
analysis was a small number of patients treated with R-DA-EPOCH since
the major population of DEL patients were older patients which could
not tolerate high-intensity chemotherapy. In the group of DEL patients,
non-GCB patients had significantly better OS than GCB-DLBCL patients in
the univariate analysis; nevertheless, the median age of GCB patients
was 70 years (range, 48-86 years) and all of whom receiving R-CHOP
therapy with 53% of recorded deaths from disease progression. Frontline
rituximab-based chemotherapy combined with RT did not show benefit on
DFS and OS in our DEL patients with either MTD> 5 or 7.5 cm. In the
study of Japanese patients with relapsed/refractory DEL, poor outcomes
in OS and EFS were seen even in patients who underwent allogeneic stem
cell transplantation.[17] Although FISH is a standard
test for diagnosis of DHL, it is expensive and time-consuming;
therefore, we performed FISH testing for MYC/BCL2/BCL6 rearrangement
only in DLBCL patients with MYC protein expression> 40%, since the
report of Zhang et al. illustrated that MYC translocation was found
only in DLBCL with MYC protein expression and the other previous
studies showed that MYC protein expression> 50% and > 70% were
predicted to have a rearrangement of MYC gene.[14,17,18]
The limitations of our study were the retrospective study population,
the small number of DE-DLBCL patients receiving R-DA-EPOCH therapy, and
poor FISH quality on formalin-fixed paraffin-embedded tissues that have
been stored for a long period. Therefore, it is impossible to draw
definitive conclusions regarding the best treatment for these patients.
Conclusions
A
high incidence of double-expressor lymphoma was observed in this study,
especially in patients aged 60 years or older and non-GCB subtype.
Patients with DEL showed dismal DFS and OS. Poor performance status,
high LDH and extranodal involvement >1 site, DHL, high IPI, and
stage III-IV were significantly associated with dismal OS and DFS in
DE-DLBCL patients.
Acknowledgements
We
would like to thank all patients who participated in the study. We want
to thank Ramathibodi Comprehensive Cancer Center, Ramathibodi Hospital,
Mahidol University for providing research funding.
Funding
This work was supported by Ramathibodi Comprehensive Cancer Center, Ramathibodi Hospital, Mahidol University.
Ethics Approval and Consent to Participate
This
retrospective study was approved by the Local Ethics Committee on Human
Rights related to research involving human subjects at Ramathibodi
Hospital, Mahidol University.
Authors' Contributions
SR
collected clinical data, FISH testing results, analyzed the data, and
wrote the manuscript. PB performed the histological examination. PC,
TP, SP, SP, KB, PW, PA, AU, SC carried out the experiment. PN designed
the study, analyzed the data, and edited the manuscript. All authors
read and approved the final manuscript.
References
- Bunworasate U, Siritanaratanakul N, Khuhapinant A,
Lekhakula A, Rujirojindakul P, Sirijerachai C, Chansung K, Suwanban T,
Chuncharunee S, Niparuck P, Nawarawong W, Norasetthada L, Kanitsap N,
Mongkonsritragoon W, Numbenjapon T, Prayongratana K, Pornvipavee R,
Intragumtornchai T. A nationwide prospective multicenter study of
clinical features and outcomes of non-Hodgkin lymphoma in Thailand: an
analysis of 939 cases. 2011:2064-64. https://doi.org/10.1182/blood.V118.21.2064.2064
- Hans
CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G,
Müller-Hermelink HK, Campo E, Braziel RM, Jaffe ES, Pan Z, Farinha P,
Smith LM, Falini B, Banham AH, Rosenwald A, Staudt LM, Connors JM,
Armitage JO, Chan WC. Confirmation of the molecular classification of
diffuse large B-cell lymphoma by immunohistochemistry using a tissue
microarray. Blood. 2004;103(1):275-82. https://doi.org/10.1182/blood-2003-05-1545 PMid:14504078
- Aggarwal
A, Rafei H, Alakeel F, Nafez Finianos A, LingLiu M, Bahesh E, LAscensao
J, Mobarek D. Outcome of Patients with Double-Expressor Lymphomas
(DELs) Treated with R-CHOP or R-EPOCH. Blood. 2016;128:5396. https://doi.org/10.1182/blood.V128.22.5396.5396
- Swerdlow
SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R,
Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the
World Health Organization classification of lymphoid neoplasms. Blood.
2016;127:2375-90. https://doi.org/10.1182/blood-2016-01-643569 PMid:26980727 PMCid:PMC4874220
- Nowakowski
GS, Laplant BR, Macon WR, Reeder CB, Foran JM, Nelson GD, Thompson CA,
Rivera CE, Inwards DJ, Micallef IN, Johnston PB, Porrata LF, Ansell SM,
Gascoyne RD, Habermann TM, Witzig TE. Lenalidomide Combined with R-CHOP
(R2CHOP) overcomes negative prognostic impact of ABC molecular subtype
in newly diagnosed diffuse large B-cell lymphoma. Blood. 2016;128:3035.
https://doi.org/10.1182/blood.V128.22.3035.3035
- Dodero
A, Guidetti A, Tucci A, Barretta F, Novo M, Devizzi L, Re A, Passi A,
Pellegrinelli A, Pruneri G, Miceli R, Testi A, Pennisi M, Di Chio MC,
Matteucci P, Carniti C, Facchetti F, Rossi G, Corradini P.
Dose-adjusted EPOCH plus rituximab improves the clinical outcome of
young patients affected by double expressor diffuse large B-cell
lymphoma. Leukemia. 2019;33(4):1047-51. https://doi.org/10.1038/s41375-018-0320-9 PMid:30631117 PMCid:PMC6756077
- Hans
CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G,
Müller-Hermelink HK, Campo E, Braziel RM, Jaffe ES, Pan Z, Farinha P,
Smith LM, Falini B, Banham AH, Rosenwald A, Staudt LM, Connors JM,
Armitage JO, Chan WC. Confirmation of the molecular classification of
diffuse large B-cell lymphoma by immuno-histochemistry using a tissue
microarray. Blood. 2004;103:275-82. https://doi.org/10.1182/blood-2003-05-1545 PMid:14504078
- Choi
WW, Weisenburger DD, Greiner TC, Piris MA, Banham AH, Delabie J,
Braziel RM, Geng H, Iqbal J, Lenz G, Vose JM, Hans CP, Fu K, Smith LM,
Li M, Liu Z, Gascoyne RD, Rosenwald A, Ott G, Rimsza LM, Campo E, Jaffe
ES, Jaye DL, Staudt LM, Chan WC. A new immuno-stain algorithm
classifies diffuse large B-cell lymphoma into molecular subtypes with
high accuracy. Clin Cancer Res. 2009;15(17): 5494-502. https://doi.org/10.1158/1078-0432.CCR-09-0113 PMid:19706817 PMCid:PMC7289055
- Schmitz
N, Zeynalova S, Nickelsen M, Kansara R, Villa D, Sehn LH, Glass B,
Scott DW, Gascoyne RD, Connors JM, Ziepert M, Pfreundschuh M, Loeffler
M, Savage KJ. CNS International Prognostic Index: A Risk Model for CNS
Relapse in Patients With Diffuse Large B-Cell Lymphoma Treated With
R-CHOP. J Clin Oncol. 2016;34(26):3150-6. https://doi.org/10.1200/JCO.2015.65.6520 PMid:27382100
- Johnson
NA, Slack GW, Savage KJ, Connors JM, Ben-Neriah S, Rogic S, Scott DW,
Tan KL, Steidl C, Sehn LH, Chan WC, Iqbal J, Meyer PN, Lenz G, Wright
G, Rimsza LM, Valentino C, Brunhoeber P, Grogan TM, Braziel RM, Cook
JR, Tubbs RR, Weisenburger DD, Campo E, Rosenwald A, Ott G, Delabie J,
Holcroft C, Jaffe ES, Staudt LM, Gascoyne RD. Concurrent expression of
MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab
plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin
Oncol. 2012;30:3452-9. https://doi.org/10.1200/JCO.2011.41.0985 PMid:22851565 PMCid:PMC3454768
- Hu
S, Xu-Monette ZY, Tzankov A, Green T, Wu L, Balasubramanyam A, Liu WM,
Visco C, Li Y, Miranda RN, Montes-Moreno S, Dybkaer K, Chiu A, Orazi A,
Zu Y, Bhagat G, Richards KL, Hsi ED, Choi WW, Zhao X, van Krieken JH,
Huang Q, Huh J, Ai W, Ponzoni M, Ferreri AJ, Zhou F, Slack GW, Gascoyne
RD, Tu M, Variakojis D, Chen W, Go RS, Piris MA, Møller MB, Medeiros
LJ, Young KH. MYC/BCL2 protein coexpression contributes to the inferior
survival of activated B-cell subtype of diffuse large B-cell lymphoma
and demonstrates highrisk gene expression signatures: a report from the
International DLBCL Rituximab-CHOP Consortium Program. Blood.
2013;121:4021-31. https://doi.org/10.1182/blood-2012-10-460063 PMid:23449635 PMCid:PMC3709650
- Riedell PA, Smith SMJC. Double hit and double expressors in lymphoma: definition and treatment. 2018;124(24):4622-32. https://doi.org/10.1002/cncr.31646 PMid:30252929
- Liu
Y, Barta SK. Diffuse large B-cell lymphoma: 2019 update on diagnosis,
risk stratification, and treatment. Am J Hematol. 2019;94:604-16. https://doi.org/10.1002/ajh.25460 PMid:30859597
- Green
TM, Young KH, Visco C, Xu-Monette ZY, Orazi A, Go RS, Nielsen O,
Gadeberg OV, Mourits-Andersen T, Frederiksen M, Pedersen LM, Møller MB.
Immunohistochemical double-hit score is a strong predictor of outcome
in patients with diffuse large B-cell lymphoma treated with rituximab
plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin
Oncol. 2012;30(28): 3460-7. https://doi.org/10.1200/JCO.2011.41.4342 PMid:22665537
- Sathyanarayanan
V, Oki Y, Issa AK, Amin Ahmed M, Noorani M, A Fanale M, B. Hagemeister
F, S. Neelapu S, J. Nastoupil L, Fowler N, Turturro F, Davis R,
Rodriguez A, Wang M, Feng MS L, H.Young K, J.McDonnell T, CPinnix C,
Westin JR. High risk diffuse large B cell lymphoma: a comparison of
aggressive subtypes treated with dose adjusted chemotherapy-the
University of Texas MD Anderson Experience. American Society of
Hematology. Washington, DC; 2016. https://doi.org/10.1182/blood.V128.22.106.106
- Kawashima
I, Inamoto Y, Maeshima AM, Nomoto J, Tajima K, Honda T, Shichijo T,
Kawajiri A, Takemura T, Onishi A, Ito A, Tanaka T, Fuji S, Kurosawa S,
Kim SW, Maruyama D, Tobinai K, Kobayashi Y, Fukuda T. Double-Expressor
Lymphoma is associated with poor outcomes after allogeneic
hematopoietic cell transplantation. Biol Blood Marrow Transplant.
2018;24:294-300. https://doi.org/10.1016/j.bbmt.2017.10.013 PMid:29037890
- Zhang
Y, Wang H, Ren C, Yu H, Fang W, Zhang N, Gao S, Hou Q. Correlation
Between C-MYC, BCL-2, and BCL-6 protein expression and gene
translocation as biomarkers in diagnosis and prognosis of diffuse large
B-cell lymphoma. Front Pharmacol. 2019;9:1497. https://doi.org/10.3389/fphar.2018.01497 PMid:30666200 PMCid:PMC6330311
- Kluk
MJ, Chapuy B, Sinha P, Roy A, Dal Cin P, Neuberg DS, Monti S, Pinkus
GS, Shipp MA, Rodig SJ. Immunohistochemical detection of MYC-driven
diffuse large B-cell lymphomas. PLoS ONE. 2012;7:e33813. https://doi.org/10.1371/journal.pone.0033813 PMid:22511926 PMCid:PMC3325231
[TOP]