Roberta Zanotti1,2, Ilaria Tanasi1,2, Lara Crosera1, Massimiliano Bonifacio1,2 Donatella Schena2,3, Giovanni Orsolini2,4, Francesca Mastropaolo2,4, Morena Tebaldi2,5, Elisa Olivieri2,6 and Patrizia Bonadonna2,6.
1 Hematology Unit, Department of Medicine, Azienda Ospedaliera Universitaria Integrata di Verona, Verona, Italy.
2 Interdisciplinary Study Group for Mastocytosis (GISM), Azienda Ospedaliera Universitaria Integrata di Verona, Verona, Italy.
3 Dermatology Unit, Department of Medicine, Azienda Ospedaliera Universitaria Integrata di Verona, Verona, Italy.
4 Rheumatology Unit, Department of Medicine, Azienda Ospedaliera Universitaria Integrata di Verona, Verona, Italy.
5 Gastroenterology Unit, Azienda Ospedaliera Universitaria Integrata di Verona, Verona, Italy.
6 Allergy Unit, Azienda Ospedaliera Universitaria Integrata di Verona, Verona, Italy.
Correspondence to:
Roberta Zanotti. Department of Medicine and Interdisciplinary Study
Group for Mastocytosis (GISM), Azienda Ospedaliera Universitaria
Integrata di Verona, Verona, Italy. E-mail:
roberta.zanotti@univr.it
Published: November 1, 2021
Received: September 23, 2021
Accepted: October 20, 2021
Mediterr J Hematol Infect Dis 2021, 13(1): e2021068 DOI
10.4084/MJHID.2021.068
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Systemic
Mastocytosis (SM) is a heterogeneous group of diseases that affect
almost exclusively adults and are defined by the proliferation and
accumulation of clonal mast cells (MC) in various tissues. Disease
subtypes range from indolent to rare but aggressive forms. Although SM
is classified as a rare disease, it is believed to be likely
underdiagnosed. Major signs and symptoms mainly depend on MC activation
and less frequent organ infiltration, typical of more aggressive
variants. Diagnosis may be challenging, and symptoms can be aspecific
and involve several organs. Therefore, it is advisable to refer
patients to specialized centers, having sufficient knowledge of the
disease, sensitive diagnostic procedures, offering a personalized and
multidisciplinary diagnostic approach, including at least
hematological, allergological, dermatological, and rheumatological
evaluations. A precise and timely diagnosis is required for: a)
adequate counseling of patients and their physicians; b) beginning of
symptomatic treatment (anti-mediator therapy); c) prevention of severe
manifestations of the disease (i.e., recurrent anaphylaxis,
osteoporosis, and bone fractures); d) cytoreductive treatment of
advanced SM variants.
This review summarizes the disease's main
manifestations and describes the ideal diagnostic approach for adult
patients with suspected SM, giving physicians the main notions for
correct patient diagnosis and management. This review also highlights
the importance of a multidisciplinary approach in this very complex
disease.
|
Introduction
The
term mastocytosis comprises a heterogeneous group of clonal diseases
characterized by proliferation and accumulation of mast cells (MC) in
different tissues, mainly skin and bone marrow (BM).[1] Cutaneous Mastocytosis (CM) is a skin-limited disease, typical of childhood, that may spontaneously regress during puberty.[2]
Conversely, more than 90% of adults have a systemic disease (SM)
involving one or more extracutaneous organs [BM, gastrointestinal tract
(GI), lymph nodes, and spleen)], with or without skin involvement.[1] The great majority of SM shows a "self-activating" somatic point mutation at codon 816 of the c-Kit receptor gene.[3,4]
SM
may present with a variety of clinical manifestations due to
inappropriate release of MC mediators (i.e., pruritus, urticaria,
angioedema, flushing, nausea, vomiting, abdominal pain, diarrhea,
episodic anaphylactoid attacks, osteopenia or osteoporosis) and skin
diseases (urticaria pigmentosa – UP or maculopapular CM - MPCM); in the
rare cases of aggressive disease, clinical features are due to MC
tissue infiltration and subsequent organ dysfunction (i.e.,
hypersplenism, pathological bone fractures, ascites, malabsorption,
cytopenia).[1]
Classification
The
2016 World Health Organization (WHO) distinguishes upon major
categories and subvariants of Mastocytosis. Diagnosis of SM is based on
one major and four minor criteria (Table 1) and requires the presence of the major histological criterion, together with at least one minor criterion.
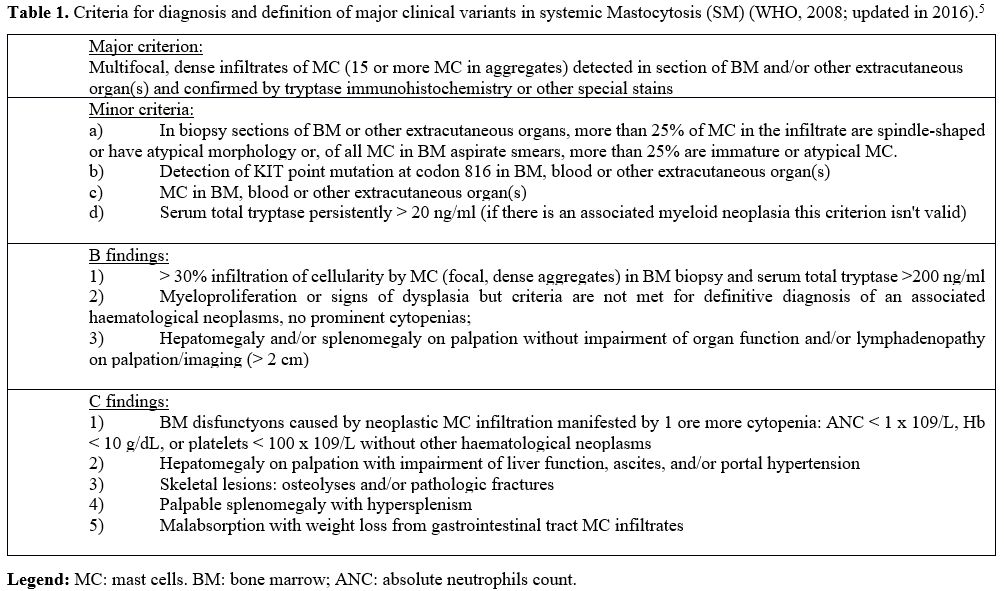 |
Table 1. Criteria
for diagnosis and definition of major clinical variants in systemic
Mastocytosis (SM) (WHO, 2008; updated in 2016).[5] |
In the absence of the major criterion, at least three out of four minor criteria need to be satisfied.[5,6]
SM patients can be further sub-classified depending on the presence of
B or C findings, defining the MCs' burden and the disease
aggressiveness, respectively (Table 2).[5,6]
Based on histological criteria, clinical parameters, and organ
involvement, SM is divided into indolent SM (ISM), smoldering SM (SSM),
and advanced SM variants (AdvSM). AdvSM are subclassified into SM with
associated hematologic neoplasia (SM-AHN), aggressive SM (ASM), and MC
leukemia (MCL) (Table 2). ISM
is the most common subtype and has a relatively benign prognosis,
although a small percentage of patients progress to SSM or AdvSM. Bone
marrow mastocytosis (BMM) represents a provisional variant of ISM,
without skin involvement, and characterized by low MC burden, low serum
tryptase levels, and frequent association with anaphylaxis, mainly
after hymenoptera sting.[7] The prognosis of this
variant is considered particularly good, but no significant difference
in survival compared to typical ISM with skin involvement has been
documented until now.[8] Recent data from the European
Competence Network on Mastocytosis (ECNM) suggest that patients with
BMM and low disease burden (defined as the absence of any B-Finding and
a tryptase level <125 ng/ml) have a better prognosis than patients
with typical ISM. As a consequence, the authors proposed redefining BMM
with these characteristics as a different SM variant.[9]
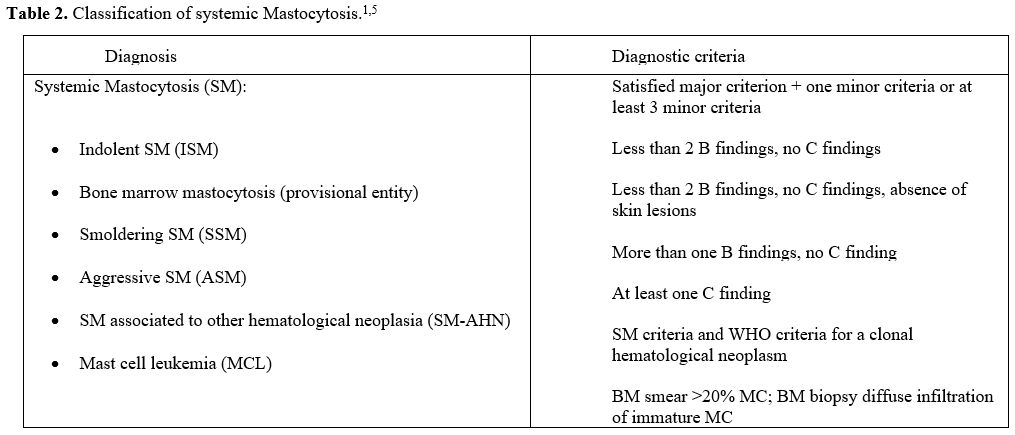 |
Table 2. Classification of systemic Mastocytosis.[1,5] |
SSM
is characterized by a higher disease burden than ISM, but the actual
prognostic relevance of SSM is debated: a first report documented an
inferior survival of SSM with respect to ISM,[10] but in a recent large retrospective study by the ECNM, the estimated survival of SSM patients was similar to that of ISM.[8]
ASM
is characterized by signs of organ damage due to MC infiltration, and
patients have a reduced life expectancy and poor prognosis.
SM-AHN
represents a very complex SM variant. In 85-90% of cases, SM is
associated with a myeloid disease (i.e.,
myelodysplastic/myeloproliferative neoplasms, myelodysplasia, acute
myeloid leukemia, myeloproliferative neoplasms), more rarely with
lymphomas or myelomas.[5,6] It is generally considered
an advanced variant, multilineage-mutated myeloid neoplasia with a
fatal outcome. However, the prognosis of SM-AHN depends on both the SM
variant and the associated disease. For example, the prognosis may not
be severe when ISM is associated with a relatively indolent disease
(such as essential thrombocythemia). It is also often challenging to
accurately correlate the B/C findings to the SM or the associated
disease, and therefore the correct classification of the SM variant.
Therefore, the incidence of SM-AHN is probably underestimated,
especially when the MC burden is low.
ASM accounts for approximately 5% of all SM and has a poor prognosis, with an average survival rate of 2-4 years.[11]
Skin lesions are frequently absent, while "C" findings such as
hepatosplenomegaly associated with signs of organ failure,
malabsorption with weight loss, blood count cytopenias or extensive
osteolysis may be dominant aspects of the clinical picture.[1]
MCL
is the leukemic form of SM, a rare entity characterized by diffuse
medullary infiltration of MCs (> 20% in the medullary smear), often
with an immature or blastic appearance.[1,12]
Skin lesions are absent in almost all patients, and the course is
fatal, with a reported prognosis of fewer than six months. However, a
"chronic" form of MCL was recently defined, characterized by the
absence of "C" findings and less aggressive clinical course, with
survival even longer than two years.
Epidemiology
Reports on epidemiological aspects of Mastocytosis in adults are
limited, and it is widely believed that the disease is highly
underdiagnosed due to a lack of knowledge about it and the very
heterogeneous clinical presentation. A nationwide study based on Danish
health registries reported an estimated prevalence of SM in adults of
10 per 100.000 inhabitants,[13] and a similar prevalence of ISM had
been reported in the Groningen region adult population (13.0 per
100,000 inhabitants).[14]
We believe that the growing diffusion of
sensitive diagnostic methods and the creation of multidisciplinary
groups dedicated to Mastocytosis will allow us to document a greater
number of SM diagnoses. Based on the database of our Interdisciplinary
Study Group for Mastocytosis (GISM), the estimated prevalence of SM in
the adult population of the Italian province of Verona is 16.1 per
100.000 inhabitants (unpublished data based on ISTAT report of Verona
province population at Jan 1, 2021).
No apparent gender predominance has been documented in adult mastocytosis.
Although
Mastocytosis is considered a non-hereditary somatic disease, familial
cases have been reported in pediatric series, with an estimated
frequency of 11–13%.[15,16] Recently, a study conducted on a large
series of adult patients reported an estimated prevalence of familial
cases of 1.5%.[17]
Pathogenesis
MC
progenitor cells express the tyrosine kinase receptor KIT, involved in
the development of MC by binding its ligand, the stem cell factor
(SCF).[18] The majority of adult patients with SM
harbor an activating KIT receptor mutation responsible for the
autonomous growth and expansion of neoplastic MC.[3] D816V mutation of KIT
is the most frequently detected, independently of the SM variant and
the aggressiveness; moreover, it is documented in about 40% of
pediatric CM.[4,15,19] Although KIT D816V mutation is undoubtedly the major driver of SM pathogenesis, it is not considered a fully transforming oncoprotein.
Additional non-KIT mutations not specific for SM (i.e., ASXL1, SRFS2, RUNX1, CBL, EZH2 mutations) are detected mainly in advanced SM and associated with poorer prognosis.[20–22] The role of these mutations in SM pathogenesis is still unclear.
Hematologic Diagnostic Workup
The
diagnostic pathway should begin with the search for characteristic skin
lesions, that even if isolated constitute an indication to perform a
complete BM assessment.[23] Skin biopsy is not strictly necessary if skin lesions are typical.[2]
In
the absence of typical skin lesions, the diagnostic pathway varies
according to the tryptase value detected. However, it must be taken
into account that, during and following an anaphylactic event, tryptase
increases, and therefore, it is necessary to re-evaluate it 24 hours
after the complete resolution of the symptoms.[23,24]
If the basal serum tryptase value is higher than 25 ng/ml, a complete
BM evaluation is immediately indicated, while if the value is inferior
to 15 ng/ml, it could be monitored over time. For tryptase values
ranging from 15 to 25 ng/ml, the indication to proceed with the BM
evaluation depends upon additional parameters, such as a REMA score ≥2 (Table 3), the detection of the D816V mutation on peripheral blood (PB), or the presence of extra symptoms suggestive of the disease.[25]
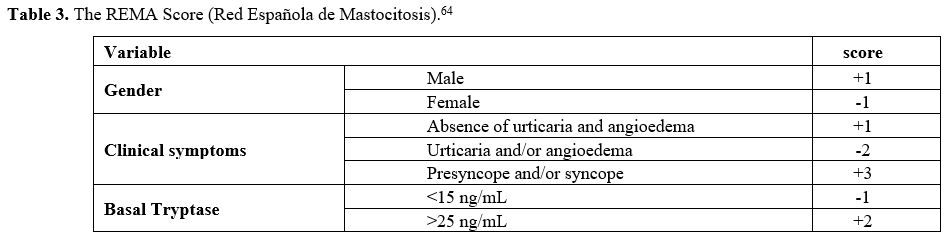 |
Table 3. The REMA Score (Red Española de Mastocitosis).[64] |
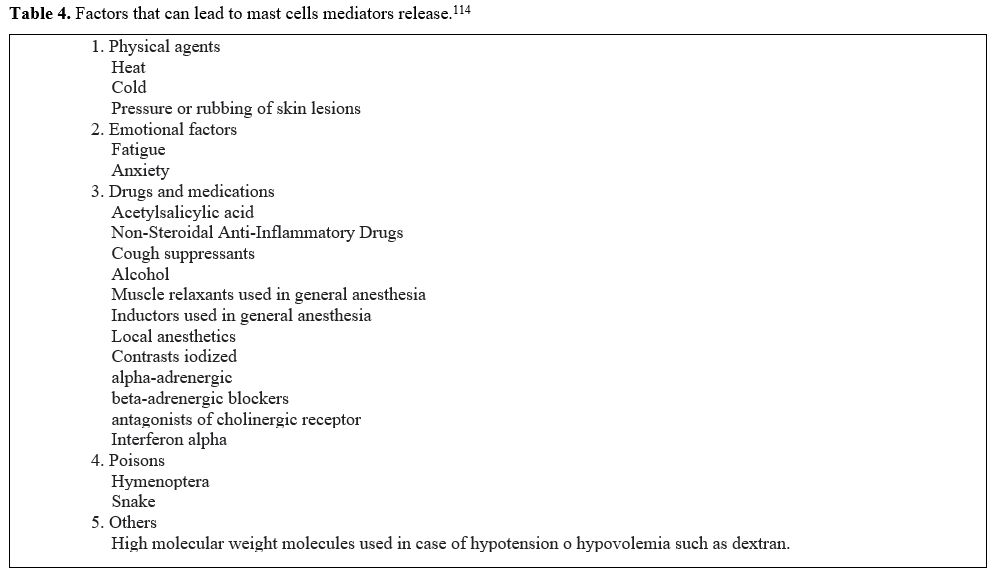 |
Table 4. Factors that can lead to mast cells mediators release.[114] |
BM and PB diagnostic workup is detailed in Table 5. They should include:
o Morphological examination of BM smear, using the classic staining with May Grunwald-Giemsa.[26]
BM smear examination is necessary to evaluate the percentage of MC and
their morphology that it was classified in four subtypes: a) spindly
shaped with hypo-granulated cytoplasm, b) well-differentiated (round,
hyper-granulated), c) immature (promastocytes with a bilobed or
indented nucleus), and d) metachromatic blasts.[26]
The BM smear should also be reviewed for AHN features. The stain with
Toluidine Blue could allow to easily identify atypical MC distinguish
them from basophils for the presence of the typical metachromatic
granules.[6]
o Morphological examination of PB smear.
PB smears should be examined for the presence
of circulating MC and eventually for excluding signs of an associated
hematological neoplasm (i.e., dysplasia, monocytosis, eosinophilia).[1]
o Histological and immunohistochemical examination of the BM biopsy
that must include, in addition to the normal Giemsa and
Hematoxylin-eosin stains, the following immunohistochemical
investigations: tryptase, CD117, CD25 (and CD2).[1,27]
In fact, neoplastic MC aberrantly express, in addition to CD117 and
Tryptase, antigens related to the lymphoid lineage, such as CD2 and
CD25, absent in the normal MC.[28,29] In particular, CD25 showed high sensitivity and specificity (close to 100%) for the diagnosis of SM[30] and is therefore considered the best immunohistochemical diagnostic marker.[27] Recently, the abnormal MC expression of CD30 has emerged as a useful feature, especially in advanced forms of SM.[31]
Furthermore, recent reports have shown that CD30 is also frequently
expressed in CM and all subtypes of SM, being useful in diagnosing CD25
negative well-differentiated SM.[32] The BM
evaluation should also include the extent of infiltration, the atypia
of MCs, the presence of fibrosis, and signs of other associated
hematological neoplasms.[1] An accurate diagnosis of
SM can be challenging in some cases, particularly when the major
histological criterion is not fulfilled and only isolated atypical MC
or small sub-diagnostic aggregates are observed. (Figure 1)
o Multi-parameter flow cytometry on BM samples requires a panel including at least monoclonal antibodies anti-CD45, CD34, CD117, CD25, CD2 (and optionally CD30).[27,32,33] In case of minimal MC infiltration, a high number of events should be acquired (up to 3-6 million events).[33,34]
o Detection of KIT D16V mutation in BM with highly sensitive KIT mutation assay
to minimize the risk of false-negative results. For instance, in ISM
the allelic burden can be very low and often less than 0.01%.[25,35]
Currently, the allele-specific oligonucleotide quantitative polymerase
chain reaction (ASO-qPCR) is considered the most sensitive technique
(0.01%). Droplet Digital PCR (ddPCR) is a promising method for
quantifying KIT D816V mutation with similar sensitivity to RT-qPCR
(0.01%) and without the need for standardization.[36]
Both these techniques detect the D816V KIT mutation only. At this
moment, Next Generation Sequencing (NGS) is not routinely recommended
for the search of the D816V KIT mutation, as it has been proved to be
less sensitive than RT-qPCR (sensitivity 1-5%).[37] Approximately 5-10% of SM are negative for the D816V KIT mutation,[4]
generally because of a) false-negative results due to low MC burden or
sub-optimal sample or the use of a less sensitive method; b) Wild-type
KIT; c) presence of another rare KIT mutation at codon 816 (F/ H/ I/Y)
or in other codons. In the case of D816V negativity and strong
suspicion of SM, other KIT mutations at position 816 (i.e., D816Y,
D816H, etc.) should be ruled out with PNA-mediated PCR technique on BM
samples or sorted MC with a sensitivity of 0.1%.[4,19,38–40] Moreover, mutations in other regions of a KIT gene may be assessed by NGS.[40]
Molecular testing in PB with an RT-qPCR-based method or ddPCR may be
used as a screening method in patients with suspicion of SM.[36,41]
However, it must be taken into account that about 30% of BMM and 10% of
ISM patients result negative for the mutation assessed in PB, also with
sensitive techniques.[39]
o BM conventional cytogenetics analysis is indicated in patients with suspected or confirmed advanced SM, especially in SM-AHN cases.[27]
o NGS study for other myeloid gene mutations is recommended to search for mutations of other myeloid genes in advanced forms, mainly for prognostic purposes.[27,42,43]
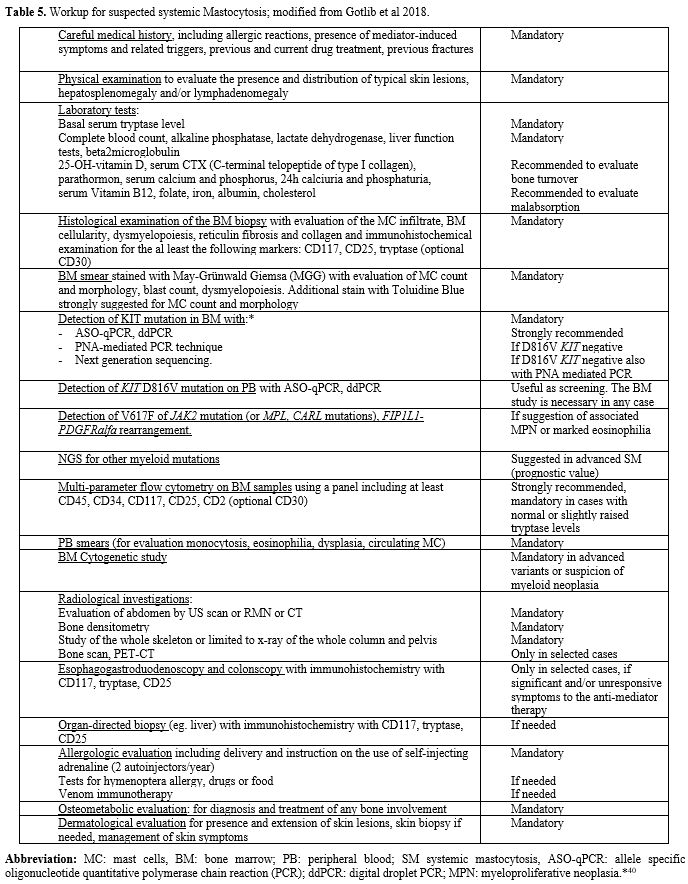 |
Table 5. Workup for suspected systemic Mastocytosis; modified from Gotlib et al 2018. |
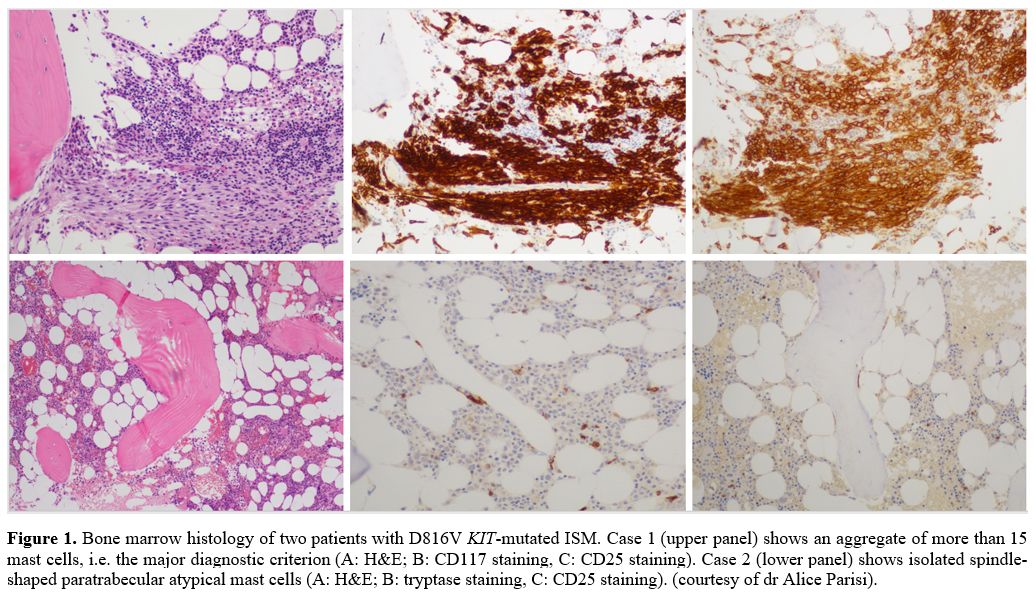 |
Figure 1. Bone marrow histology of two patients with D816V KIT-mutated
ISM. Case 1 (upper panel) shows an aggregate of more than 15 mast
cells, i.e. the major diagnostic criterion (A: H&E; B: CD117
staining, C: CD25 staining). Case 2 (lower panel) shows isolated
spindle-shaped paratrabecular atypical mast cells (A: H&E; B:
tryptase staining, C: CD25 staining). (courtesy of dr Alice Parisi). |
Prognosis
Several
clinical, serological, cytomorphological, immunological have been
reported to be prognostic in SM. Some of these variables have been
included in the WHO classification, such as cytopenia and organomegaly.[1,5]
Other variables associated with poorer prognosis are age >60 years,
low albumin serum level, increased serum β2-microglobulin and alkaline
phosphatase.[44–46]
Some biological parameters
have been proposed and tested in SM to improve WHO-based risk
stratification: poorer prognosis is associated with a multilineage KIT mutation involvement, high KIT D816V allele burden, and presence and number of mutations in genes other than KIT (e.g., SRSF2, ASXL1, RUNX1).[20,46,47]
Several integrated prognostic models, as IPSM, MAPS, MARS, GPSM score
have been still proposed, all demonstrated on large retrospective
patient cohorts and also confirmed in external series.[48–51]
Some models include only clinical information and are particularly
useful in routine practice, while other scores include both mutational
and clinical data. Further longitudinal studies in a large series of SM
patients with long follow-up may help select better scores for adequate
patient stratification, treatment decisions, and clinical management of
MS patients.
Multidisciplinary Evaluation and Treatment
General considerations.
Mastocytosis is a complex disease presenting with several clinical
manifestations and with a variable clinical course. In non-advanced
variants, patients mainly refer to skin symptoms (i.e., pruritus,
flushing) or other mediators-release symptoms (i.e., anaphylaxis,
osteoarticular pain, GI symptoms), and management often relies upon
symptoms control and elimination of additional risk factors and
comorbidities. In advanced forms of SM, organ damage prevails, and
cytoreductive treatment is needed. Thus, a multidisciplinary diagnostic
approach is needed for all these reasons, and a treatment strategy
should be tailored to a single patient. Additionally, these patients
may need psychological or psychiatric support.
The approach to the
patient with SM must include adequate counseling addressed to patients
and relatives and physicians involved in the treatment. In addition,
information should be given on the natural history of disease and
related problems, the drugs to be avoided, and the management of
patients requiring anesthesia or surgical treatment. Furthermore,
patients should be instructed to avoid triggers for acute mediator
release (Table 5) and the
emergency use of steroids associated with fast-acting antihistamines
and self-injectable adrenaline, mainly if they present a high risk of
anaphylaxis (i.e., patients allergic to Hymenoptera venom).
Complete workup of patients with SM at diagnosis is detailed in Table 5.
History,
physical exam, assessment of symptoms burden, laboratory evaluations
(annually for patients with ISM and every six months for SSM), and DEXA
scan every 1-3 years are recommended for ISM and SSM patients. In
addition, patients with ISM and SSM should also be monitored for the
development of signs of disease progression to advanced SM (e.g.,
development of B or C findings).[27] The frequency of
monitoring patients with advanced variants of SM should be
individualized on the patient conditions and cytoreductive treatment.
Skin manifestations. The classification of CM includes the following variants[2]
•
Maculopapular Cutaneous Mastocytosis (MPCM) or Urticaria Pigmentosa,
subclassified into monomorphic and polymorphic variants.
• Diffuse Cutaneous Mastocytosis.
• Isolated Mastocytoma.
The
presence of telangiectasia, along with MPCM-like skin lesions, has
traditionally been termed Telangiectasia Macularis Eruptiva Perstans
(TMEP), though the term TMEP should no longer be used.[2]
Adult patients with CM who have not undergone BM assessment are more
correctly and provisionally referred to as having Mastocytosis in the
skin (MIS).[1]
The most common presenting symptom
in adult mastocytosis is the gradual onset of pigmented, reddish-brown,
monomorphic, small-sized (up to 5 mm in diameter) skin lesions (MPCM) (Figure 2).[2]
Some cases result from the persistence in adulthood of pediatric CM.
The frequency of these cases remains to be determined; in clinical
practice, monomorphic MCPM generally persists in adulthood, while
polymorphic MPCM tends to resolve in puberty, as well as the cutaneous
Mastocytoma.[2,52] Skin lesions typically show a wheal-and-flare reaction upon rubbing or stroking, the so-called Darier's sign.[2]
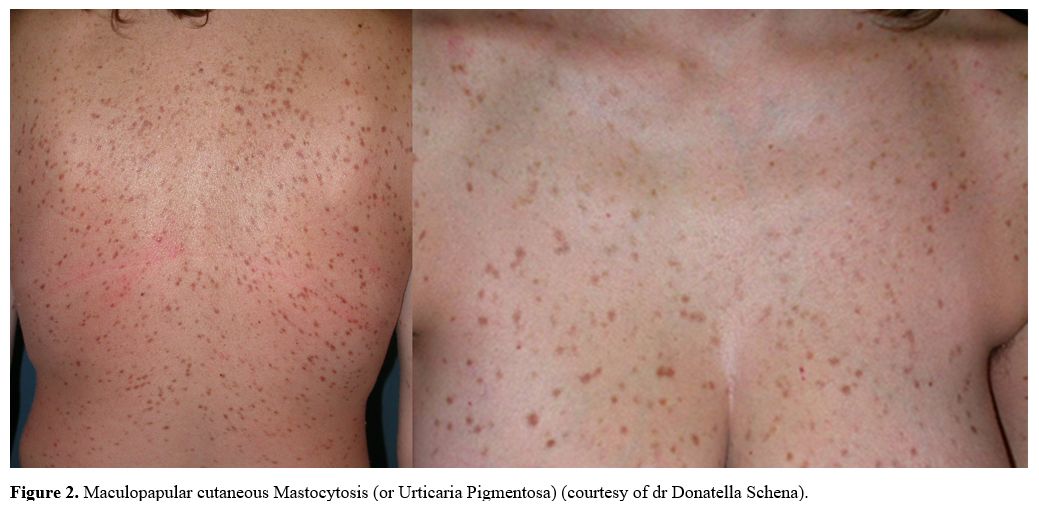 |
Figure 2. Maculopapular cutaneous Mastocytosis (or Urticaria Pigmentosa) (courtesy of dr Donatella Schena). |
Skin
symptoms include pruritus, flushing, and wheeling, often triggered by
physical stimuli, such as heat, cold, sunlight, and friction.[1]
Skin symptoms due to MC mediators release can usually be controlled by
H1- antihistamines alone or in association with H2-inhibitors.
Cosmetic
complaints could be a significant issue in many patients, especially
younger adults, and should not be minimized. Moreover, symptoms could
not respond to anti-mediator therapy. At present, ultraviolet A therapy
or Psoralen plus ultraviolet A (PUVA) photochemotherapy can be
employed,[1,43,53,54]
to reduce MC mediator skin symptoms and visibility of skin lesions,
although the effect is temporary. This treatment should be used with
caution due to its potential cutaneous side effects.
Anaphylaxis. The prevalence of anaphylaxis in adult patients with Mastocytosis ranges from 20% to 49%,[55–57] much higher than the 0.05–2% estimated frequency in the general population.[58,59]
Discrepancies between different studies might be a result of the
heterogeneity of the patient cohorts, the definition of anaphylaxis,
varying recruitment strategies but also be due to the sensitivity of
the diagnostic techniques. Allergic/anaphylactic symptoms are mostly
present in patients with BMM, often representing the initial clinical
manifestation and the indication for BM evaluation in most cases.[60]
The
triggers that can induce a massive degranulation of MC and subsequent
anaphylaxis in adult SM patients are numerous, Hymenoptera stings being
the more frequent (19-60% of cases of anaphylaxis), followed by foods
(3-16% of cases) and drugs (5-9%).[55–57,60]
alcohol, exercise and temperature changes are other possible triggering
factors of anaphylaxis in Mastocytosis, acting mainly as co-factors.[56]
Idiopathic anaphylaxis is not infrequent in SM, reported in up to 29-39% of cases.[57,60]
The
higher prevalence of HVA confirms a significant association between HVA
and SM in SM patients (up to 20-30%) compared to the general population
(00.5-3.3% of adults in the US and 0.3-7.5% of adults in Europe).[61–63]
Patients with SM and severe HVA are typically affected by indolent SM
lacking skin lesions in the majority of cases (i.e., BMM), are mainly
of the male gender, have not very high basal tryptase levels, and
allergic reaction is characterized by hypotension with the absence of
urticaria and angioedema.[64,65] Early diagnosis of
SM in patients with severe HVA represents a substantial advantage
because they are at high risk of severe osteoporosis, and early therapy
can be immediately started to prevent vertebral fracture. Furthermore,
proper advice and prescription of adrenaline (two autoinjectors) can be
assessed. Finally, venom immunotherapy should be continued lifelong to
prevent fatal events.[66]
Gastrointestinal symptoms. Patients with SM frequently complain of GI symptoms, including abdominal pain, cramping, diarrhea and gastritis.[1,67]
Symptoms may be mild or severe, and differential diagnosis with other
GI diseases could be difficult. The pathogenesis of GI symptoms in SM
is, in most cases, related to MC mediator release, but especially in
advanced forms, it could also involve the accumulation of clonal MC in
the GI tract. Various stimuli may trigger GI symptoms, i.e., foods,
alcohol, stress, but they also can occur spontaneously.
In
patients with SM, endoscopic abnormalities are frequently nonspecific.
Histological features may include the presence of MC in aggregates or
sheets in the lamina propria and the detection of aberrant
CD25-expressing MC.[68] However, the involvement can
be focal and subtle, making the diagnosis challenging. Moreover,
clinical symptoms do not always correlate with histological findings.
Treatment
options include H2 blockers, proton-pump inhibitors, and oral sodium
cromolyn, alone or in combination. Complete patient history is helpful
to identify the triggers of symptoms. In the rare, aggressive subtypes
of SM, severe MC infiltration in the GI tract may lead to malabsorption
and weight loss. In these cases, cytoreductive therapy is indicated.[1]
Bone involvement: diagnosis and treatment of related symptoms. According to the cohort of Hermans et al.,[69]
osteoporosis and bone lesions are among the most frequent
manifestations of SM. On the other hand, a recent study on over 8000
osteoporotic patients recognized SM as its cause in 0.5% of the
population.[70] Osteoporosis' prevalence settles down to 8-40% in patients affected by SM while fractures to 3-41%.[71–76]
Useful imaging modalities in this context are dual-energy X-ray
absorptiometry, radiography (skeleton in toto or at least axial), CT,
and magnetic resonance imaging advanced techniques such as PET could be
reserved for special cases.[77] Alongside imaging, it
is therefore very important to evaluate laboratory tests (such as the
serum dosage of 25-OH-vitamin D, s-CTX, Parathormone, alkaline
phosphatase, calcemia, phosphoremia, 24-hour calciuria, and
phosphaturia, see Table 3).
SM-related fragility involves most prominently the spine, provoking frequently vertebral fragility fractures.[77]
Given this, SM diagnosis should be considered when approaching a
patient with unexplained fragility fractures, osteoporosis, or
inappropriate low bone mineral density (BMD), as a possible underlying
etiology.[78] The presence of osteoporosis in male patients or young premenopausal women should induce the suspect of SM.[70]
Osteoporosis secondary to SM frequently causes a lower BMD at the
lumbar spine than the hip, indicating a greater loss of trabecular with
respect to cortical bone. This could be due to either a higher
sensitivity of trabecular bone to local factors synthesized by MC or
MC's development from BM, which is predominantly localized in the
trabecular bone.[70] Furthermore, both the Z-score
and the T-score should be considered in these patients since the latter
could be misleading due to the low sensitivity and specificity to
detect secondary bone disease, especially in men and premenopausal
women.[79] When facing a low BMD with a Z-score <
-2, secondary causes of osteoporosis should be excluded with further
diagnostic examinations. Likewise, fragility fractures occurring in the
context of normal or almost normal BMD values should be investigated to
rule out possible secondary causes.[78] Bone
involvement in patients affected by Mastocytosis comprises both a
qualitative and a quantitative problem, as shown by the occurrence of
fractures in osteopenic patients or those with normal values of BMD.
Patients affected by osteoporosis secondary to SM often display a high
bone turnover. Interestingly, many studies evidenced a correlation of
C-telopeptide and osteoprotegerin to tryptase levels, highlighting a
possible correlation to the number of MC.[70,80,81]
Of note, serum tryptase elevation (>25 ng/mL) is considered a useful
screening tool to predict systemic Mastocytosis and select patients who
should undergo BM biopsy. In cases of mild increase of serum tryptase
levels (15-25 ng/mL), BM biopsy should also be considered based on the
concomitant clinical elements of suspicion. However, it is not
completely reliable as with any biomarker due to possible false
positive and negative results. Data from Carosi et al. described serum
tryptase elevation in a large cohort of patients with osteoporosis, but
only a small percentage (19%) of those who underwent BM biopsy was
confirmed to have Mastocytosis.[81] Therefore, as
serum tryptase values lack the high reliability needed in this group of
patients when a high clinical suspicion is present, BM biopsy should be
considered regardless of serum tryptase value. The picture is even more
complicated because, according to recent data, the absence of skin
involvement represents a significant risk factor for fractures in SM.
This is probably due to a higher diagnostic delay and misdiagnosis in
patients not presenting typical skin lesions.[74,79]
It must be remembered that osteoporosis and fragility fractures are not
the only bone manifestations of SM. A minority of patients present with
a diffuse osteosclerotic pattern, characterized by high BMD, high bone
turnover, and diffuse bone scintigraphy uptake ("super scan").[78,82]
In most cases, these patients also display a very high level of serum
tryptase and have a higher chance to be affected by advanced forms of
SM.[83] It may also happen that in the context of a
sclerotic bone, small lytic lesions may appear, not having the
classical appearance of "C-criteria" lytic lesions. A small percentage
of patients may also have single or multiple focal sclerotic or lytic
lesions.[77,78,82] Antiresorptive
drugs are the mainstay of treatment in osteoporosis induced by
Mastocytosis. In particular, 5 mg zoledronate IV per year seems to be
an effective therapeutic option to reduce bone turnover markers and
prevent bone loss,[84] but in aggressive forms with
lytic involvement and recurrent fracture a monthly protocol for myeloma
could be applied. The most frequent side effect, which occurs more
frequently than in the general population, is the acute phase reaction
to the first administration of zoledronate. However, this reaction is
transient, manageable with symptoms and with adequate patient
information before administration. Given the pivotal role that the
RANK-RANKL system seems to play in the pathogenesis of osteoporosis
caused by SM, denosumab might be a further therapeutic option.[77,85]
In
patients with severe osteoporosis or refractory to bisphosphonates,
therapy with alpha-interferon is suggested based on some studies which
reported a good efficacy of the combined use of bisphosphonates and
interferon, also if with tolerance problems.86 In the future, the role
of alpha-interferon could be replaced by more innovative drugs.
If
the diet is low in calcium, the prescription of vitamin D supplements
and even calcium supplements remains pivotal in all conditions ranging
from mild osteopenia to severe osteoporosis.
Special Aspects
Anesthesia.
Currently, there are no reliable data on the safety of anesthetic drugs
in patients with Mastocytosis, and it is impossible to provide general
recommendations on the safety of drugs, or drug families, used in the
perioperative period.The
risk appears to be greater in adult patients with Mastocytosis than in
children, regardless of skin involvement, particularly in those with a
history of previous adverse drug reactions.[87,88]
From a practical point of view, a detailed allergological history is
necessary. In patients who have undergone previous surgery under
general anesthesia, it may be advisable to consult the medical record
and learn about the drugs used during surgery and in the postoperative
period.[88] However, routine preoperative skin testing with drugs to be used is not recommended.[88]
Prudentially, a reasonable approach is to choose those drugs with low
capacity to elicit MC degranulation in each pharmacologic group and to
use drugs with known tolerance by individual patients. The following
drugs, employed for general anesthesia, are considered safe: propofol,
a sedative-hypnotic anesthetic; sevoflurane and isoflurane, inhaled
anesthetic agents; fentanyl, sufentanil, remifentanil and alfentanil,
opioid anesthetics; local anesthetics such as lidocaine and
bupivacaine; skin antiseptics such as povidone-iodine. Finally, curares
drugs such as Vecuronium, pancuronium, and cisatracurium have been less
frequently associated with the risk of adverse reactions.[88,89]In
SM patients, it is also recommended to avoid potential physical
triggers, such as sudden temperature changes, cold fluid infusions,
extensive tissue trauma, vigorous skin rubbing, and other mechanical
factors.[88] In addition, anxiety can trigger MC
degranulation so that patients could be premedicated with sedatives
(benzodiazepines) before the surgical procedure.[89]There
is no consensus about the routine administration of premedication
before anesthesia or its efficacy to prevent or reduce the severity of
reactions. Premedication is recommended in patients with previous
perioperative anaphylaxis and in general, depending on the individual
patient's risk.[87,88] Venom Immunotherapy.
Venom immunotherapy (VIT) is recognized as a life-saving treatment for
HVA patients. After some debate, mainly due to safety concerns, it is
now generally accepted that VIT should be given anyway.[90]
SM patients who had experienced systemic reactions after Hymenoptera
sting should undergo allergological workup. The tests can be performed
following the standard protocols (in vivo and in vitro), currently used
for patients without Mastocytosis. Although skin tests are generally
safe, considering the increased risk of patients with SM, they should
be performed in a hospital setting.[66] It is also
helpful to perform the dosage of specific IgE towards the whole
extracts of the poisons of the main Hymenoptera, as well as the dosage
of the specific IgE towards the single species-specific molecular
markers available (Api m1-2-3-5-10 for the bee, Ves v1, and Ves v 5 for
Vespula spp and Pol d 5 for Polistes Dominulus), and the cross-reactive
carbohydrate determinants (CCD).[91] This
helps define the individual sensitization profile and discriminate
among the poison molecules involved in the allergic reaction and the
cross-reactive ones. As SM patients may have very low specific IgE
levels, a cut-off level of specific IgE for recombinant molecules lower
than 0.10 kUA/L is preferable to ensure better sensitivity and
specificity.[92]Venom
immunotherapy (VIT) is universally considered the only life-saving and
effective treatment in patients with Hymenoptera venom allergy, even in
SM patients, protecting them from severe allergic reactions to
subsequent punctures.[90]Given
many reports on fatal Hymenoptera sting reactions after discontinuation
of treatment, it is advisable to continue the VIT life-long in patients
with SM.[66]To
reduce the number and severity of extensive local reactions or mild
systemic reactions to VIT, premedication with an H1 antihistamine can
be used in patients with Mastocytosis, while Omalizumab can be used in
case of more severe systemic reactions (i.e., urticaria or angioedema)
occurring during immunotherapy.[93,94] Pregnancy. Diagnosis of SM does not appear to affect fertility.[95] There is little evidence regarding the impact of Mastocytosis on pregnancy compared to the general population.[95]
Based on limited reports, patients with Mastocytosis seem not to show a
significant increase of maternal-fetal adverse events during pregnancy
and delivery (i.e., miscarriage, preterm birth, complications of labor
and delivery) compared to the general population.[95,96] Patients with nonaggressive categories of Mastocytosis should not be advised against pregnancy,[95]
but they must be evaluated before conception, during pregnancy, and
postpartum by a multidisciplinary team (which also includes a midwife
and an anesthetist).[95]The
management of symptoms of women with SM during pregnancy and early
postpartum should combine drugs with no or very limited teratogenic
potential to relieve symptoms of MC activation, avoid known triggers,
and eventually use prophylactic anti-mediator drugs (steroids,
antihistamines), as needed.[27] Interferon-alpha
cytoreductive therapy may be considered for pregnant women with severe
symptoms and refractory to conventional treatments. The use of
cladribine or tyrosine kinase inhibitors (TKI) is not recommended.[27]Vaccination.
Patients with Mastocytosis generally have an increased incidence of
adverse reactions to exogenous agents. Although there are no data on
the prevalence of adverse reactions to vaccines in adult patients with
Mastocytosis (i.e., influenza, hepatitis B, or travel-related
vaccinations), experts have a consensus that Mastocytosis does not
represent a contraindication to vaccinations in adults.[97] In
a few cases, reactions were observed, particularly in children.
Although patients with Mastocytosis can be vaccinated according to the
standard schedule, precautions to prevent MC activation and
degranulation have been formulated by experts, under observation with
medications available to treat anaphylaxis, particularly in cases of
diffuse skin manifestations. Protocols for premedication with steroids,
antihistamines, and leukotriene receptor antagonists or cromolyn
therapy have been applied to prevent complications, but official
guidelines have not to be released.[98] Although
the reported frequency of severe side effects is low, there is an
emerging discussion about the safety of Covid-19 vaccination in
patients with severe allergies and Mastocytosis. However, severe
adverse reactions are rare even in these patients, and the general use
of Covid-19 vaccination in patients with Mastocytosis is recommended
globally. The only well-established exception is a known or suspected
allergy against a constituent of the vaccine. Safety measures,
including premedication with anti-H1 and post-vaccination observation,
should be considered in all patients with Mastocytosis, depending on
the individual risk.[99]
Treatment of Advanced Systemic Mastocytosis
Cytoreductive
therapy is indicated in patients with advanced Mastocytosis and
includes chemotherapeutics, such as cladribine, biologics,
alpha-interferon, and tyrosine kinase (TK) inhibitors, such as
imatinib, and the multitarget oral TK inhibitor midostaurin.[43]Interferon-alpha
(IFN-α) appears to be of limited efficacy and has its indication in
treating slow-progressing forms or, in non-advanced forms, severe
osteoporosis refractory to bisphosphonate therapy.[43,86,100,101]
The frequency of major response (i.e., complete resolution of one or
more baseline "C" findings) ranges from 20% to 30%. The optimal dose
and duration of IFN-α therapy for SM is still unknown (typically 1 to 5
MU subcutaneously, three times/week), although concurrent
administration of corticosteroids (i.e., prednisone) may improve its
efficacy (up to 40% of major response rate) and tolerability.[43,100,101] The pegylated form of alpha-interferon may be better tolerated and is likely to be preferred.[43,102]Cladribine
administered at a dosage of 0.14 mg/kg/day for five days, as a 2-hours
infusion or by subcutaneous administration, and for up to 9 cycles, led
to clinical improvement by lowering the secretion of mediators and
reducing MC infiltration and serum tryptase levels.[43]
The most common side effects include myelosuppression and lymphopenia.
Reported overall response rates are 43% in ASM, 53% in SM-AHN, and 0%
in MCL; the median response duration was 2.47 years.[103] Imatinib
is indicated only in KIT wild-type SM variants or harboring rare KIT
mutations sensitive to this drug and in the rare well-differentiated
forms of SM (WDSM).[43,104]Although
the second-generation multi-kinase inhibitor, dasatinib, demonstrated
in vitro efficacy against various KIT mutants, including KIT D816V,[105] two clinical trials reported modest efficacy and significant side effects.[106–108]Midostaurin
is a potent pan-inhibitor of TK, approved in 2017 in monotherapy for
treating adult patients with advanced forms of SM, at the recommended
starting dose of 100 mg twice/day orally, regardless of the KIT
mutational status. The registration study demonstrated a high efficacy
leading to an overall response rate (including major or partial
responses) in 60% of patients, improved symptoms burden and quality of
life, and good tolerability.[109] In addition to BM
toxicity, the most common side effects are nausea and vomiting,
generally well controlled with prophylactic antiemetics, and taking the
drug with food. After a median follow-up of 26 months, the median
overall survival in the pivotal study was 28.7 months, with improvement
over historical survival data, including a median OS not reached in
ASM, of 20.7 months in SM-AHN and 9.4 months in MCL.[109]Ongoing
trials, also led in Italy, aim to evaluate the efficacy of other TK
inhibitors such as avapritinib, a potent inhibitor of D816V mutated
KIT, recently registered in the United States to treat diseases
determined by activating KIT mutations, including Mastocytosis and
gastrointestinal stromal tumors (GIST). This drug led to a rapid and
deeper response in advanced SM, with an overall response rate greater
than 70%, of whom 24% of complete response or complete hematological
response.[110] The starting treatment dose was
established at 200 mg once daily. The most frequently reported side
effects are edema, thrombocytopenia, anemia, diarrhea, nausea, and
fatigue. One patient in the pivotal study, who had pre-treatment severe
thrombocytopenia (platelets <50×109/L),
experienced Grade 4 subdural hematoma; consequently, pre-treatment
severe thrombocytopenia was defined as an exclusion criterion, and dose
interruption for severe thrombocytopenia is recommended. More
than 50% of AdvSM express CD30 on the surface of clonal MC.
Nonetheless, a recent phase 2 trial of Brentuximab Vedotin (BV), a
CD30-directed antibody-drug conjugate, on ten patients of CD30-positive
AdvSM, failed to demonstrate the single-agent activity of BV in this
setting.[111] The
treatment of SM-AHN is very complex, and the strategy relies upon both
the clinical variant of SM (advanced or indolent) and the type of
associated neoplasm. Usually, treatment is tailored against the more
aggressive form or consists of a combined treatment,[54] but responses with midostaurin and avapritinib could be obtained regardless of the presence of concomitant AHN.[42,109,110]Allogeneic
stem cell transplantation in advanced SM can be an option if the
associated hematological neoplasm itself has an indication or MCL
responsive to TK inhibitors, considering the very severe prognosis. In
other advanced forms of SM, the decision is tricky, as few studies
demonstrated the superiority of transplant over KIT inhibitor
maintenance therapy.[42]In
patients with ISM presenting with severe mediator symptoms and
refractory to standard therapy, more aggressive therapy may be
indicated; the use of KIT inhibitors in patients with non-advanced
forms of Mastocytosis will be assessed in ongoing studies.[43]
Masitinib, a TKI inhibitor of Lyn, Fyn, PDGFR-α/β, and wild-type KIT,
was recently evaluated in phase III, randomized, double-blind trial, in
non-advanced SM unresponsive to optimal symptomatic treatments.
Masitinib allowed a 75% of sustained improvement in one symptom as
pruritus, flushing, depression, or fatigue, in 18.7% of patients
compared to 7.4% of patients taking a placebo without severe toxicity.
However, an excess incidence of diarrhea and rush of 9% and 4%
respectively was reported in the masitinib group.[112]
Recent data from a study enrolling ISM patients with moderate or severe
mediators related symptoms, refractory to best supportive care drugs,
showed that low doses of avapritinib were well tolerated and produced
significantly greater symptoms reduction than placebo.[113]
Conclusions
Mastocytosis
presents with a plethora of signs and symptoms, mostly related to the
secretion of mediators from clonal MC. In many cases, mediator-release
symptoms significantly impact patients' quality of life and could be
controlled through the instauration of adequate anti-mediator therapy.
Consequently, an early and accurate diagnosis is beneficial for most
patients. Patients with a positive history of an anaphylactic reaction
to Hymenoptera venom should receive immunotherapy continuously to avoid
other systemic reactions. Patients with osteoporosis secondary to SM
may benefit from antiresorptive therapy.
Furthermore,
patients requiring surgical procedures, both in local or general
anesthesia, should be adequately managed to prevent massive
degranulation of clonal MC and subsequent severe adverse events.
Concerning more advanced forms of SM, treating physicians should be
able to handle cytoreductive therapies, considering allogeneic stem
cell transplant or enrolment in clinical trials in selected cases.
Given the complexity of this rare and often misdiagnosed disease, it is
of great importance to address the patient to well experienced and
specialized center, possibly performing a multidisciplinary diagnostic
evaluation. Additionally, the availability of sensitive molecular
techniques is essential for a precise diagnosis.
This
review provides a compendium of the current knowledge on the disease,
aiming to help physicians easily recognize patients with a high
probability of SM, which should be addressed to reference centers.
References
- Valent, P.; Akin, C.; Escribano, L.; Födinger, M.;
Hartmann, K.; Brockow, K.; Castells, M.; Sperr, W.R.; Kluin‐Nelemans,
H.C.; Hamdy, N. a. T.; et al. Standards and Standardization in
Mastocytosis: Consensus Statements on Diagnostics, Treatment
Recommendations and Response Criteria. European Journal of Clinical
Investigation 2007, 37, 435-453, doi:10.1111/j.1365-2362.2007.01807.x. https://doi.org/10.1111/j.1365-2362.2007.01807.x PMid:17537151
- Hartmann,
K.; Escribano, L.; Grattan, C.; Brockow, K.; Carter, M.C.;
Alvarez-Twose, I.; Matito, A.; Broesby-Olsen, S.; Siebenhaar, F.;
Lange, M.; et al. Cutaneous Manifestations in Patients with
Mastocytosis: Consensus Report of the European Competence Network on
Mastocytosis; the American Academy of Allergy, Asthma & Immunology;
and the European Academy of Allergology and Clinical Immunology. J
Allergy Clin Immunol 2016, 137, 35-45, doi:10.1016/j.jaci.2015.08.034. https://doi.org/10.1016/j.jaci.2015.08.034 PMid:26476479
- Longley,
B.J.; Tyrrell, L.; Lu, S.Z.; Ma, Y.S.; Langley, K.; Ding, T.G.; Duffy,
T.; Jacobs, P.; Tang, L.H.; Modlin, I. Somatic C-KIT Activating
Mutation in Urticaria Pigmentosa and Aggressive Mastocytosis:
Establishment of Clonality in a Human Mast Cell Neoplasm. Nat Genet
1996, 12, 312-314, doi:10.1038/ng0396-312. https://doi.org/10.1038/ng0396-312 PMid:8589724
- Arock,
M.; Sotlar, K.; Akin, C.; Broesby-Olsen, S.; Hoermann, G.; Escribano,
L.; Kristensen, T.K.; Kluin-Nelemans, H.C.; Hermine, O.; Dubreuil, P.;
et al. KIT Mutation Analysis in Mast Cell Neoplasms: Recommendations of
the European Competence Network on Mastocytosis. Leukemia 2015, 29,
1223-1232, doi:10.1038/leu.2015.24. https://doi.org/10.1038/leu.2015.24 PMid:25650093 PMCid:PMC4522520
- Arber,
D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Beau,
M.M.L.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 Revision
to the World Health Organization Classification of Myeloid Neoplasms
and Acute Leukemia. Blood 2016, 127, 2391-2405,
doi:10.1182/blood-2016-03-643544. https://doi.org/10.1182/blood-2016-03-643544 PMid:27069254
- Valent,
P.; Horny, H.P.; Escribano, L.; Longley, B.J.; Li, C.Y.; Schwartz,
L.B.; Marone, G.; Nuñez, R.; Akin, C.; Sotlar, K.; et al. Diagnostic
Criteria and Classification of Mastocytosis: A Consensus Proposal. Leuk
Res 2001, 25, 603-625, doi:10.1016/s0145-2126(01)00038-8. https://doi.org/10.1016/S0145-2126(01)00038-8
- Zanotti,
R.; Bonadonna, P.; Bonifacio, M.; Artuso, A.; Schena, D.; Rossini, M.;
Perbellini, O.; Colarossi, S.; Chilosi, M.; Pizzolo, G. Isolated Bone
Marrow Mastocytosis: An Underestimated Subvariant of Indolent Systemic
Mastocytosis. Haematologica 2011, 96, 482-484,
doi:10.3324/haematol.2010.034553. https://doi.org/10.3324/haematol.2010.034553 PMid:21193416 PMCid:PMC3046284
- Trizuljak,
J.; Sperr, W.R.; Nekvindová, L.; Elberink, H.O.; Gleixner, K.V.;
Gorska, A.; Lange, M.; Hartmann, K.; Illerhaus, A.; Bonifacio, M.; et
al. Clinical Features and Survival of Patients with Indolent Systemic
Mastocytosis Defined by the Updated WHO Classification. Allergy 2020,
75, 1927-1938, doi:10.1111/all.14248. https://doi.org/10.1111/all.14248 PMid:32108361 PMCid:PMC7115854
- Zanotti
R, Bonifacio M, Lucchini, G Sperr W, Scaffidi L, van Anrooij B et al.
Refined Diagnostic Criteria for Bone Marrow Mastocytosis: A Proposal of
the European Competence Network on Mastocytosis.
- Pardanani,
A.; Lim, K.-H.; Lasho, T.L.; Finke, C.M.; McClure, R.F.; Li, C.-Y.;
Tefferi, A. WHO Subvariants of Indolent Mastocytosis: Clinical Details
and Prognostic Evaluation in 159 Consecutive Adults. Blood 2010, 115,
150-151, doi:10.1182/blood-2009-10-249979. https://doi.org/10.1182/blood-2009-10-249979 PMid:20056798
- Lim,
K.-H.; Tefferi, A.; Lasho, T.L.; Finke, C.; Patnaik, M.; Butterfield,
J.H.; McClure, R.F.; Li, C.-Y.; Pardanani, A. Systemic Mastocytosis in
342 Consecutive Adults: Survival Studies and Prognostic Factors. Blood
2009, 113, 5727-5736, doi:10.1182/blood-2009-02-205237. https://doi.org/10.1182/blood-2009-02-205237 PMid:19363219
- Valentini,
C.G.; Rondoni, M.; Pogliani, E.M.; Van Lint, M.T.; Cattaneo, C.;
Marbello, L.; Pulsoni, A.; Giona, F.; Martinelli, G.; Leone, G.; et al.
Mast Cell Leukemia: A Report of Ten Cases. Ann Hematol 2008, 87,
505-508, doi:10.1007/s00277-007-0430-3. https://doi.org/10.1007/s00277-007-0430-3 PMid:18172645
- Cohen,
S.S.; Skovbo, S.; Vestergaard, H.; Kristensen, T.; Møller, M.;
Bindslev-Jensen, C.; Fryzek, J.P.; Broesby-Olsen, S. Epidemiology of
Systemic Mastocytosis in Denmark. Br J Haematol 2014, 166, 521-528,
doi:10.1111/bjh.12916. https://doi.org/10.1111/bjh.12916 PMid:24761987
- van
Doormaal, J.J.; Arends, S.; Brunekreeft, K.L.; van der Wal, V.B.;
Sietsma, J.; van Voorst Vader, P.C.; Oude Elberink, J.N.G.;
Kluin-Nelemans, J.C.; van der Veer, E.; de Monchy, J.G.R. Prevalence of
Indolent Systemic Mastocytosis in a Dutch Region. J Allergy Clin
Immunol 2013, 131, 1429-1431.e1, doi:10.1016/j.jaci.2012.10.015. https://doi.org/10.1016/j.jaci.2012.10.015 PMid:23219169
- Bodemer,
C.; Hermine, O.; Palmérini, F.; Yang, Y.; Grandpeix-Guyodo, C.;
Leventhal, P.S.; Hadj-Rabia, S.; Nasca, L.; Georgin-Lavialle, S.;
Cohen-Akenine, A.; et al. Pediatric Mastocytosis Is a Clonal Disease
Associated with D816V and Other Activating C-KIT Mutations. Journal of
Investigative Dermatology 2010, 130, 804-815, doi:10.1038/jid.2009.281.
https://doi.org/10.1038/jid.2009.281 PMid:19865100
- Ben-Amitai,
D.; Metzker, A.; Cohen, H.A. Pediatric Cutaneous Mastocytosis: A Review
of 180 Patients. Isr Med Assoc J 2005, 7, 320-322.
- Tanasi,
I.; Bonifacio, M.; Pizzolato, M.; Irene Grifoni, F.; Sciumè, M.; Elena,
C.; Benvenuti, P.; Mannelli, F.; Parente, R.; Schena, D.; et al.
Familial Occurrence of Systemic and Cutaneous Mastocytosis in an Adult
Multicentre Series. Br J Haematol 2021, 193, 845-848,
doi:10.1111/bjh.17405. https://doi.org/10.1111/bjh.17405 PMid:33754335
- Valent,
P. The Riddle of the Mast Cell: Kit(CD117)-Ligand as the Missing Link?
Immunology Today 1994, 15, 111-114, doi:10.1016/0167-5699(94)90153-8. https://doi.org/10.1016/0167-5699(94)90153-8
- Garcia-Montero,
A.C.; Jara-Acevedo, M.; Teodosio, C.; Sanchez, M.L.; Nunez, R.; Prados,
A.; Aldanondo, I.; Sanchez, L.; Dominguez, M.; Botana, L.M.; et al. KIT
Mutation in Mast Cells and Other Bone Marrow Hematopoietic Cell
Lineages in Systemic Mast Cell Disorders: A Prospective Study of the
Spanish Network on Mastocytosis (REMA) in a Series of 113 Patients.
Blood 2006, 108, 2366-2372, doi:10.1182/blood-2006-04-015545. https://doi.org/10.1182/blood-2006-04-015545 PMid:16741248
- Jawhar,
M.; Schwaab, J.; Schnittger, S.; Meggendorfer, M.; Pfirrmann, M.;
Sotlar, K.; Horny, H.-P.; Metzgeroth, G.; Kluger, S.; Naumann, N.; et
al. Additional Mutations in SRSF2, ASXL1 and/or RUNX1 Identify a
High-Risk Group of Patients with KIT D816V + Advanced Systemic
Mastocytosis. Leukemia 2016, 30, 136-143, doi:10.1038/leu.2015.284. https://doi.org/10.1038/leu.2015.284 PMid:26464169
- Muñoz-González,
J.I.; Jara-Acevedo, M.; Alvarez-Twose, I.; Merker, J.D.; Teodosio, C.;
Hou, Y.; Henriques, A.; Roskin, K.M.; Sanchez-Muñoz, L.; Tsai, A.G.; et
al. Impact of Somatic and Germline Mutations on the Outcome of Systemic
Mastocytosis. Blood Adv 2018, 2, 2814-2828,
doi:10.1182/bloodadvances.2018020628. https://doi.org/10.1182/bloodadvances.2018020628 PMid:30373888 PMCid:PMC6234367
- Pardanani,
A.; Lasho, T.; Elala, Y.; Wassie, E.; Finke, C.; Reichard, K.K.; Chen,
D.; Hanson, C.A.; Ketterling, R.P.; Tefferi, A. Next-Generation
Sequencing in Systemic Mastocytosis: Derivation of a Mutation-Augmented
Clinical Prognostic Model for Survival. Am J Hematol 2016, 91, 888-893,
doi:10.1002/ajh.24426. https://doi.org/10.1002/ajh.24426 PMid:27214377
- Valent,
P.; Akin, C.; Arock, M.; Brockow, K.; Butterfield, J.H.; Carter, M.C.;
Castells, M.; Escribano, L.; Hartmann, K.; Lieberman, P.; et al.
Definitions, Criteria and Global Classification of Mast Cell Disorders
with Special Reference to Mast Cell Activation Syndromes: A Consensus
Proposal. Int Arch Allergy Immunol 2012, 157, 215-225,
doi:10.1159/000328760. https://doi.org/10.1159/000328760 PMid:22041891 PMCid:PMC3224511
- Shanmugam,
G.; Schwartz, L.B.; Khan, D.A. Prolonged Elevation of Serum Tryptase in
Idiopathic Anaphylaxis. J Allergy Clin Immunol 2006, 117, 950-951,
doi:10.1016/j.jaci.2005.12.1356. https://doi.org/10.1016/j.jaci.2005.12.1356 PMid:16630958
- Valent,
P.; Escribano, L.; Broesby-Olsen, S.; Hartmann, K.; Grattan, C.;
Brockow, K.; Niedoszytko, M.; Nedoszytko, B.; Oude Elberink, J.N.G.;
Kristensen, T.; et al. Proposed Diagnostic Algorithm for Patients with
Suspected Mastocytosis: A Proposal of the European Competence Network
on Mastocytosis. Allergy 2014, 69, 1267-1274, doi:10.1111/all.12436. https://doi.org/10.1111/all.12436 PMid:24836395
- Sperr,
W.R.; Escribano, L.; Jordan, J.H.; Schernthaner, G.H.; Kundi, M.;
Horny, H.P.; Valent, P. Morphologic Properties of Neoplastic Mast
Cells: Delineation of Stages of Maturation and Implication for
Cytological Grading of Mastocytosis. Leuk Res 2001, 25, 529-536,
doi:10.1016/s0145-2126(01)00041-8. https://doi.org/10.1016/S0145-2126(01)00041-8
- Gotlib,
J.; Gerds, A.T.; Bose, P.; Castells, M.C.; Deininger, M.W.; Gojo, I.;
Gundabolu, K.; Hobbs, G.; Jamieson, C.; McMahon, B.; et al. Systemic
Mastocytosis, Version 2.2019, NCCN Clinical Practice Guidelines in
Oncology. J Natl Compr Canc Netw 2018, 16, 1500-1537,
doi:10.6004/jnccn.2018.0088. https://doi.org/10.6004/jnccn.2018.0088 PMid:30545997
- Escribano,
L.; Díaz-Agustín, B.; Bellas, C.; Navalón, R.; Nuñez, R.; Sperr, W.R.;
Schernthaner, G.H.; Valent, P.; Orfao, A. Utility of Flow Cytometric
Analysis of Mast Cells in the Diagnosis and Classification of Adult
Mastocytosis. Leuk Res 2001, 25, 563-570,
doi:10.1016/s0145-2126(01)00050-9. https://doi.org/10.1016/S0145-2126(01)00050-9
- Metcalfe,
D.D.; Mekori, Y.A. Pathogenesis and Pathology of Mastocytosis. Annu Rev
Pathol 2017, 12, 487-514, doi:10.1146/annurev-pathol-052016-100312. https://doi.org/10.1146/annurev-pathol-052016-100312 PMid:28135563
- Morgado,
J.M.T.; Sánchez-Muñoz, L.; Teodósio, C.G.; Jara-Acevedo, M.;
Alvarez-Twose, I.; Matito, A.; Fernández-Nuñez, E.; García-Montero, A.;
Orfao, A.; Escribano, L. Immunophenotyping in Systemic Mastocytosis
Diagnosis: "CD25 Positive" Alone Is More Informative than the "CD25
and/or CD2" WHO Criterion. Mod Pathol 2012, 25, 516-521,
doi:10.1038/modpathol.2011.192. https://doi.org/10.1038/modpathol.2011.192 PMid:22222639
- Sotlar,
K.; Cerny-Reiterer, S.; Petat-Dutter, K.; Hessel, H.; Berezowska, S.;
Müllauer, L.; Valent, P.; Horny, H.-P. Aberrant Expression of CD30 in
Neoplastic Mast Cells in High-Grade Mastocytosis. Mod Pathol 2011, 24,
585-595, doi:10.1038/modpathol.2010.224. https://doi.org/10.1038/modpathol.2010.224 PMid:21186345
- Morgado,
J.M.; Perbellini, O.; Johnson, R.C.; Teodósio, C.; Matito, A.;
Álvarez-Twose, I.; Bonadonna, P.; Zamò, A.; Jara-Acevedo, M.; Mayado,
A.; et al. CD30 Expression by Bone Marrow Mast Cells from Different
Diagnostic Variants of Systemic Mastocytosis. Histopathology 2013, 63,
780-787, doi:10.1111/his.12221. https://doi.org/10.1111/his.12221 PMid:24111625
- Escribano,
L.; Diaz‐Agustin, B.; López, A.; López, R.N.; García‐Montero, A.;
Almeida, J.; Prados, A.; Angulo, M.; Herrero, S.; Orfao, A.
Immunophenotypic Analysis of Mast Cells in Mastocytosis: When and How
to Do It. Proposals of the Spanish Network on Mastocytosis (REMA).
Cytometry Part B: Clinical Cytometry 2004, 58B, 1-8,
doi:10.1002/cyto.b.10072. https://doi.org/10.1002/cyto.b.10072 PMid:14994369
- Perbellini,
O.; Zamò, A.; Colarossi, S.; Zampieri, F.; Zoppi, F.; Bonadonna, P.;
Schena, D.; Artuso, A.; Martinelli, G.; Chilosi, M.; et al. Primary
Role of Multiparametric Flow Cytometry in the Diagnostic Work-up of
Indolent Clonal Mast Cell Disorders. Cytometry B Clin Cytom 2011, 80,
362-368, doi:10.1002/cyto.b.20606. https://doi.org/10.1002/cyto.b.20606 PMid:21656905
- Kristensen,
T.; Broesby-Olsen, S.; Vestergaard, H.; Bindslev-Jensen, C.; Møller,
M.B.; Mastocytosis Centre Odense University Hospital (MastOUH)
Circulating KIT D816V Mutation-Positive Non-Mast Cells in Peripheral
Blood Are Characteristic of Indolent Systemic Mastocytosis. Eur J
Haematol 2012, 89, 42-46, doi:10.1111/j.1600-0609.2012.01789.x. https://doi.org/10.1111/j.1600-0609.2012.01789.x PMid:22469616
- Greiner,
G.; Gurbisz, M.; Ratzinger, F.; Witzeneder, N.; Simonitsch-Klupp, I.;
Mitterbauer-Hohendanner, G.; Mayerhofer, M.; Müllauer, L.; Sperr, W.R.;
Valent, P.; et al. Digital PCR: A Sensitive and Precise Method for KIT
D816V Quantification in Mastocytosis. Clin Chem 2018, 64, 547-555,
doi:10.1373/clinchem.2017.277897. https://doi.org/10.1373/clinchem.2017.277897 PMid:29237714 PMCid:PMC7115889
- Kristensen,
T.; Broesby-Olsen, S.; Vestergaard, H.; Bindslev-Jensen, C.; Møller,
M.B.; Mastocytosis Centre Odense University Hospital (MastOUH) Targeted
Ultradeep Next-Generation Sequencing as a Method for KIT D816V Mutation
Analysis in Mastocytosis. Eur J Haematol 2016, 96, 381-388,
doi:10.1111/ejh.12601. https://doi.org/10.1111/ejh.12601 PMid:26095448
- Sotlar,
K.; Escribano, L.; Landt, O.; Möhrle, S.; Herrero, S.; Torrelo, A.;
Lass, U.; Horny, H.-P.; Bültmann, B. One-Step Detection of c-Kit Point
Mutations Using Peptide Nucleic Acid-Mediated Polymerase Chain Reaction
Clamping and Hybridization Probes. Am J Pathol 2003, 162, 737-746. https://doi.org/10.1016/S0002-9440(10)63870-9
- Jara-Acevedo,
M.; Teodosio, C.; Sanchez-Muñoz, L.; Álvarez-Twose, I.; Mayado, A.;
Caldas, C.; Matito, A.; Morgado, J.M.; Muñoz-González, J.I.; Escribano,
L.; et al. Detection of the KIT D816V Mutation in Peripheral Blood of
Systemic Mastocytosis: Diagnostic Implications. Mod Pathol 2015, 28,
1138-1149, doi:10.1038/modpathol.2015.72. https://doi.org/10.1038/modpathol.2015.72 PMid:26067933
- Monaldi,
C.; De Santis, S.; Mancini, M.; Bruno, S.; Cavo, M.; Soverini, S.
Systemic Mastocytosis: Molecular Landscape and Implications for
Treatment. Mediterr J Hematol Infect Dis 2021, 13, e2021046,
doi:10.4084/MJHID.2021.046. https://doi.org/10.4084/MJHID.2021.046 PMid:34276915 PMCid:PMC8265368
- Kristensen,
T.; Vestergaard, H.; Bindslev-Jensen, C.; Mortz, C.G.; Kjaer, H.F.;
Ollert, M.; Møller, M.B.; Broesby-Olsen, S.; Mastocytosis Centre Odense
University Hospital (MastOUH) Prospective Evaluation of the Diagnostic
Value of Sensitive KIT D816V Mutation Analysis of Blood in Adults with
Suspected Systemic Mastocytosis. Allergy 2017, 72, 1737-1743,
doi:10.1111/all.13187. https://doi.org/10.1111/all.13187 PMid:28432683
- Reiter,
A.; George, T.I.; Gotlib, J. New Developments in Diagnosis,
Prognostication, and Treatment of Advanced Systemic Mastocytosis. Blood
2020, 135, 1365-1376, doi:10.1182/blood.2019000932. https://doi.org/10.1182/blood.2019000932 PMid:32106312
- Pardanani,
A. Systemic Mastocytosis in Adults: 2021 Update on Diagnosis, Risk
Stratification and Management. Am J Hematol 2021, 96, 508-525,
doi:10.1002/ajh.26118. https://doi.org/10.1002/ajh.26118 PMid:33524167
- Lim,
K.-H.; Tefferi, A.; Lasho, T.L.; Finke, C.; Patnaik, M.; Butterfield,
J.H.; McClure, R.F.; Li, C.-Y.; Pardanani, A. Systemic Mastocytosis in
342 Consecutive Adults: Survival Studies and Prognostic Factors. Blood
2009, 113, 5727-5736, doi:10.1182/blood-2009-02-205237. https://doi.org/10.1182/blood-2009-02-205237 PMid:19363219
- Jawhar,
M.; Schwaab, J.; Hausmann, D.; Clemens, J.; Naumann, N.; Henzler, T.;
Horny, H.-P.; Sotlar, K.; Schoenberg, S.O.; Cross, N.C.P.; et al.
Splenomegaly, Elevated Alkaline Phosphatase and Mutations in the
SRSF2/ASXL1/RUNX1 Gene Panel Are Strong Adverse Prognostic Markers in
Patients with Systemic Mastocytosis. Leukemia 2016, 30, 2342-2350,
doi:10.1038/leu.2016.190. https://doi.org/10.1038/leu.2016.190 PMid:27416984
- Escribano,
L.; Alvarez-Twose, I.; Sánchez-Muñoz, L.; Garcia-Montero, A.; Núñez,
R.; Almeida, J.; Jara-Acevedo, M.; Teodósio, C.; García-Cosío, M.;
Bellas, C.; et al. Prognosis in Adult Indolent Systemic Mastocytosis: A
Long-Term Study of the Spanish Network on Mastocytosis in a Series of
145 Patients. J Allergy Clin Immunol 2009, 124, 514-521,
doi:10.1016/j.jaci.2009.05.003. https://doi.org/10.1016/j.jaci.2009.05.003 PMid:19541349
- Hoermann,
G.; Gleixner, K.V.; Dinu, G.E.; Kundi, M.; Greiner, G.; Wimazal, F.;
Hadzijusufovic, E.; Mitterbauer, G.; Mannhalter, C.; Valent, P.; et al.
The KIT D816V Allele Burden Predicts Survival in Patients with
Mastocytosis and Correlates with the WHO Type of the Disease. Allergy
2014, 69, 810-813, doi:10.1111/all.12409. https://doi.org/10.1111/all.12409 PMid:24750133 PMCid:PMC4896381
- Sperr,
W.R.; Kundi, M.; Alvarez-Twose, I.; van Anrooij, B.; Oude Elberink,
J.N.G.; Gorska, A.; Niedoszytko, M.; Gleixner, K.V.; Hadzijusufovic,
E.; Zanotti, R.; et al. International Prognostic Scoring System for
Mastocytosis (IPSM): A Retrospective Cohort Study. Lancet Haematol
2019, 6, e638-e649, doi:10.1016/S2352-3026(19)30166-8. https://doi.org/10.1016/S2352-3026(19)30166-8
- Pardanani,
A.; Shah, S.; Mannelli, F.; Elala, Y.C.; Guglielmelli, P.; Lasho, T.L.;
Patnaik, M.M.; Gangat, N.; Ketterling, R.P.; Reichard, K.K.; et al.
Mayo Alliance Prognostic System for Mastocytosis: Clinical and Hybrid
Clinical-Molecular Models. Blood Adv 2018, 2, 2964-2972,
doi:10.1182/bloodadvances.2018026245. https://doi.org/10.1182/bloodadvances.2018026245 PMid:30413432 PMCid:PMC6234360
- Jawhar,
M.; Schwaab, J.; Álvarez-Twose, I.; Shoumariyeh, K.; Naumann, N.;
Lübke, J.; Perkins, C.; Muñoz-González, J.I.; Meggendorfer, M.;
Kennedy, V.; et al. MARS: Mutation-Adjusted Risk Score for Advanced
Systemic Mastocytosis. J Clin Oncol 2019, 37, 2846-2856,
doi:10.1200/JCO.19.00640. https://doi.org/10.1200/JCO.19.00640 PMid:31509472 PMCid:PMC6823885
- Muñoz-González,
J.I.; Álvarez-Twose, I.; Jara-Acevedo, M.; Zanotti, R.; Perkins, C.;
Jawhar, M.; Sperr, W.R.; Shoumariyeh, K.; Schwaab, J.; Greiner, G.; et
al. Proposed Global Prognostic Score for Systemic Mastocytosis: A
Retrospective Prognostic Modelling Study. Lancet Haematol 2021, 8,
e194-e204, doi:10.1016/S2352-3026(20)30400-2. https://doi.org/10.1016/S2352-3026(20)30400-2
- Schena,
D.; Galvan, A.; Tessari, G.; Girolomoni, G. Clinical Features and
Course of Cutaneous Mastocytosis in 133 Children. Br J Dermatol 2016,
174, 411-413, doi:10.1111/bjd.14004. https://doi.org/10.1111/bjd.14004 PMid:26138896
- Godt,
O.; Proksch, E.; Streit, V.; Christophers, E. Short- and Long-Term
Effectiveness of Oral and Bath PUVA Therapy in Urticaria Pigmentosa and
Systemic Mastocytosis. Dermatology 1997, 195, 35-39,
doi:10.1159/000245681. https://doi.org/10.1159/000245681 PMid:9267734
- Valent,
P.; Akin, C.; Gleixner, K.V.; Sperr, W.R.; Reiter, A.; Arock, M.;
Triggiani, M. Multidisciplinary Challenges in Mastocytosis and How to
Address with Personalized Medicine Approaches. Int J Mol Sci 2019, 20,
E2976, doi:10.3390/ijms20122976. https://doi.org/10.3390/ijms20122976 PMid:31216696 PMCid:PMC6627900
- González
de Olano, D.; de la Hoz Caballer, B.; Núñez López, R.; Sánchez Muñoz,
L.; Cuevas Agustín, M.; Diéguez, M.C.; Alvarez Twose, I.; Castells,
M.C.; Escribano Mora, L. Prevalence of Allergy and Anaphylactic
Symptoms in 210 Adult and Pediatric Patients with Mastocytosis in
Spain: A Study of the Spanish Network on Mastocytosis (REMA). Clin.
Exp. Allergy 2007, 37, 1547-1555, doi:10.1111/j.1365-2222.2007.02804.x.
https://doi.org/10.1111/j.1365-2222.2007.02804.x PMid:17883734
- Brockow,
K.; Jofer, C.; Behrendt, H.; Ring, J. Anaphylaxis in Patients with
Mastocytosis: A Study on History, Clinical Features and Risk Factors in
120 Patients. Allergy 2008, 63, 226-232,
doi:10.1111/j.1398-9995.2007.01569.x. https://doi.org/10.1111/j.1398-9995.2007.01569.x PMid:18186813
- Gülen,
T.; Hägglund, H.; Dahlén, B.; Nilsson, G. High Prevalence of
Anaphylaxis in Patients with Systemic Mastocytosis - a Single-Centre
Experience. Clin Exp Allergy 2014, 44, 121-129, doi:10.1111/cea.12225. https://doi.org/10.1111/cea.12225 PMid:24164252
- Lieberman,
P.; Camargo, C.A.; Bohlke, K.; Jick, H.; Miller, R.L.; Sheikh, A.;
Simons, F.E.R. Epidemiology of Anaphylaxis: Findings of the American
College of Allergy, Asthma and Immunology Epidemiology of Anaphylaxis
Working Group. Ann Allergy Asthma Immunol 2006, 97, 596-602,
doi:10.1016/S1081-1206(10)61086-1. https://doi.org/10.1016/S1081-1206(10)61086-1
- Muraro,
A.; Roberts, G.; Worm, M.; Bilò, M.B.; Brockow, K.; Fernández Rivas,
M.; Santos, A.F.; Zolkipli, Z.Q.; Bellou, A.; Beyer, K.; et al.
Anaphylaxis: Guidelines from the European Academy of Allergy and
Clinical Immunology. Allergy 2014, 69, 1026-1045,
doi:10.1111/all.12437. https://doi.org/10.1111/all.12437 PMid:24909803
- Pieri,
L.; Bonadonna, P.; Elena, C.; Papayannidis, C.; Grifoni, F.I.; Rondoni,
M.; Girlanda, S.; Mauro, M.; Magliacane, D.; Elli, E.M.; et al.
Clinical Presentation and Management Practice of Systemic Mastocytosis.
A Survey on 460 Italian Patients. Am J Hematol 2016, 91, 692-699,
doi:10.1002/ajh.24382. https://doi.org/10.1002/ajh.24382 PMid:27060898
- Biló,
B.M.; Rueff, F.; Mosbech, H.; Bonifazi, F.; Oude-Elberink, J.N.G.;
EAACI Interest Group on Insect Venom Hypersensitivity Diagnosis of
Hymenoptera Venom Allergy. Allergy 2005, 60, 1339-1349,
doi:10.1111/j.1398-9995.2005.00963.x. https://doi.org/10.1111/j.1398-9995.2005.00963.x PMid:16197464
- Niedoszytko,
M.; de Monchy, J.; van Doormaal, J.J.; Jassem, E.; Oude Elberink,
J.N.G. Mastocytosis and Insect Venom Allergy: Diagnosis, Safety and
Efficacy of Venom Immunotherapy. Allergy 2009, 64, 1237-1245,
doi:10.1111/j.1398-9995.2009.02118.x. https://doi.org/10.1111/j.1398-9995.2009.02118.x PMid:19627278
- Bonadonna,
P.; Lombardo, C.; Zanotti, R. Mastocytosis and Allergic Diseases. J
Investig Allergol Clin Immunol 2014, 24, 288-297; quiz 3 p preceding
297.
- Alvarez-Twose, I.; González
de Olano, D.; Sánchez-Muñoz, L.; Matito, A.; Esteban-López, M.I.; Vega,
A.; Mateo, M.B.; Alonso Díaz de Durana, M.D.; de la Hoz, B.; Del Pozo
Gil, M.D.; et al. Clinical, Biological, and Molecular Characteristics
of Clonal Mast Cell Disorders Presenting with Systemic Mast Cell
Activation Symptoms. J Allergy Clin Immunol 2010, 125, 1269-1278.e2,
doi:10.1016/j.jaci.2010.02.019. https://doi.org/10.1016/j.jaci.2010.02.019 PMid:20434205
- Bonadonna,
P.; Perbellini, O.; Passalacqua, G.; Caruso, B.; Colarossi, S.; Dal
Fior, D.; Castellani, L.; Bonetto, C.; Frattini, F.; Dama, A.; et al.
Clonal Mast Cell Disorders in Patients with Systemic Reactions to
Hymenoptera Stings and Increased Serum Tryptase Levels. J Allergy Clin
Immunol 2009, 123, 680-686, doi:10.1016/j.jaci.2008.11.018. https://doi.org/10.1016/j.jaci.2008.11.018 PMid:19135713
- Bonadonna,
P.; Bonifacio, M.; Lombardo, C.; Zanotti, R. Hymenoptera Allergy and
Mast Cell Activation Syndromes. Curr Allergy Asthma Rep 2016, 16, 5,
doi:10.1007/s11882-015-0582-5. https://doi.org/10.1007/s11882-015-0582-5 PMid:26714690
- Sokol,
H.; Georgin-Lavialle, S.; Canioni, D.; Barete, S.; Damaj, G.; Soucie,
E.; Bruneau, J.; Chandesris, M.-O.; Suarez, F.; Launay, J.-M.; et al.
Gastrointestinal Manifestations in Mastocytosis: A Study of 83
Patients. J Allergy Clin Immunol 2013, 132, 866-873.e1-3,
doi:10.1016/j.jaci.2013.05.026. https://doi.org/10.1016/j.jaci.2013.05.026 PMid:23890756
- Doyle,
L.A.; Sepehr, G.J.; Hamilton, M.J.; Akin, C.; Castells, M.C.; Hornick,
J.L. A Clinicopathologic Study of 24 Cases of Systemic Mastocytosis
Involving the Gastrointestinal Tract and Assessment of Mucosal Mast
Cell Density in Irritable Bowel Syndrome and Asymptomatic Patients. Am
J Surg Pathol 2014, 38, 832-843, doi:10.1097/PAS.0000000000000190. https://doi.org/10.1097/PAS.0000000000000190 PMid:24618605 PMCid:PMC4086834
- Hermans,
M.A.W.; Rietveld, M.J.A.; van Laar, J.A.M.; Dalm, V.A.S.H.; Verburg,
M.; Pasmans, S.G.M.A.; Gerth van Wijk, R.; van Hagen, P.M.; van Daele,
P.L.A. Systemic Mastocytosis: A Cohort Study on Clinical
Characteristics of 136 Patients in a Large Tertiary Centre. Eur J
Intern Med 2016, 30, 25-30, doi:10.1016/j.ejim.2016.01.005. https://doi.org/10.1016/j.ejim.2016.01.005 PMid:26809706
- Gehlen
M, Schmidt N, Pfeifer M, Balasingam S, Schwarz-Eywill M, Maier A,
Werner M, Siggelkow H. Osteoporosis Caused by Systemic Mastocytosis:
Prevalence in a Cohort of 8392 Patients with Osteoporosis.Calcif Tissue
Int. 2021 Jul 5. doi: 10.1007/s00223-021-00887-4. Epub ahead of print.
PMID: 34223956. https://doi.org/10.1007/s00223-021-00887-4 PMid:34223956
- Escribano,
L.; Alvarez-Twose, I.; Sánchez-Muñoz, L.; Garcia-Montero, A.; Núñez,
R.; Almeida, J.; Jara-Acevedo, M.; Teodósio, C.; García-Cosío, M.;
Bellas, C.; et al. Prognosis in Adult Indolent Systemic Mastocytosis: A
Long-Term Study of the Spanish Network on Mastocytosis in a Series of
145 Patients. J Allergy Clin Immunol 2009, 124, 514-521,
doi:10.1016/j.jaci.2009.05.003. https://doi.org/10.1016/j.jaci.2009.05.003 PMid:19541349
- Rossini,
M.; Zanotti, R.; Bonadonna, P.; Artuso, A.; Caruso, B.; Schena, D.;
Vecchiato, D.; Bonifacio, M.; Viapiana, O.; Gatti, D.; et al. Bone
Mineral Density, Bone Turnover Markers and Fractures in Patients with
Indolent Systemic Mastocytosis. Bone 2011, 49, 880-885,
doi:10.1016/j.bone.2011.07.004. https://doi.org/10.1016/j.bone.2011.07.004 PMid:21782049
- Barete,
S.; Assous, N.; de Gennes, C.; Grandpeix, C.; Feger, F.; Palmerini, F.;
Dubreuil, P.; Arock, M.; Roux, C.; Launay, J.M.; et al. Systemic
Mastocytosis and Bone Involvement in a Cohort of 75 Patients. Ann Rheum
Dis 2010, 69, 1838-1841, doi:10.1136/ard.2009.124511. https://doi.org/10.1136/ard.2009.124511 PMid:20570833
- van
der Veer, E.; Arends, S.; van der Hoek, S.; Versluijs, J.B.; de Monchy,
J.G.R.; Oude Elberink, J.N.G.; van Doormaal, J.J. Predictors of New
Fragility Fractures after Diagnosis of Indolent Systemic Mastocytosis.
J Allergy Clin Immunol 2014, 134, 1413-1421,
doi:10.1016/j.jaci.2014.05.003. https://doi.org/10.1016/j.jaci.2014.05.003 PMid:24985401
- Degboé,
Y.; Eischen, M.; Nigon, D.; Apoil, P.-A.; Mailhol, C.; Tournier, E.;
Laurent, C.; Hanssens, K.; Hermine, O.; Paul, C.; et al. Prevalence and
Risk Factors for Fragility Fracture in Systemic Mastocytosis. Bone
2017, 105, 219-225, doi:10.1016/j.bone.2017.09.005. https://doi.org/10.1016/j.bone.2017.09.005 PMid:28919366
- Broesby-Olsen,
S.; Kristensen, T.K.; Møller, M.B.; Bindslev-Jensen, C.; Vestergaard,
H. Adult-Onset Systemic Mastocytosis in Monozygotic Twins with KIT
D816V and JAK2 V617F Mutations. Journal of Allergy and Clinical
Immunology 2012, 130, 806-808, doi:10.1016/j.jaci.2012.04.013. https://doi.org/10.1016/j.jaci.2012.04.013 PMid:22608575
- Orsolini,
G.; Viapiana, O.; Rossini, M.; Bonifacio, M.; Zanotti, R. Bone Disease
in Mastocytosis. Immunol Allergy Clin North Am 2018, 38, 443-454,
doi:10.1016/j.iac.2018.04.013. https://doi.org/10.1016/j.iac.2018.04.013 PMid:30007462
- Zanotti,
R.; Tanasi, I.; Bernardelli, A.; Orsolini, G.; Bonadonna, P. Bone
Marrow Mastocytosis: A Diagnostic Challenge. J Clin Med 2021, 10, 1420,
doi:10.3390/jcm10071420. https://doi.org/10.3390/jcm10071420 PMid:33915965 PMCid:PMC8037514
- Rossini,
M.; Zanotti, R.; Viapiana, O.; Tripi, G.; Orsolini, G.; Idolazzi, L.;
Bonadonna, P.; Schena, D.; Escribano, L.; Adami, S.; et al. Bone
Involvement and Osteoporosis in Mastocytosis. Immunol Allergy Clin
North Am 2014, 34, 383-396, doi:10.1016/j.iac.2014.01.011. https://doi.org/10.1016/j.iac.2014.01.011 PMid:24745681
- Guillaume,
N.; Desoutter, J.; Chandesris, O.; Merlusca, L.; Henry, I.;
Georgin-Lavialle, S.; Barete, S.; Hirsch, I.; Bouredji, D.; Royer, B.;
et al. Bone Complications of Mastocytosis: A Link between Clinical and
Biological Characteristics. Am J Med 2013, 126, 75.e1-7,
doi:10.1016/j.amjmed.2012.07.018. https://doi.org/10.1016/j.amjmed.2012.07.018 PMid:23200108
- Carosi,
G.; Guabello, G.; Longhi, M.; Grifoni, F.; Passeri, E.; Corbetta, S.
Hypertryptasemia and Mast Cell-Related Disorders in Severe Osteoporotic
Patients. Mediators Inflamm 2020, 2020, doi:10.1155/2020/5785378. https://doi.org/10.1155/2020/5785378 PMid:33144848 PMCid:PMC7599415
- Leone,
A.; Criscuolo, M.; Gullì, C.; Petrosino, A.; Carlo Bianco, N.;
Colosimo, C. Systemic Mastocytosis Revisited with an Emphasis on
Skeletal Manifestations. Radiol Med 2020,
doi:10.1007/s11547-020-01306-8. https://doi.org/10.1007/s11547-020-01306-8 PMid:33242205
- Riffel,
P.; Schwaab, J.; Lutz, C.; Naumann, N.; Metzgeroth, G.; Fabarius, A.;
Schoenberg, S.O.; Hofmann, W.-K.; Valent, P.; Reiter, A.; et al. An
Increased Bone Mineral Density Is an Adverse Prognostic Factor in
Patients with Systemic Mastocytosis. J Cancer Res Clin Oncol 2020, 146,
945-951, doi:10.1007/s00432-019-03119-3. https://doi.org/10.1007/s00432-019-03119-3 PMid:31980928 PMCid:PMC7085471
- Rossini,
M.; Zanotti, R.; Viapiana, O.; Tripi, G.; Idolazzi, L.; Biondan, M.;
Orsolini, G.; Bonadonna, P.; Adami, S.; Gatti, D. Zoledronic Acid in
Osteoporosis Secondary to Mastocytosis. Am J Med 2014, 127,
1127.e1-1127.e4, doi:10.1016/j.amjmed.2014.06.015. https://doi.org/10.1016/j.amjmed.2014.06.015 PMid:24954632
- Greene,
L.W.; Asadipooya, K.; Corradi, P.F.; Akin, C. Endocrine Manifestations
of Systemic Mastocytosis in Bone. Rev Endocr Metab Disord 2016, 17,
419-431, doi:10.1007/s11154-016-9362-3. https://doi.org/10.1007/s11154-016-9362-3 PMid:27239674
- Laroche,
M.; Livideanu, C.; Paul, C.; Cantagrel, A. Interferon Alpha and
Pamidronate in Osteoporosis with Fracture Secondary to Mastocytosis. Am
J Med 2011, 124, 776-778, doi:10.1016/j.amjmed.2011.02.038. https://doi.org/10.1016/j.amjmed.2011.02.038 PMid:21787907
- Matito,
A.; Morgado, J.M.; Sánchez-López, P.; Álvarez-Twose, I.; Sánchez-Muñoz,
L.; Orfao, A.; Escribano, L. Management of Anesthesia in Adult and
Pediatric Mastocytosis: A Study of the Spanish Network on Mastocytosis
(REMA) Based on 726 Anesthetic Procedures. IAA 2015, 167, 47-56,
doi:10.1159/000436969. https://doi.org/10.1159/000436969 PMid:26160029
- Carter,
M.C.; Uzzaman, A.; Scott, L.M.; Metcalfe, D.D.; Quezado, Z. Pediatric
Mastocytosis: Routine Anesthetic Management for a Complex Disease.
Anesth Analg 2008, 107, 422-427, doi:10.1213/ane.0b013e31817e6d7c. https://doi.org/10.1213/ane.0b013e31817e6d7c PMid:18633019 PMCid:PMC2736554
- Bonadonna,
P.; Zanotti, R.; Varani, A.B.; Pagani, M. Mast Cell Diseases and Drug
Hypersensitivity Reactions. Current Treatment Options in Allergy 2017,
2, 258-267, doi:10.1007/s40521-017-0130-8. https://doi.org/10.1007/s40521-017-0130-8
- Bonadonna,
P.; Gonzalez-de-Olano, D.; Zanotti, R.; Riccio, A.; De Ferrari, L.;
Lombardo, C.; Rogkakou, A.; Escribano, L.; Alvarez-Twose, I.; Matito,
A.; et al. Venom Immunotherapy in Patients with Clonal Mast Cell
Disorders: Efficacy, Safety, and Practical Considerations. J Allergy
Clin Immunol Pract 2013, 1, 474-478, doi:10.1016/j.jaip.2013.06.014. https://doi.org/10.1016/j.jaip.2013.06.014 PMid:24565619
- Bilò,
M.B.; Pravettoni, V.; Bignardi, D.; Bonadonna, P.; Mauro, M.; Novembre,
E.; Quercia, O.; Cilia, M.; Cortellini, G.; Costantino, M.T.; et al.
Hymenoptera Venom Allergy: Management of Children and Adults in
Clinical Practice. J Investig Allergol Clin Immunol 2019, 29, 180-205,
doi:10.18176/jiaci.0310. https://doi.org/10.18176/jiaci.0310 PMid:30183660
- Michel,
J.; Brockow, K.; Darsow, U; Ring, J.; Schmidt-Weber, C.B.; Grunwald,
T.; Blank, S.; Ollert, M. Added Sensitivity of Component-Resolved
Diagnosis in Hymenoptera Venom-Allergic Patients with Elevated Serum
Tryptase and/or Mastocytosis. Allergy 2016, 71, 651-660,
doi:10.1111/all.12850. https://doi.org/10.1111/all.12850 PMid:26836051
- Kontou-Fili,
K. High Omalizumab Dose Controls Recurrent Reactions to Venom
Immunotherapy in Indolent Systemic Mastocytosis. Allergy 2008, 63,
376-378, doi:10.1111/j.1398-9995.2007.01604.x. https://doi.org/10.1111/j.1398-9995.2007.01604.x PMid:18269681
- Galera,
C.; Soohun, N.; Zankar, N.; Caimmi, S.; Gallen, C.; Demoly, P. Severe
Anaphylaxis to Bee Venom Immunotherapy: Efficacy of Pretreatment and
Concurrent Treatment with Omalizumab. J Investig Allergol Clin Immunol
2009, 19, 225-229.
- Matito, A.;
Álvarez-Twose, I.; Morgado, J.M.; Sánchez-Muñoz, L.; Orfao, A.;
Escribano, L. Clinical Impact of Pregnancy in Mastocytosis: A Study of
the Spanish Network on Mastocytosis (REMA) in 45 Cases. Int. Arch.
Allergy Immunol. 2011, 156, 104-111, doi:10.1159/000321954. https://doi.org/10.1159/000321954 PMid:21447966
- Worobec,
A.S.; Akin, C.; Scott, L.M.; Metcalfe, D.D. Mastocytosis Complicating
Pregnancy. Obstet Gynecol 2000, 95, 391-395,
doi:10.1016/s0029-7844(99)00591-8. https://doi.org/10.1016/S0029-7844(99)00591-8
- Zanoni,
G.; Zanotti, R.; Schena, D.; Sabbadini, C.; Opri, R.; Bonadonna, P.
Vaccination Management in Children and Adults with Mastocytosis. Clin
Exp Allergy 2017, 47, 593-596, doi:10.1111/cea.12882. https://doi.org/10.1111/cea.12882 PMid:28079293
- Carter,
M.C.; Metcalfe, D.D.; Matito, A.; Escribano, L.; Butterfield, J.H.;
Schwartz, L.B.; Bonadonna, P.; Zanotti, R.; Triggiani, M.; Castells,
M.; et al. Adverse Reactions to Drugs and Biologics in Patients with
Clonal Mast Cell Disorders: A Work Group Report of the Mast Cells
Disorder Committee, American Academy of Allergy, Asthma &
Immunology. J Allergy Clin Immunol 2019, 143, 880-893,
doi:10.1016/j.jaci.2018.10.063. https://doi.org/10.1016/j.jaci.2018.10.063 PMid:30528617
- Bonadonna,
P.; Brockow, K.; Niedoszytko, M.; Elberink, H.O.; Akin, C.; Nedoszytko,
B.; Butterfield, J.H.; Alvarez-Twose, I.; Sotlar, K.; Schwaab, J.; et
al. COVID-19 Vaccination in Mastocytosis: Recommendations of the
European Competence Network on Mastocytosis (ECNM) and American
Initiative in Mast Cell Diseases (AIM). J Allergy Clin Immunol Pract
2021, 9, 2139-2144, doi:10.1016/j.jaip.2021.03.041. https://doi.org/10.1016/j.jaip.2021.03.041 PMid:33831618 PMCid:PMC8019658
- Casassus,
P.; Caillat-Vigneron, N.; Martin, A.; Simon, J.; Gallais, V.; Beaudry,
P.; Eclache, V.; Laroche, L.; Lortholary, P.; Raphaël, M.; et al.
Treatment of Adult Systemic Mastocytosis with Interferon-α: Results of
a Multicentre Phase II Trial on 20 Patients. British Journal of
Haematology 2002, 119, 1090-1097, doi:10.1046/j.1365-2141.2002.03944.x.
https://doi.org/10.1046/j.1365-2141.2002.03944.x PMid:12472593
- Lim,
K.H.; Pardanani, A.; Butterfield, J.H.; Li, C.-Y.; Tefferi, A.
Cytoreductive Therapy in 108 Adults with Systemic Mastocytosis: Outcome
Analysis and Response Prediction during Treatment with
Interferon-Alpha, Hydroxyurea, Imatinib Mesylate or
2-Chlorodeoxyadenosine. Am J Hematol 2009, 84, 790-794,
doi:10.1002/ajh.21561. https://doi.org/10.1002/ajh.21561 PMid:19890907
- Arun,
V.A.; Soni, D.; Bal, A.; Jain, A. Aggressive Systemic Mastocytosis
Presenting as Rapidly Progressive Ascites, Generalised Lymphadenopathy
and Osteosclerosis. BMJ Case Rep 2021, 14, e238034,
doi:10.1136/bcr-2020-238034. https://doi.org/10.1136/bcr-2020-238034 PMid:33558379
- Barete,
S.; Lortholary, O.; Damaj, G.; Hirsch, I.; Chandesris, M.O.; Elie, C.;
Hamidou, M.; Durieu, I.; Suarez, F.; Grosbois, B.; et al. Long-Term
Efficacy and Safety of Cladribine (2-CdA) in Adult Patients with
Mastocytosis. Blood 2015, 126, 1009-1016; quiz 1050,
doi:10.1182/blood-2014-12-614743. https://doi.org/10.1182/blood-2014-12-614743 PMid:26002962
- Alvarez-Twose,
I.; Matito, A.; Morgado, J.M.; Sánchez-Muñoz, L.; Jara-Acevedo, M.;
García-Montero, A.; Mayado, A.; Caldas, C.; Teodósio, C.;
Muñoz-González, J.I.; et al. Imatinib in Systemic Mastocytosis: A Phase
IV Clinical Trial in Patients Lacking Exon 17 KIT Mutations and Review
of the Literature. Oncotarget 2016, 8, 68950-68963,
doi:10.18632/oncotarget.10711. https://doi.org/10.18632/oncotarget.10711 PMid:28978170 PMCid:PMC5620310
- Gleixner,
K.V.; Mayerhofer, M.; Sonneck, K.; Gruze, A.; Samorapoompichit, P.;
Baumgartner, C.; Lee, F.Y.; Aichberger, K.J.; Manley, P.W.; Fabbro, D.;
et al. Synergistic Growth-Inhibitory Effects of Two Tyrosine Kinase
Inhibitors, Dasatinib and PKC412, on Neoplastic Mast Cells Expressing
the D816V-Mutated Oncogenic Variant of KIT. Haematologica 2007, 92,
1451-1459, doi:10.3324/haematol.11339. https://doi.org/10.3324/haematol.11339 PMid:18024392
- Verstovsek,
S.; Tefferi, A.; Cortes, J.; O'Brien, S.; Garcia-Manero, G.; Pardanani,
A.; Akin, C.; Faderl, S.; Manshouri, T.; Thomas, D.; et al. Phase II
Study of Dasatinib in Philadelphia Chromosome-Negative Acute and
Chronic Myeloid Diseases, Including Systemic Mastocytosis. Clin Cancer
Res 2008, 14, 3906-3915, doi:10.1158/1078-0432.CCR-08-0366. https://doi.org/10.1158/1078-0432.CCR-08-0366 PMid:18559612 PMCid:PMC5018899
- Purtill,
D.; Cooney, J.; Sinniah, R.; Carnley, B.; Cull, G.; Augustson, B.;
Cannell, P. Dasatinib Therapy for Systemic Mastocytosis: Four Cases.
Eur J Haematol 2008, 80, 456-458, doi:10.1111/j.1600-0609.2008.01048.x.
https://doi.org/10.1111/j.1600-0609.2008.01048.x PMid:18284618
- Bibi,
S.; Arock, M. Tyrosine Kinase Inhibition in Mastocytosis: KIT and
Beyond KIT. Immunol Allergy Clin North Am 2018, 38, 527-543,
doi:10.1016/j.iac.2018.04.007. https://doi.org/10.1016/j.iac.2018.04.007 PMid:30007468
- Gotlib,
J.; Kluin-Nelemans, H.C.; George, T.I.; Akin, C.; Sotlar, K.; Hermine,
O.; Awan, F.T.; Hexner, E.; Mauro, M.J.; Sternberg, D.W.; et al.
Efficacy and Safety of Midostaurin in Advanced Systemic Mastocytosis. N
Engl J Med 2016, 374, 2530-2541, doi:10.1056/NEJMoa1513098. https://doi.org/10.1056/NEJMoa1513098 PMid:27355533
- Radia
D, Deininger M, Gotlib J, et al. Avapritinib, a potent and selective
inhibitor of kit d816v, induces complete and durable responses in
patients (pts) with advanced systemic mastocytosis (ADVSM).
- Gotlib,
J.; Baird, J.H.; George, T.I.; Langford, C.; Reyes, I.; Abuel, J.;
Perkins, C.; Schroeder, K.; Bose, P.; Verstovsek, S. A Phase 2 Study of
Brentuximab Vedotin in Patients with CD30-Positive Advanced Systemic
Mastocytosis. Blood Adv 2019, 3, 2264-2271,
doi:10.1182/bloodadvances.2019000152. https://doi.org/10.1182/bloodadvances.2019000152 PMid:31350306 PMCid:PMC6693006
- Lortholary,
O.; Chandesris, M.O.; Livideanu, C.B.; Paul, C.; Guillet, G.; Jassem,
E.; Niedoszytko, M.; Barete, S.; Verstovsek, S.; Grattan, C.; et al.
Masitinib for Treatment of Severely Symptomatic Indolent Systemic
Mastocytosis: A Randomised, Placebo-Controlled, Phase 3 Study. Lancet
2017, 389, 612-620, doi:10.1016/S0140-6736(16)31403-9. https://doi.org/10.1016/S0140-6736(16)31403-9
- Maurer,
M.; Elberink, H.O.; Gotlib, J.; Sabato, V.; Hartmann, K.;
Broesby-Olsen, S.; Castells, M.; Deininger, M.W.; Heaney, M.L.; George,
T.I.; et al. Results from PIONEER: A Randomized, Double-Blind,
Placebo-Controlled, Phase 2 Study of Avapritinib in Patients with
Indolent Systemic Mastocytosis (ISM). Oncology Research and Treatment
2020, 43, 77.
- Escribano, L.; Akin, C.;
Castells, M.; Orfao, A.; Metcalfe, D.D. Mastocytosis: Current Concepts
in Diagnosis and Treatment. Ann. Hematol. 2002, 81, 677-690,
doi:10.1007/s00277-002-0575-z. https://doi.org/10.1007/s00277-002-0575-z PMid:12483363
[TOP]






