Monthly of cases of HSP and EBV infection. The incidence of HSP cases was lower in summer and higher in winter, while EBV infection and EBV-triggered HSP had no obvious seasonal characteristics (Figure 1A).
Age and gender distribution of HSP and EBV infection. A peak prevalence, mainly in the 2–5 years age range, was observed in the EBV infection cases, and after this age period, the prevalence decreased dramatically. However, a bell-shaped distribution pattern was also seen with a peak prevalence mainly in the 6–10 years age range in the HSP and EBV-triggered HSP cases (Figure 1B). There was no significant difference in the male-to-female ratio in the three groups (χ2 = 0.418, p = 0.811, Figure 1C).
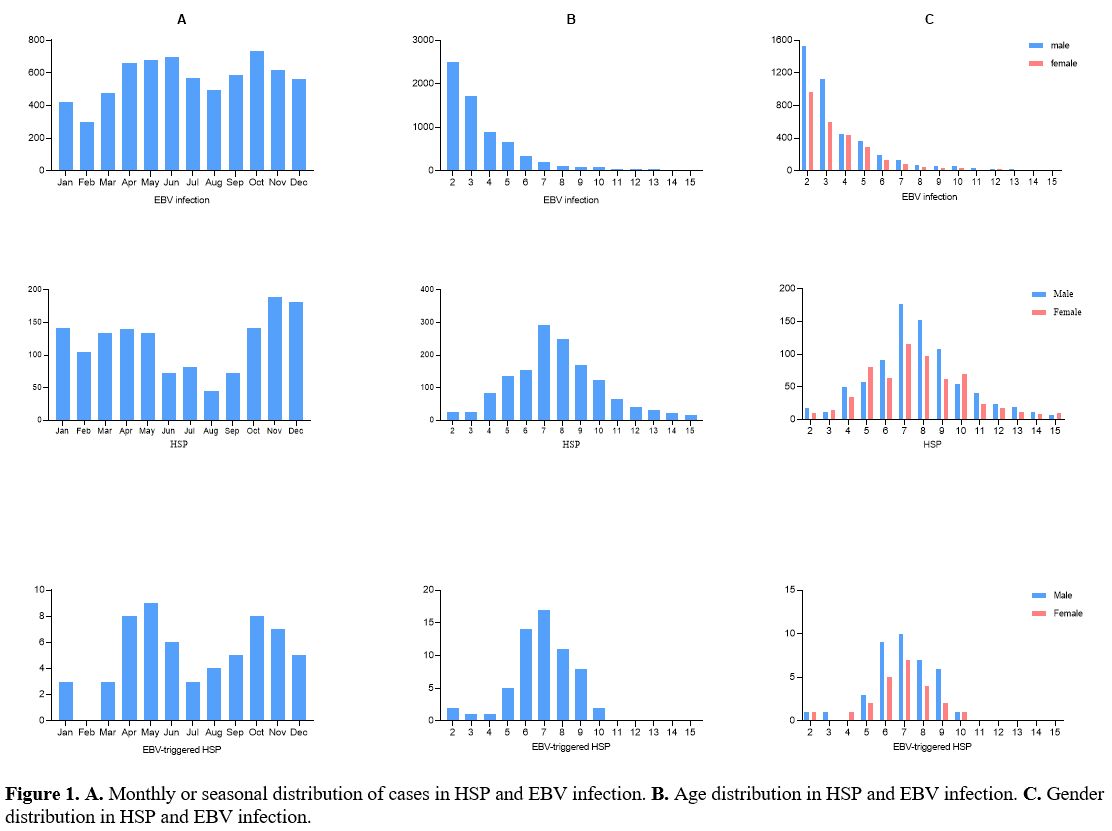 |
Figure
1. A.
Monthly or seasonal distribution of cases in HSP and EBV infection. B. Age distribution
in HSP and EBV infection. C.
Gender distribution in HSP and EBV infection. |
Clinical manifestations of EBV infection. Six thousand seven hundred ninety-four children were determined with positive results, including 4098 males and 2696 females (male: female=1.52;1). A total of 63807 children were tested for EBV, and the rate of EBV infection was 10.6% (6794/63807) (Table 1). The frequency of EBV infection developing HSP in children was 0.9% (61/6794). In the cases of EBV infection, a significantly greater frequency of EBV-triggered HSP was found in the age of 6-10 years (χ2 = 356.808, p < 0.001).
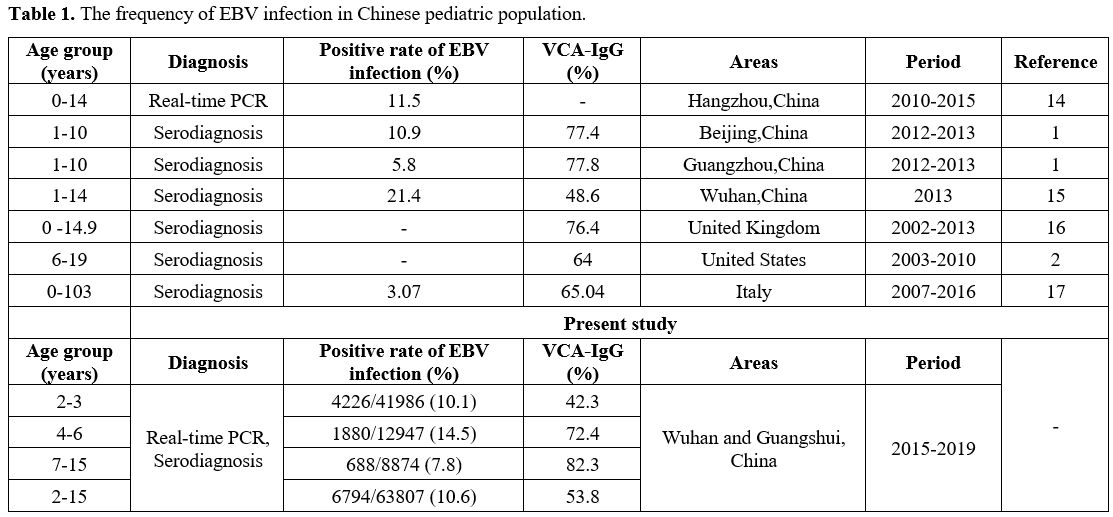 |
Table 1. The frequency of EBV infection in Chinese pediatric population. |
Clinical manifestations of HSP with EB infection. The EBV-triggered HSP cases most frequently exhibited abdominal pain (39/61, 63.9%), followed by arthritis/arthralgia (34/61, 55.7%), and renal involvement (12/61, 20.0%) (Table 2). The clinical manifestations presented no significant heterogenicity between the EBV-triggered HSP group and the non-infectious group. However, the EBV-triggered HSP cases had a significantly higher frequency of abdominal pain than the MP-triggered HSP group (χ2 = 8.024, p = 0.005) (Table 3).
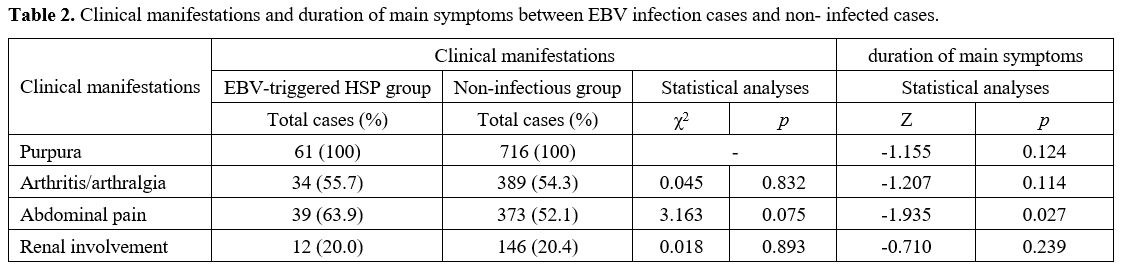 |
Table 2. Clinical manifestations and duration of main symptoms between EBV infection cases and non- infected cases. |
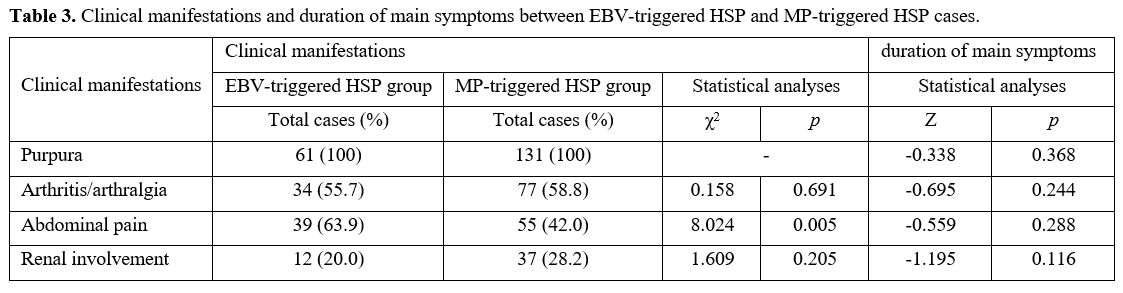 |
Table 3. Clinical manifestations and duration of main symptoms between EBV-triggered HSP and MP-triggered HSP cases. |
Duration of main symptoms. The comparison of the duration of main symptoms of the non-infectious cases with infectious cases is presented in Table 2. Significant differences were observed in the duration of abdominal pain (Z = -1.935, p = 0.027) between the two groups.
Laboratory tests. As shown in Figure 2, C3 (t = 9.709, p < 0.001), IgA (t = 20.39, p < 0.001) and IgG (t = 6.407, p < 0.001) were significantly higher in the EBV-infected group than those in the healthy control group. Notably, a significantly higher proportion of CD19 (t = 6.773, p < 0.001) and lower proportion of CD56 (t = 11.13, p < 0.001) was also found in EBV-infected group compared with healthy control group.
The IgA level of the infected group was also significantly higher than that of the non- infected group (t = 2.162, p = 0.032), while CD4/CD8 ratio (t = 10.070, p < 0.001) and CD56 proportion (t = 2.096, p = 0.037) were significantly lower than those of the non- infected group.
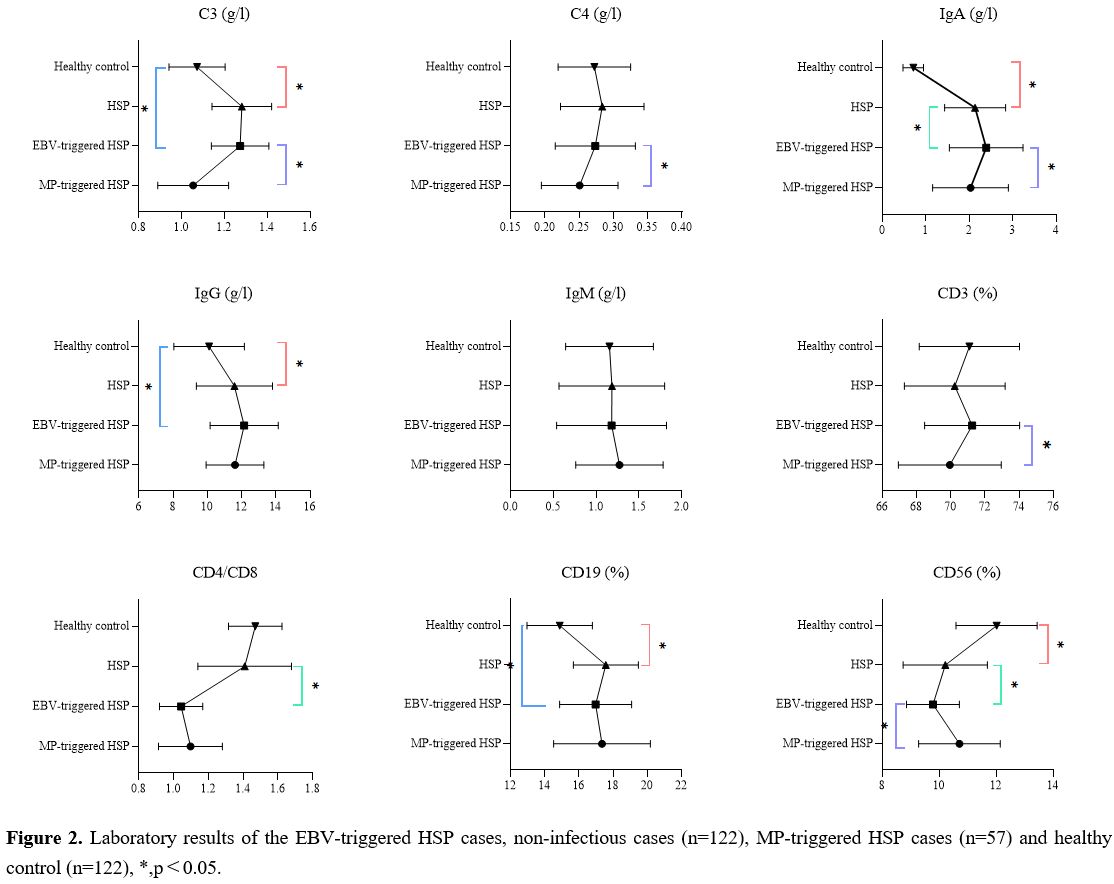 |
Figure 2. Laboratory results of the EBV-triggered HSP cases, non-infectious cases (n=122), MP-triggered HSP cases (n=57) and healthy control (n=122), *, p<0.05. |
The laboratory results of C3 (t = 7.926, p < 0.001), C4 (t = 2.168, p = 0.032), IgA (t = 2.278, p = 0.025) and CD3 proportion (t = 2.450, p = 0.016) were significantly decreased in the EBV infection group than in the MP-triggered HSP group, while the CD56 proportion (t =4.228, p < 0.001) increased significantly.