Moussa Seck1,3, Alioune Badara Senghor2, Mossane Loum3, Sokhna Aissatou Touré3, Blaise Félix Faye1,3, Alioune Badara Diallo3, Mohamed Keita3, Seydi Elimane Bousso3, Sérigne Mourtalla Guèye2,3, Macoura Gadji1,2, Abibatou Sall1,4, Awa Oumar Touré1,5 and Saliou Diop1,2,3.
1 Department of Hematology, Cheikh Anta Diop University, Dakar, Senegal.
2 National Blood Transfusion Center, Dakar, Senegal.
3 Clinical Hematology Department, CNTS, Dakar, Senegal.
4 Hematology Laboratory, Dalal Jamm Hospital, Dakar, Senegal.
5 Hematology Laboratory, Aristide Le Dantec Hospital, Dakar, Senegal.
Correspondence to:
Seck Moussa (Seck M). Department of Hematology, Cheikh Anta Diop
University, Dakar, Senegal. Clinical Hematology Department, CNTS,
Dakar, Senegal. BP 5002 Dakar-Fann. Phone: (+221) 77 557 28 86
Published: January 1, 2022
Received: September 3, 2021
Accepted: December 8, 2021
Mediterr J Hematol Infect Dis 2022, 14(1): e2022004 DOI
10.4084/MJHID.2022.004
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Context and Objectives:
Blood transfusions (BT) remain a mainstay of therapy for patients with
sickle cell disease (SCD) but pose significant clinical challenges. We
aim to assess infectious markers, red cell alloimmunization, and iron
overload secondary to BT in SCD patients.
Materials and Methods:
This case-control study included 253 SCD (153 SCD-transfused and 100
SCD non-transfused). We evaluated the transfusion practice (modalities,
indications), post-transfusion complications (infections,
alloimmunization, iron overload), and risk factors of these
complications (socio-demographic, clinical, biological).
Results:
Median age was 28.5 years (5 - 59). The sex ratio was 0.86. Homozygous
SCD was the most common (95.3%). Simple BT was performed in 92.8% and
transfusion exchange in 18.9%. Transfusion indications were dominated
by acute anemia (57.06%) and vaso-occlusive crisis (VOCs) (14%). Red
blood cell concentrates (RBCSs) were administered to 93.46%. The median
RBCs received per patient was 10 (2 - 48). The prevalence of VHC in
SCD-transfused was 1.33% and 2% for VHB. Anti-HIV antibodies were not
found. Red cell alloimmunization frequency was 16%. The most common
alloantibodies were anti-rhesus (34.19%) and anti-Kell (23.67%). Iron
overload was detected in 7.84%. The number of RBCs transfused was the
only risk factor for alloimmunization (p = 0.03) and iron overload (p =
0.023). BT frequency was not related to infectious transmission.
Conclusion:
BT therapy is still a risk for SCD polytransfused patients despite
advances in blood safety. Although infectious transmission has rare,
the risk of alloimmunization and iron overload is high in these
patients.
|
Introduction
SCD
is one of the most common worldwide hereditary disorders characterized
by the substitution of hemoglobin A (HbA) with the abnormal HbS. In
Africa, 10% to 40% are carriers of HbS, and each year 200 000 to 300
000 newborns have the homozygous form.[1] SCD is
characterized by high morbidity and significant mortality at the onset
of acute or chronic complications whose treatment often resorts to BT.[2]
BT is currently a major therapeutic option indispensable in the management of severe forms of SCD.[3,4] It exists in three methods to treat SCD anemia: simple BT, punctual transfusion exchange, and long-term transfusion exchange.[5,6]
Despite
the benefits, multi-transfused patients are at an increased risk of
complications of red cell alloimmunization, iron overload, and
blood-transmitted infections.[7,8,9] The frequency of these complications correlates with RBCs units transfused.[3,4]
The presence of one of these complications is a major risk factor for
morbidity and mortality in SCD patients requiring specialized
therapeutic management.[8,10]
Preventing these complications can be achieved by optimization of BT
indications, extensive phenotyping of RBCs and patient’s blood group,
and strengthening infectious blood safety.[11,12]
Systematic screening for these complications in SCD-polytransfused is
recommended by screening for irregular agglutinins, for infectious
transmitted (HIV, VHB, VHC) before and after BT, and ferritin assay
even if serum ferritin constitutes an indirect marker to detect
post-transfusion iron overload.[13,14,15] In Africa,
there are many shortcomings in transfusion availability and safety.
These constraints could have consequences in polytransfused patients
such as SCD patients. Unfortunately, data on the frequency of
post-transfusion complications are scarce, especially in Senegal.
The objective of this study was to report on the practical aspects of BT and post-transfusion complications in SCD patients.
Materials and Methods
The
study included 253 SCD patients (homozygous SS, heterozygous SC, Sβ0
thalassemia, Sβ+ thalassemia) consisting of 153 transfused and 100
non-transfused SCD.
SCD-transfused had undergone at least two
transfusions during follow-up; blood samples were taken at least one
month before from any vaso occlusives crisis (VOCs) or infections. VOCs
were defined as bone pain that lasted 48 hours or required
hospitalization. All patients were diagnosed by hemoglobin
electrophoresis at alkaline pH, regularly monitored every three months,
and a medical record included socio-demographic, clinical, and
biological data. Informed consent was obtained for all patients before
the samples.
This case-control study was carried out at the
Clinical Hematology Department (Dakar, Senegal) over six months. Three
samples (5ml) were taken; EDTA samples for detection and identification
of irregular antibodies, citrate samples for viral hepatitis B (HBsAg),
hepatitis C (VHC antibodies), and HIV (HIV 1 and 2 antibodies, and P24
antigen), and a serum sample for ferritin assay. Blood samples
collected were immediately centrifuged at 3500 rpm. The serum was
aliquoted and stored at -80°C.
Viral markers screening (HBsAg,
VHC antibodies, HIV antibodies) was performed using chemiluminescence
methods (Architect i1000sr, Abbott, USA). Searching for irregular
antibodies was carried out by gel filtration test (Bio-Rad reagents,
USA). Ferritin assay was performed by immunoassay (PLC Axsym, Abbott,
USA). The reference values of ferritin level were defined between 7 and
250 ng/ml (Women) and 20-300 ng/ml (Men). Iron overload was defined
when ferritin level was greater than or equal to 1000 ng/ml.
Socio-demographic
variables were age and sex. Clinical variables consisted of the
duration of follow-up, number of VOCs, acute complications (acute
anemia, priapism, acute chest syndrome, infections, stroke), chronic
complications (biliary lithiasis, leg ulcer, osteonecrosis, cardiac
failure, renal failure). Laboratory variables consisted of blood count
and hemoglobin electrophoresis data. Transfusion data were the
frequency of transfusions, type of blood product, transfusion
indications, and transfusion modalities. In addition, risk factors for
the occurrence of transfusion complications were studied.
Data
were collected and analyzed using Epi Info version 3.5.4. Means were
calculated with a 95% confidence interval. Chi2 test was used to study
the frequency data (significance of p <0.05). In addition, the
correlation coefficient (r) between ferritin level and RBCs number
received was determined (r between -1 and 1).
All patients
signed an informed consent form prior to participation in the study.
For minors, the signature was obtained from one of the parents.
Results
SCD Patients Baseline Characteristics.
The mean age was 28.5 years (5 - 59), and the sex ratio was 0.86.
Homozygous SCD was more common (95.3%), Sβ0 thalassemia (2.62%), SC
form (1.31%), and Sβ+ thalassemia (0.65%). The mean duration of
follow-up was 9.1 years (2 - 26). The mean number of VOCs per year was
2 (1 - 6). Acute complications consisted of anemia (63.98%), infections
(26.69%), priapism (5.51%), stroke (2.12%), acute chest syndrome
(0.85%) and renal failure (0.85%). Chronic complications were found in
39.87% of SCD-transfused and consisted of femoral head osteonecrosis
(27.87%), biliary lithiasis (32.79%), leg ulcers (16.39%), pulmonary
arterial hypertension (16.39%), and renal failure (6.56%). We had no
statistical difference in baseline characteristics comparing transfused
and non-transfused SCD patients (Table 1).
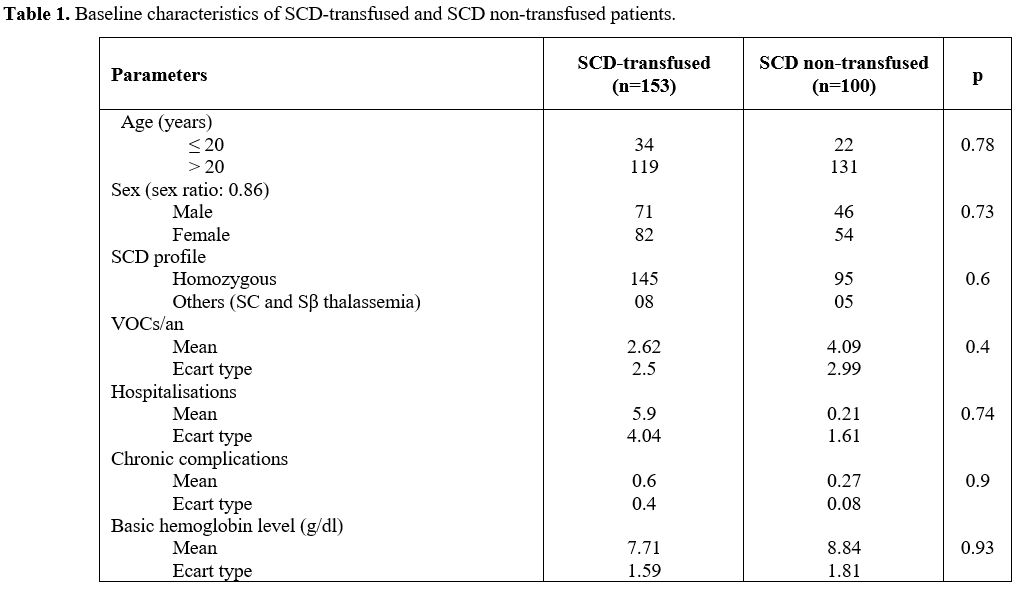 |
Table 1. Baseline characteristics of SCD-transfused and SCD non-transfused patients.
|
Blood Transfusion Practice.
According to transfusion methods, simple transfusion was performed in
92.81%, transfusion exchange in 18.95%; 14.37% of the patients were
submitted to a blood transfusion program. Transfusion indications
consisted of acute anemia (57.06%), prolonged VOCs (14%), pregnancy
management (10.5%), surgery (7%), leg ulcers (4.2%), infections (2.8%),
priapism (1.4%), acute chest syndrome (2.1%) and stroke (0.7%). RBCs
were administered to 93.46% and whole blood to 6.54%. Mean number of
RBCs transfused by patient was 10 (2 - 48); 43.14% had received between
2 and 5 RBCs (Figure 1).
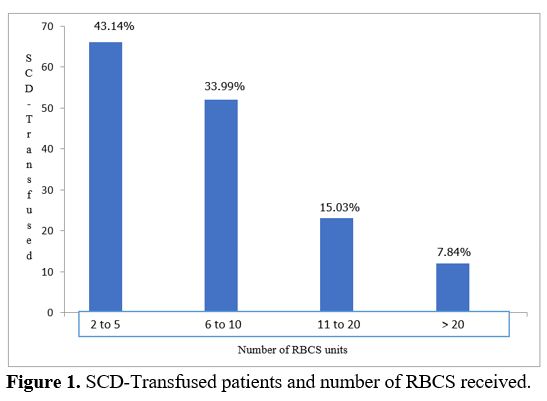 |
Figure
1. SCD-Transfused patients and number of RBCS received.
|
Post-Transfusion Red Cell Alloimmunization (RCA). The prevalence of RCA was 16%. Anti-Rhesus and anti-Kell alloantibodies were predominant with 34.19% and 23.67% respectively (Table 2).
According
to the distribution of alloantibodies, 14 SCD-transfused had a single
alloantibody (58.3%), 5 patients had two alloantibodies (20.83%) and 5
other patients had three alloantibodies (20.83%).
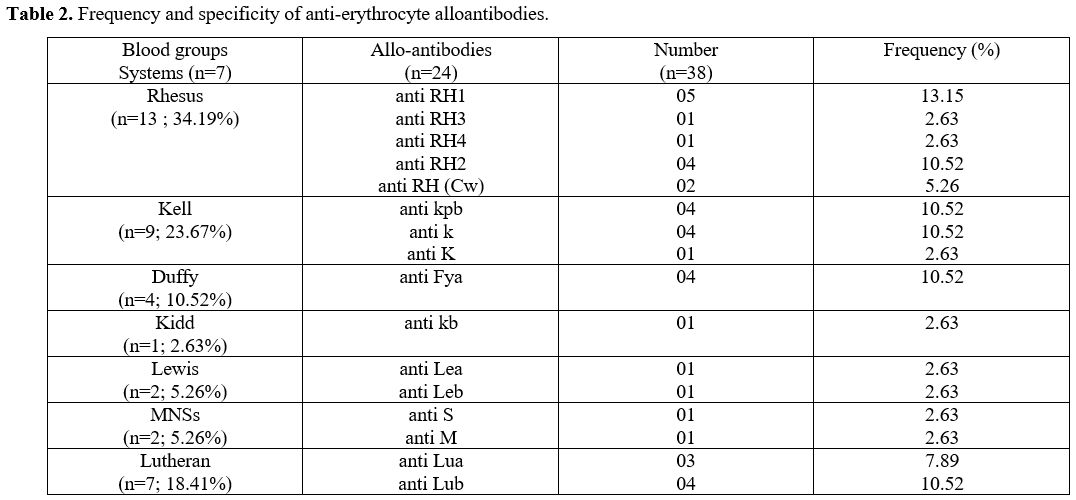 |
Table 2. Frequency and specificity of anti-erythrocyte alloantibodies..
|
Post-Transfusion Iron Overload.
Median value of ferritin level was 339.5 ng/ml (16 - 5941). Twelve
patients (7.84%) had ferritin levels more than 1000 ng/ml and received
more than 20 RBCSs. We observed that ferritin level was correlated of
blood transfusions frequency (r = 0.8) (Figure 2).
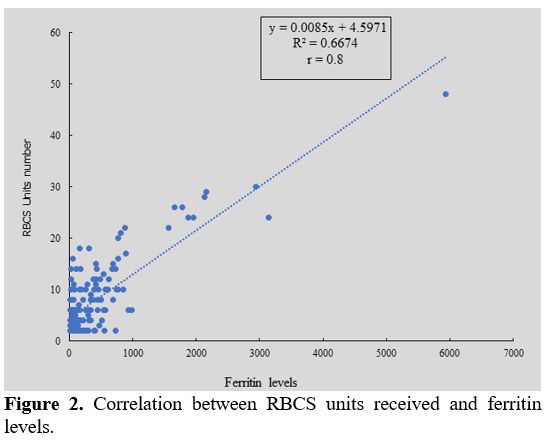 |
Figure 2. Correlation between RBCS units received and ferritin levels.
|
Transfusion-Transmitted Infections (TTI).
The prevalence of VHC in SCD-transfused was 1.33% and 1% in
non-transfused (p = 0.64). The prevalence of VHB was 2% in
SCD-transfused and 3% in non-transfused (p = 0.45). Anti-HIV antibodies
were not found neither in SCD-transfused than non-transfused (Table 3).
Risk Factors Occurred Post-Transfusion Complications.
The number of RBCs transfused was the only risk factor for RCA (p=0.03)
and for iron overload (p=0.023). In this study transmission of
infectious agents was not observed after blood transfusions (Table 3).
 |
Table 3. Risk factors associated with infectious markers, RCA and iron overload.
|
Discussion
BT
is an essential treatment in the management of SCD. Its usage is
different from one country to another depending on the type of
indication.[12,16,17] The objective
of BT in SCD is not only correct chronic anemia but also to decrease
HBS level and therefore to prevent or treat SCD complications whereas
in case, of a simple or a transfusion exchange, either in an acute
context or during a transfusion program.[3,6]
Iterative transfusions are exposed to RCA, infectious and iron overload
which pose a great risk of morbidity and mortality for SCD patients.[4,5]
In
this study, simple transfusion was performed in the majority of cases
and few patients had a chronic transfusion program. We confirm that our
patients are less transfused as compared to other SCD cohort in Africa
and in the world.[16,17] The lower severity of the
Senegal’s haplotype means that patients have few symptoms with less
than 3 VOCs per year and transfusion indications dominated by acute
anemia and prolonged VOCs, which are more random than scheduled.[12]
BT
is an important treatment for some complications of SCD. On the
contrary, transfusion may lead to alloimmunization to red blood cell
antigens, with such alloantibodies putting patients at risk for acute
or delayed hemolysis and increasing difficulty finding compatible RBCs.
In addition, SCD patients are more susceptible to developing RBCs
alloantibodies than non-SCD multiply-transfused for not completely
understood reasons.[18,19]
The phenotypic and
antigenic disparity between blood donors and SCD patients partly
explain the appearance of RBCs alloantibodies.[20] In our study, the RCA rate was 16%. RCA frequency has been shown to increase with the BT.[10,15,21] In Africa, where SCD-patients are rarely on a long-term transfusion, RCA rates are lower.[23,24,25]
Unlikely in developed countries, in which SCD-patients receive more
RBCs, alloimmunization is higher, often reaching half of SCD-patients.[15,26]
These high rates of RBCs in developed countries could be explained by
genetic differences in blood group antigens distribution between black
SCD-patients and Caucasian blood donors.[18,27] Extended phenotyping blood group systems between blood donors and SCD-patients would reduce the risk of RCA.[22,28]
Thirty-eight different antibodies were identified in 24 SCD-transfused
with positive irregular antibodies detection. These alloantibodies were
mainly directed against Rhesus and Kell systems antigens. Anti-Rhesus
and anti-Kell specificities were reported by most studies in Africa or
developed countries.[21,29] The high immunogenicity of these two systems other than the ABO system could partially explain the high incidence.
Given
the importance of polymorphism in blood group systems, the number of
epitopes defining an erythrocyte antigen, BT may only be one of many
RCA risk factors.[18] BT remains a critical component
of care for acute and chronic SCD complications. Randomized clinical
trials demonstrated the benefits of transfusion therapy to prevent
primary and secondary strokes and post-operative acute chest syndrome.
Despite overall improvements in blood inventory safety, adverse effects
of BT are prevalent among SCD-patients and include RCA, acute and
delayed hemolytic BT reactions.[9]
BT plays a
prominent role in the management of SCD-patients but causes significant
iron overload. As transfusions are used to treat severe complications
in SCD, it remains difficult to distinguish whether organ damage is a
consequence of iron overload or is due to the complications treated by
BT. Better BT management has resulted in increased survival, but
prolonged exposure to iron puts SCD-patients at greater risk for
iron-related complications that should be treated.[13,30]
Post-transfusion iron overload causes serious organ damage. Excess iron
accumulates in the parenchymal tissues of different organs and causes
degenerative lesions due to its toxicity.[31,34,35]
Less than 10% of SCD-transfused received more than twenty RBCs units
during follow-up. All these patients had a very high ferritin level
(> 1000 ng/ml). Literature data show that organs’ iron overload risk
appears when ferritin level is greater than 1000 ng/ml,[32] confirming our hypothesis that iron overload is correlated with the frequency of RBCs transfused.[33]
However, it should be noted that serum ferritin assay is a good
sensitivity marker but of poorer specificity for detecting iron
overload in SCD-polytransfused patients because the inflammatory
phenomena are more frequent in SCD.[35] The choice of
serum ferritin assay as iron overload evaluation method in our study is
guided by the fact that this method is more accessible and less costly
than others. However, it can have drawbacks because hyperferritinemia
can be related not only to organs’ iron overload but also to
pathologies frequent in SCD like liver disease, inflammation or VOCs.[36]
Another alternative would be the measurement of unbound plasma iron
which evaluates toxic iron fraction, but unfortunately, this test is
not available in current practice.[37] Direct methods
such as the determination of the intrahepatic iron concentration by
hepatic biopsy, magnetic susceptometry or nuclear magnetic resonance
imaging (MRI), and myocardial iron overload by MRI make it possible to
accurately assess the organs iron overload.[30] Direct methods are more reliable but rarely accessible, so serum ferritin assay is more widely used in Africa.[32,38]
SCD-patients
often require RBCs transfusion for clinical complications and then may
be exposed to transfusion-transmitted infections.[14]
The prevalence of VHB and VHC was low in SCD-transfused in Senegal, and
no HIV antibodies were found in any patient. This prevalence is much
lower than those found in other countries in Africa.[43,44]
This low prevalence is explained by the progress in the medical
selection of blood donors and the strengthening of infectious
transfusion safety through the systematic screening of these infectious
agents.[11] Comparing the prevalence of infections in
the Senegalese population, we find that HBsAg was lower (11%) and
anti-HCV (2.2%) and anti-HIV antibodies (0.7%) were
slightly higher than in other African countries.[45] We confirm that TTI is more frequent in Africa.[46] VHB is more common, affecting more than 20% of SCD-polytransfused in some countries,[47,48] followed by VHC.[49]
HIV antibodies were not found in this study but were present in studies
carried out in several Africa countries with sometimes very high rates.[48,50] This infectious risk in multi-transfused patients is lower in developed countries.[51,52]
Establishing infectious blood safety by the genomic screening of
infectious markers is the real goal. In Africa, infectious tests which
are used do not make it possible to cover the serological period
resulting in a persistent residual risk of TTI.[46,52]
Risk
factors of RBCs transfused were age, gender, chronic complications,
mean baseline hemoglobin level, and number of RBCs-transfused. The only
risk factor associated with RCA and iron overload was the number of
RBCs transfused. No factors were associated with TTI occurrence by
comparing SCD-transfused and non-transfused.
The pathogenesis of
alloimmunization is not well understood, and initiatives that aim to
reduce the incidence of alloimmunization are generally expensive and
either ineffective or unproven. Future reductions in the costs
associated with genotype matching could make a largescale program
economically feasible. Novel techniques to identify patients at the
highest risk for alloimmunization could improve the cost-effectiveness
of antigen matching programs.[39] Judicious use of
BT, optimization of red cell antigen matching, and the use of
erythrocytapheresis and iron chelation can minimize adverse effects.
Early recognition and management of hemolytic transfusion reactions can
avert poor clinical outcomes.[9] Identifying genetic
markers may help predict which patients are at risk of forming
alloantibodies. This study found 19 moderately associated SNPs, among
others, SNPs in TLR1/TANK and MALT1 were associated with a higher
alloimmunization risk, while SNPs in STAM/IFNAR1 and STAT4 conferred a
lower alloimmunization risk.[40]
The strong correlation between ferritin level and RBCs received is confirmed in some studies,[32] while others emphasized a lack of relationship between the two parameters.[16,38]
Therefore, ferritin assay must be serially performed, and the screening
must be made in the basal state without VOCs. The prevention of
post-transfusion iron overload is based on the optimization and
regulation of BT in managing chronic anemia.[41] In
addition, Erythrocytapheresis reduces iron overload and allows a longer
interval between procedures without a higher RBCs requirement from the
second year on automated RBCs exchange.[42]
Conclusions
Post-transfusion
iron overload and RCA strongly correlate with BT frequency, which is
not the case with infectious agents (HIV, HBV, HCV). So, we recommend
optimization of transfusion practices, extensive phenotyping blood
groups, serial ferritin screening after twenty RBCs-transfused, and TTI
screening before and after transfusion for improved blood safety in
SCD-patients.
Acknowledgments
The
authors thank all the staff of the hematology department of Cheikh Anta
Diop University and the clinical hematology department and the National
Blood Transfusion Center laboratory in Dakar, Senegal.
References
- Diop S, Pirenne F. Transfusion and sickle cell anemia in Africa. Transfus Clin Biol. 2021 May;28(2):143-145 https://doi.org/10.1016/j.tracli.2021.01.013 PMid:33515732
- Samir
KB. Sickle cell disease: Classification of clinical complications and
approaches to preventive and therapeutic management. Clin Hemorheol
Microcirc. 2018;68(2-3):105-128 https://doi.org/10.3233/CH-189002 PMid:29614627
- Stella
TC, Ross MF. Management of Patients with Sickle Cell Disease Using
Transfusion Therapy. Guidelines and Complications. Hematol Oncol Clin N
Am 30 (2016) 591-608. https://doi.org/10.1016/j.hoc.2016.01.011 PMid:27112998
- Stella
TC, Mouaz A, Ross MF, Joshua JF, Jeanne EH, Jo H, Kameka M, Kwiatkowski
JL, Pirenne F, Shi PA, Stowell SR, Thein SL, Westhoff CM, Wong TE, Akl
EA. American Society of Hematology 2020 guidelines for sickle cell
disease: transfusion support. Blood Advances 2019:4(2): 327-355. Blood
Adv. 2020 Jan 28;4(2):327-355 https://doi.org/10.1182/bloodadvances.2019001143 PMid:31985807 PMCid:PMC6988392
- Sharma
D, Ogbenna AA, Kassim A, Andrews J. Transfusion support in patients
with sickle cell disease. Semin Hematol. 2020 Apr;57(2):39-50 https://doi.org/10.1053/j.seminhematol.2020.07.007 PMid:32892842
- Faye
BF, Sow D, Seck M, Dieng N, Touré SA, Gadji M, Senghor AB, Gueye YB, Sy
D, Sall A, Dieye TN, Toure AO, Diop S . Efficacy and Safety of Manual
Partial Red Cell Exchange in the Management of Severe Complications of
Sickle Cell Disease in a Developing Country. Adv Hematol.
2017;2017:3518402. https://doi.org/10.1155/2017/3518402 PMid:28584527 PMCid:PMC5443989
- Mabien
AP, Brown B, Herbert DE, Haynes J. Iron overload in adults with sickle
cell disease who have received intermittent red blood cell
transfusions. J Am Assoc Nurse Pract. 2015; 27(10):591-6. https://doi.org/10.1002/2327-6924.12221 PMid:25711464
- Pirenne
F. The cause and pathogenesis of hemolytic transfusion reactions in
sickle-cell disease. Curr Opin Hematol. 2019 Nov;26(6):488-494. https://doi.org/10.1097/MOH.0000000000000546 PMid:31589171
- Grace
EL and Stella TC. Red cell transfusion and alloimmunization in sickle
cell disease. Haematologica. 2021 Jul 1;106(7):1805-1815 https://doi.org/10.3324/haematol.2020.270546 PMid:33792218 PMCid:PMC8252926
- Allali
S, Peyrard T, Amiranoff D, Jérémie FC, Chalumeau M, Brousse V,
Montalembert M. Prevalence and risk factors for red blood cell
alloimmunization in 175 children with sickle cell disease in a French
university hospital reference centre. Br J Haematol.2017
May;177(4):641-647. https://doi.org/10.1111/bjh.14609 PMid:28402005
- Seck
M, Diéye B, Guéye YB, Faye BF, Senghor AB, Toure SA, Dieng N, Sall A,
Touré AO, Diéye TN, Diop S. Evaluation of the efficacy of medical
screening of blood donors on preventing blood transfusion-transmitted
infectious agents. Transfus Clin Biol. 2016;23(2):98-102 https://doi.org/10.1016/j.tracli.2015.11.001 PMid:26681660
- Seck
M, Tall A, Faye BF, Bah DS, Guéye Y, Sall A, Touré AO, Diop S.
Evaluation of transfusion practices in sickle cell disease in Senegal:
cohort study of 1078 patients with sickle cell disease. Med Sante Trop.
2017 Nov 1;27(4):402-406 https://doi.org/10.1684/mst.2017.0744 PMid:29313508
- Thomas DC, John CW. How we manage iron overload in sickle cell patients. Br J Haematol.2020. https://doi.org/10.1111/bjh.14575 PMid:28295188 PMCid:PMC5444974
- Blatyta
PF, Kelly S, Sabino E, Preiss L, Mendes F, Carneiro-Proietti AB.
Prevalence of serological markers of transfusion and sexually
transmitted infections and their correlation with clinical features in
a large cohort of Brazilian sickle cell disease patients. Transfusion.
2020; 60(2): 343-350. https://doi.org/10.1111/trf.15619 PMid:31804727 PMCid:PMC8010912
- Sally
ACL, Kristina G, Mia CK, Yi-Fan C, Santosh LS, Lewis LH, Gordeuk VR,
Ronald G Strauss RG, Triulzi DJ. Red blood cell alloimmunization in
sickle cell disease: assessment of transfusion protocols during two
time periods. Transfusion. 2018 Jul;58(7):1588-1596 https://doi.org/10.1111/trf.14588 PMid:29570817 PMCid:PMC7193458
- Boulat C. La transfusion du drépanocytaire. Transfus. Clin. Biol. 2013 ; 20 :68-71 https://doi.org/10.1016/j.tracli.2013.02.014 PMid:23597585
- Elira
Dokekias A, Ngolet Ossini L, Atipo Tsiba FO. Blood transfusion
assessment to 112 homozygous sickle-cell disease patients in university
hospital of Brazzaville. Transfus Clin Biol. Nov-Dec
2009;16(5-6):464-70. https://doi.org/10.1016/j.tracli.2009.01.003 PMid:19369104
- Krystalyn
EH, Ross MF, Matthew SK, Jeanne EH, Richard OF. Mechanisms of
alloimmunization in sickle cell disease. Curr Opin Hematol 2019,
26:434-441 https://doi.org/10.1097/MOH.0000000000000540 PMid:31483335
- Ross
MF, Erin KM, Jane B, Mia SW, Robert WG, James RE. Impact of Red Blood
Cell Antigen Matching on Alloimmunization and Transfusion Complications
in Patients with Sickle Cell Disease: A Systematic Review. Ytmrv
(2018), doi:10.1016/j.tmrv.2018.07.003. https://doi.org/10.1016/j.tmrv.2018.07.003 PMid:30122266
- Ben
AI, Louati N, Khemekhem H, Dhieb A, Rekik H, Mdhaffar M, Gargouri J.
Red blood cell immunization in haemoglobinopathie: about 84 cases.
Transfus Clin Biol. 2012 Dec;19(6):345-52 https://doi.org/10.1016/j.tracli.2012.06.006 PMid:23103424
- Zalpuri
S, Zwaginga JJ, le Cessie S, Elshuis J, Schonewille H, Van der bom JG.
Red-blood-cell allo-immunization and number of red-blood-cell
transfusions. Vox Sang. 2012 Feb;102(2):144-9. https://doi.org/10.1111/j.1423-0410.2011.01517.x PMid:21729098
- Balbuena-Merle
R, Hendrickson JE. Red blood cell alloimmunization and delayed
hemolytic transfusion reactions in patients with sickle cell disease.
Transfus Clin Biol, 2019 ;26 : 112-115 https://doi.org/10.1016/j.tracli.2019.02.003 PMid:30857806
- Noizat-Pirenne
F. Immunohematologic characteristics in the Afro-caribbean population.
Consequences for transfusion safety. Transfus Clin Biol. 2003
Jun;10(3):185-91. https://doi.org/10.1016/S1246-7820(03)00042-9
- Kangiwa
U, Ibegbulam O, Ocheni S, Madu A, Mohammed N. Pattern and prevelence of
alloimmunization in multiply transfused patients with sickle cell
disease in Nigeria. Biomark Res. 2015 Oct 13; 3: 26. https://doi.org/10.1186/s40364-015-0050-3 PMid:26464798 PMCid:PMC4603770
- Dias
Zanette AM, de Souza Goncalves M, Vilasboas Schettini L, Magalhaes
Agguiar L, Santos Bahia RC, Vasconcelos Nogueira LA, de Freitas Brandão
CJ, Neves de Azevedo AC, Ramos de Aragao L, Marcos Arruda S.
Alloimmunisation and clinical profile of sickle cell disease patients
from Salvador-Brazil. Ethn Dis. Spring 2010;20(2):136-41.
- Aygun
B, Padmanabhan S, Paley C, Chandrasekaran V. Clinical significance of
RBCS alloantibodies and autoantibodies in sickle cell patients who
received transfusions. Transfusion 2002; 42:37-43. https://doi.org/10.1046/j.1537-2995.2002.00007.x PMid:11896310
- Higgins
JM, Sloan SR. Stochastic modeling of human RBCS alloimmunization:
evidence for a distinct population of immunologic responders. Blood.
2008; 112:2546-53. https://doi.org/10.1182/blood-2008-03-146415 PMid:18535200
- Lilian
AB, Andrew DC, Robertson DD, Alex OA, Sheri HA, Henk S. Red blood cell
alloimmunization and minor red blood cell antigen phenotypes in
transfused Ghanaian patients with sickle cell disease. Transfusion.
2019;9999;1-7
- Boateng LA, Ngoma AM,
Bates I, Schonewille H. Red Blood Cell Alloimmunization in Transfused
Patients With Sickle Cell Disease in Sub-Saharan Africa; a Systematic
Review and Meta-Analysis. Transfus Med Rev. 2019 Jul;33(3):162-169 https://doi.org/10.1016/j.tmrv.2019.06.003 PMid:31345590
- Stanley
HM, Friedman DF, Webb J, Kwiatkowski JL. Transfusional Iron Overload in
a Cohort of Children with Sickle Cell Disease: Impact of Magnetic
Resonance Imaging, Transfusion Method, and Chelation. Pediatr Blood
Cancer 2016;63:1414-1418 https://doi.org/10.1002/pbc.26017 PMid:27100139 PMCid:PMC5132054
- Oduor
H, Minniti CP, Brofferio A, Gharib AM, Abd-Elmoniem KZ, Hsieh MM,
Tisdale JF, Fitzhugh CD. Severe cardiac iron toxicity in two adults
with sickle cell disease. Transfusion. 2017 Mar;57(3):700-704. https://doi.org/10.1111/trf.13961 PMid:28019032 PMCid:PMC5352507
- Hafsia
R, Belakhal F, Ben Salah N, Gouider E, Elborji W. Iron overload in
sickle cell anemia : a study of 94 patients. Tunis Med. 2011
Jun;89(6):548-52.
- Leo-Kodeli S,
Renaudier P, Lassale B. Evaluation of transfusion hemochromatosis
prevalence, SFVTT-01 study: preliminary results of the SFVTT working
group. Transfus Clin Biol. 2014 Nov;21(4-5):182-8. https://doi.org/10.1016/j.tracli.2014.08.002 PMid:25277422
- Ginwalla
M, AlMasoud A, Tofovic D, Alin T, Al-Kindi S, Oliveira G, Rajagopalan
S, Schilz R, Little J. Cardiovascular Evaluation and Management of Iron
Overload Cardiomyopathy in Sickle Cell Disease. Am J Hematol. 2018
Jan;93(1):E7-E9. doi: 10.1002/ajh.24924. Epub 2017 Oct 23. https://doi.org/10.1002/ajh.24924 PMid:28971490
- Yassin
M, Soliman A, De Sanctis V, Nashwan A, Abusamaan S, Moustafa A, Samah
K, Soliman D. Liver iron content (LIC) in adults with sickle cell
disease (SCD): correlation with serum ferritin and liver enzymes
concentrations in trasfusion dependent (TD-SCD) and non-transfusion
dependent (NT-SCD) patients. Mediterr J Hematol Infect Dis. 2017 Jun
20;9(1):e2017037. https://doi.org/10.4084/mjhid.2017.037 PMid:28698780 PMCid:PMC5499497
- Thuret
I, Barlogis V, Michel G. Current concepts in the management of
transfusional iron overload. Arch Pediatr. 2009 Jun;16(6):559-61. https://doi.org/10.1016/S0929-693X(09)74066-7
- Walter
PB, Fung EB, Killilea DW, Jiang Q, Hudes M, Madden J, Porter J,
Patricia E, Vichinsky E, Harmatz P. Oxidative stress and inflammation
in iron-overloaded patients with beta-thalassaemia or sickle cell
disease. Br J Haematol. 2006 Oct;135(2):254-63. https://doi.org/10.1111/j.1365-2141.2006.06277.x PMid:17010049 PMCid:PMC2185791
- Akinbami
AA, Dosunmu AO, Adediran AA, Oshinaike OO, Osunkalu VO, Ajibola SO,
Arogundade OM. Serum ferritin levels in adults with sickle cell disease
in Lagos, Nigeria. J Blood Med. 2013 May 22;4:59-63 https://doi.org/10.2147/JBM.S42212 PMid:23723723 PMCid:PMC3666661
- Gehrie
EA, Ness PM, Bloch EM, Kacker S, Tobian AA. Medical and economic
implications of strategies to prevent alloimmunization in sickle cell
disease. Transfusion. 2017 Sep;57(9):2267-2276. https://doi.org/10.1111/trf.14212 PMid:28653325 PMCid:PMC5695925
- Meinderts
SM, Gerritsma JJ, Sins JW, de Boer M, van Leeuwen K, Biemond BJ,
Rijneveld AW, Kerkhoffs Jean-Louis H, Habibi A, Bruggen RV, Kuijpers
TW, Schoot EVD, Pirenne F, Fijnvandraat K, Tanck MW, Van den Berg TK.
Identification of genetic biomarkers for alloimmunization in sickle
cell disease. Br J Haematol. 2019 Sep;186(6):887-899. https://doi.org/10.1111/bjh.15998 PMid:31168801
- Noizat-Pirenne
F. Transfusion and sickle cell disease: axes of transfusion safety
optimization. Transfus Clin Biol. 2014 May;21(2):77-84. https://doi.org/10.1016/j.tracli.2014.03.005 PMid:24811565
- Dedeken
L, Quoc LP, Rozen L, El Kenz H, Huybrechts S, Devalck C, Diallo S,
Heijmans C, Ferster A. Automated RBCs exchange compared to manual
exchange transfusion for children with sickle cell disease is
cost-effective and reduces iron overload. Transfusion. 2018
Jun;58(6):1356-1362. https://doi.org/10.1111/trf.14575 PMid:29574950
- Diarra
AB, Guindo A, Kouriba B, Dorie A. Sickle cell anemia and transfusion
safety in Bamako, Mali. Seroprevalence of HIV, HBV and HCV infections
and alloimmunization belonged to Rh and Kell systems in sickle cell
anemia patients. Transfus Clin Biol. 2013 Dec;20(5-6):476-81. https://doi.org/10.1016/j.tracli.2013.03.067
- Ngo-Sack
F, Noah D, Zouhaïratou H, Mbanya D. Prevalence of HBsAg and anti-HCV
antibodies in homozygous sickle cell patients at Yaounde Central
Hospital. Pan Afr Med J. 2013;14:40.doi: 10.11604/pamj.2013.14.40.2069.
Epub 2013 Jan 28. https://doi.org/10.11604/pamj.2013.14.40.2069 PMid:23560123 PMCid:PMC3612872
- Agence National de la Statistique et de la Démographie (ANSD): Rapport sur la prévalence des infections au Sénégal en 2016. https://www.sec.gouv.sn/agence-nationale
- Kissou
SA, Koura M, Sawadogo A, Ouédraogo AS, Traoré H, Kamboulé K, Zogona
WWF, Nacro B. Serological Markers of Viral Hepatitis B and C in
Children with Sickle Cell Disease Monitored in the Pediatrics
Department at the University Hospital of Bobo-Dioulasso (Burkina Faso).
Bull Soc Pathol Exot. 2017 Aug;110(3):160-164. https://doi.org/10.1007/s13149-017-0555-4 PMid:28417347
- Fasola
FA, Odaibo GN, Aken'Ova YA, Olaleye OD. Hepatitis B and C viral markers
in patients with sickle cell disease in Ibadan, Nigeria. Afr J Med Sci,
2003; 32: 293 -295.
- Séka-séka J,
Yapo-Crezoit AC, Dasse-Sery R, Akre-Draga P, Sorho F, Sombo Mambo F.
Etude de la séroprévalence de l'hépatite virale C dans la population
drépanocytaire en Côte d'Ivoire. Méd Afr N, 1998; 45 (1):102-10
- Schreiber
GB, Busch MP, Kleinman SH, Korelitz JJ. The risk of
transfusion-transmitted viral infections. The Retrovirus Epidemiology
Donor Study. N Engl J Med. 1996 Jun 27;334(26):1685-90. https://doi.org/10.1056/NEJM199606273342601 PMid:8637512
- Uwingabiye
J, Zahid H, Unyendie L, Hadef R. Seroprevalence of viral markers among
blood donors at the Blood Donor Center of Mohammed V Military Teaching
Hospital of Rabat, Morocco. Pan Afr Med J. 2016 Nov 24;25:185. https://doi.org/10.11604/pamj.2016.25.185.6266 PMid:28292147 PMCid:PMC5326047
- Karafina
MS, Carpenterb E, Panb A, Simpsonb P, Field JJ. Older red cell units
are associated with an increased incidence ofinfection in chronically
transfused adults with sickle cell disease. Transfus Apher Sci. 2017
Jun;56(3):345-351 https://doi.org/10.1016/j.transci.2017.01.008 PMid:28279592
- Touré-Fall
AO, Dièye TND, Sall A, Diop M, Diop S, Thiam D, Diakhate L. Residual
risk of transmission of HIV and HBV, in Senegalese national blood bank
from 2003 to 2005. Transfus Clin Biol. Nov-Dec 2009;16(5-6):439-43. https://doi.org/10.1016/j.tracli.2009.09.005 PMid:19926508
[TOP]




