Chimeric
antigen receptor (CAR) T cells represent one of the newest frontiers of
cell therapy. Their application currently involves relapsed/refractory
aggressive B cell lymphoma and leukemia as a standard of care, while
several studies explore CAR-T cells to treat multiple myeloma and other
hematological malignancies. Here we describe the cytomorphology of
CAR-T cells collected from the leftovers of infusion bags, and
therefore before the encounter with the antigen, with, among others, a
peculiar population of giant lymphoid cells with blastoid features and
hypertrophic Golgi clear para-nuclear area.
The CAR is a
chimeric transmembrane receptor with an extra-membrane domain,
responsible for antigen CD19 recognition via a single-chain variable
fragment structure. It is linked through a transmembrane domain to an
intracellular component responsible for the T cell activation against
its target. Commercial CAR-T cells are obtained from autologous
lymphocytes passing through a positive selection of T cells and viral
transduction of the CAR. During the manufacturing process, lymphocytes
are stimulated with a culture medium containing interleukin 2. Such an
expansion process aims at obtaining a target dose that varies according
to the commercial product. Second-generation CAR-T cells activation is
mediated by co-stimulation of CD-3zeta. Despite the deepening knowledge
of CAR-T cells expansion kinetics and resistance mechanisms, few data
are available concerning morphological features of these artificially
modified cells in ex vivo samples before infusion.
We collected
samples of CAR-T cells from the leftovers of infusion bags of two
patients with refractory aggressive non-Hodgkin B cell lymphoma,
treated at our center with commercial axicabtagene ciloleucel CAR-T
cells. After the complete infusion, residual CAR-T cells were obtained
from the infusion bags by injection and washing with five milliliters
of saline solution. The recovered material was then divided into three
aliquots for the analyses. First, a nucleated cell count was performed
on an ADVIA® 120 automated cytometer. Second, cells were analyzed by
flow cytometry according to the method described by Magnani et al.[1]
Data were acquired on BD FACSCantoII flow cytometer and analyzed by BD
FACSDiva software (BD Biosciences). Finally, a third aliquot was used
to prepare cytospins (800 rpm for 3 min). Peripheral blood samples and
smears of treated patients were similarly analyzed during a two-week
follow-up. Smears and cytospins were stained according to
May-Grümwald-Giemsa and observed at the optical microscope.
The nucleated cell counts of the residual samples were 0.5 and 0.6 x109/L,
respectively. Therefore, the analyzer classified all the cells as
either lymphocytes or large unstained (myeloperoxidase-negative) cells
(LUCs).
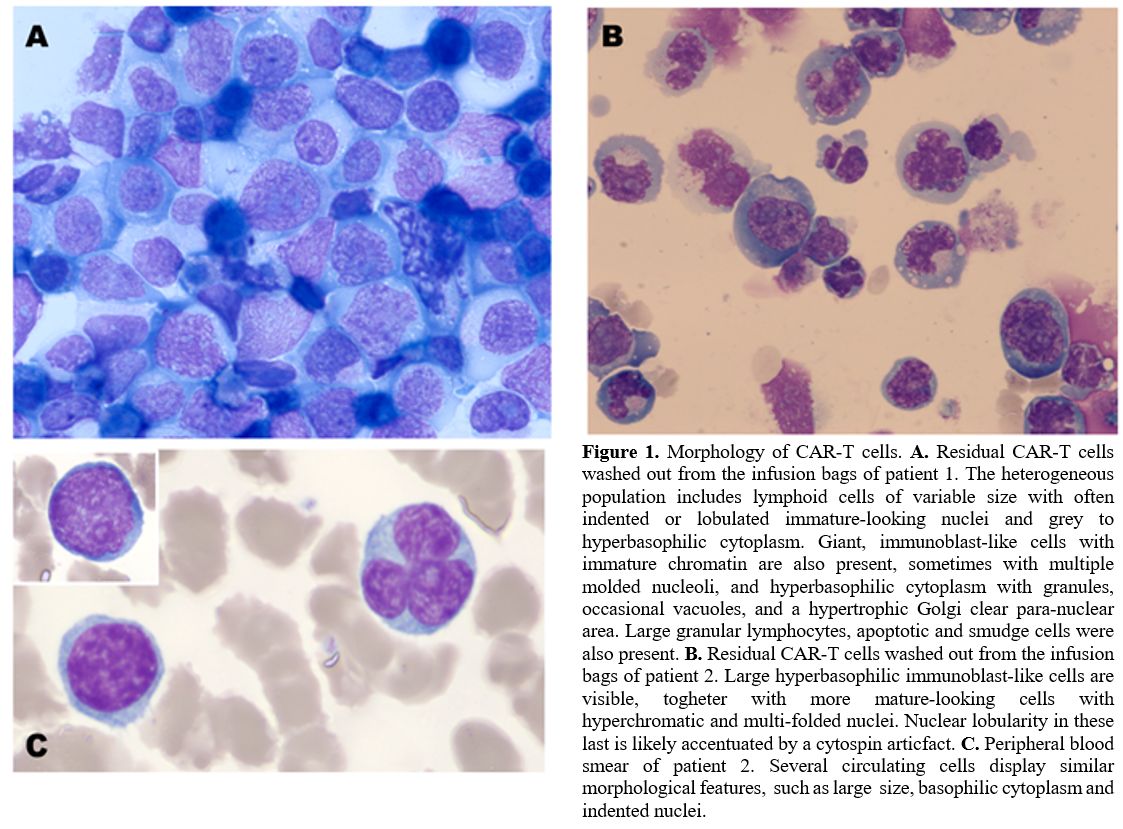
At the microscopic examination of cytospin preparations,
the lymphoid cells appeared morphologically heterogeneous. We
identified the following subtypes: i) large granular lymphocytes (LGL),
often with prominent granulations (less than 5%); ii)
small-to-medium-sized lymphocytes (30-40%), with moderately abundant
basophilic cytoplasm, thickened and hyperchromatic chromatin, and
pleiomorphism of the nucleus profile, accentuated indeed by the
cytospin preparation; nuclear indentation was however sometimes visible
in cells from the peripheral blood smears (Figure 1C);
iii) large lymphocytes (25-30%) with promonocytoid features (nuclear
indentation, immature chromatin, grey cytoplasm with fine scanty
granules); iv) giant/enormous lymphoid cells with blastic features
(25-30%), immature chromatin, multiple molded nucleoli, often with
reinforced chromatin border, abundant immunoblast-like hyperbasophilic
cytoplasm, with numerous purple granulations, with frequent vacuoles
and a hypertrophic clear para-nuclear Golgi zone (Figure 1). Apoptotic and smudge cells were also present and not considered in the cell counts.
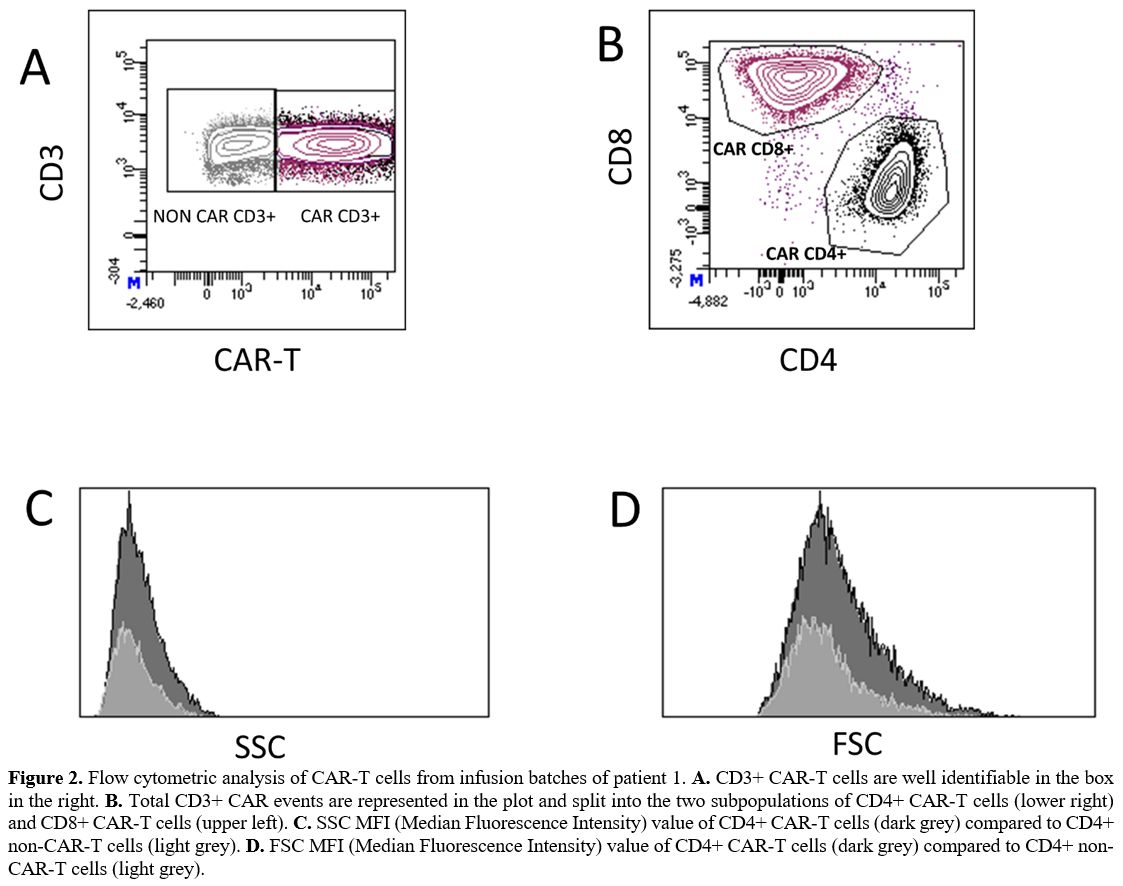
The
immunophenotype evaluation of the two residual samples showed that 76%
and 70% of cells, respectively, were CD3+ CAR-T cells. Of these 59% and
63%, respectively, were CD8+ and 39% and 35%, respectively, were CD4+.
The median values of side scatter (SSC) and forward scatter (FSC) were
increased in both the CD4+ and the CD8+ CAR-T compared to non-CAR-T
cells (Figure 2). The largest
cells with the highest FSC values mostly consisted of CD4+ CAR-T
cells. Their morphology is consistent with giant hyperbasophilic cells
with hypertrophic Golgi clear para-nuclear area, as described in
population iv.
In
conclusion, here we describe CAR-T cells' morphological and flow
cytometry characteristics from the cell product of two patients at the
time of infusion. We identified, among others, a peculiar population of
giant lymphoid cells with blastoid features and hypertrophic Golgi
clear para-nuclear area. As we identified these cells as CD4+ CAR-T
cells, these morphological features most likely reflect massive helper
T-cell activation. This activation involves microtubules and the
actomyosin cytoskeleton leading to an expansion of the
microtubule-organizing center (MTOC).[2,3] Notably,
similar large or giant cells, sometimes with nuclear indentations, were
also visible with very low frequency on peripheral blood smears ten
days after the infusion, consistent with flow cytometric detection of
rare CAR-T cells on the same PB samples. Similar cell images have also
been reported in bone marrow or peripheral blood smears.[4–6]
Our
direct observation of lymphoid subsets with peculiar morphological
characteristics in the infusion bag provides a description of
morphological features of CAR-T cells and a proof of concept that these
are not acquired in vivo secondary to the encounter with the antigen
but already present at the time of infusion secondary to viral
transduction and in-vitro stimulation. Further studies will be critical
to verify if different activation protocols and co-stimulatory
molecules in CAR construct, period of resting before sample freezing,
and other preparation factors have a role in determining the
heterogeneous morphology of these cells.
From a practical
standpoint, we underline that the observation of apparently abnormal or
immature cells in peripheral blood smears obtained after CAR-T cell
infusions should not be immediately be ascribed to the possible
occurrence or relapse of a malignant disorder.