Luca Guarnera1, Federico Meconi1, Roberto Secchi1, Maria Rosaria Pascale1, Fabiana Esposito1, Annagiulia Zizzari1, Vito Mario Rapisarda1, Manuela Rizzo1, Livio Pupo1 and Maria Cantonetti1.
1 Hematology, Department of Biomedicine and Prevention, Tor Vergata University, Rome, Italy.
Correspondence to:
Luca Guarnera. Hematology, Department of Biomedicine and Prevention,
Tor Vergata University, Rome, Italy. Tel: 06/20908503. E-mail:
lucaguarnera@live.com
Published: March 1, 2022
Received: September 26, 2021
Accepted: February 6, 2022
Mediterr J Hematol Infect Dis 2022, 14(1): e2022017 DOI
10.4084/MJHID.2022.017
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Gastric Diffuse large B‐cell lymphoma (DLBCL) is the most common
extranodal site of lymphoma's involvement (30%-40% of all extranodal
lymphomas and 55%-65% of all gastrointestinal lymphomas). However,
gastric localizations are also sometimes found in systemic DLBCL.
Gastric complications such as bleeding, perforation, and stenosis under
chemotherapy are well documented.
Methods:
We retrospectively analyzed 15 patients with newly diagnosed DLBCL with
gastrointestinal involvement. Endoscopies were performed in these
patients before and after treatment. Treatment consisted of
cyclophosphamide low-dose pre-phase chemotherapy before
conventional-dose chemotherapy.
Results:
Endoscopy at staging detected ulcers in 12 patients (80%). After
low-dose pre-phase chemotherapy, GI ulcers healed in 91.6% of cases (1
ulcer detected). After the whole treatment (Low-dose pre-phase +
chemotherapy) 9 patients (60%) achieved complete response, 4 patients
(26.6%) partial response, 2 (13,3%) patients presented disease
progression. The most frequent adverse event was neutropenia (73.3%);
the most frequent non-hematological adverse event was transaminases
elevation (20%).
Conclusion:
Cyclophosphamide low-dose pre-phase chemotherapy resulted in a safe and
effective way to prevent adverse events in systemic DLBCL with
gastrointestinal involvement.
|
Introduction
Diffuse
large B‐cell lymphoma (DLBCL) is the most common subtype of aggressive
non‐Hodgkin Lymphoma (NHL), accounting for about 40% of all NHLs.[1]
Primary gastric DLBCL (PG-DLBCL) is NHL's most common extranodal site
(30%-40% of all extranodal lymphomas and 55%-65% of all
gastrointestinal lymphomas).[2] Primary gastric
lymphoma is a rare tumor, with an incidence of 4% to 20% of NHL and
approximately 5% of primary gastric neoplasms.[3] The small intestine and ileocecal regions follow in frequency.[4]
However, gastric localizations are also sometimes found in systemic
DLBCL. Nowadays, R-CHOP (Rituximab, Cyclophosphamide, Doxorubicin,
Vincristine, and Prednisone) has been established as the first‐line
treatment for DLBCL.[5] The current standard therapy for DLBCL with gastric lesions is six to eight cycles of R‐CHOP.[6]
It is now well documented that gastric complications such as bleeding,
perforation, and stenosis can occur under chemotherapy.[7]
For this reason, several strategies have been used to minimize adverse
events, like fractioned chemotherapy or pre-phase chemotherapy, with
positive results.[8-9]
Given the recent evidence highlighting differences between systemic DLBCL and PG-DLBCL on histological and prognostic levels,[10-11]
we decided to review newly diagnosed patients with DLBCL with
gastrointestinal (GI) involvement treated with Cyclophosphamide
low-dose pre-phase chemotherapy at our institution to verify if the
strategies used for PG-DLBCL could have also been applied to systemic
DLBCL with GI localizations.
Methods
We
retrospectively analyzed newly diagnosed patients with DLBCL with
secondary GI involvement in Policlinico Tor Vergata, Rome,
between February 2016 and April 2020. Patients with PG-DLBCL were
excluded. All patients with DLBCL and concurrent extranodal GI lesions,
highlighted with CT-PET scan and histologically diagnosed, were
considered. Endoscopies (Esophagogastroduodenoscopy if uptake at
gastric level, Rectocolonsigmoidoscopy if uptake at colon level) were
performed in these patients before and after treatment to carry out
biopsies for histological exams and to evaluate the presence of ulcers
or mucosal alterations. Fluorescence in situ hybridization (FISH) for
MYC/BCL2 and BCL6 translocations were performed on Paraffin-embedded
tissue with the dual-color break-apart FISH assay.
Blood chemistry
tests were performed at diagnosis, inflammatory indices were dosed, and
Helicobacter Pylori (HP) infection was investigated.
Statistical
analysis was performed through IBM SPSS Statistics 27 (IBM Corp. in
Armonk, NY). Mann-Whitney U test was used to compare variables. Cut-off
of statistical significance was set at p <0.05.
Prior to treatment, patients signed informed consent.
Treatment.
All treatments were carried out in our institution. The patients
enrolled received a low-dose pre-phase therapy before conventional-dose
chemotherapy (CT). Before treatment HBV, HCV, and HIV status was
studied. None of the patients presented viral infections.
Low-dose pre-phase chemotherapy consisted of Cyclophosphamide 0.2 g intravenously (IV) on days 1, 3, 5, 7, and 9.
Endoscopies
were then performed again to reassess the state of mucosa and the
possible presence or evolution of ulcers at a minimum of 48 hours from
the last Cyclophosphamide dose.
Conventional-dose CT consisted of R-CHOP, six to eight cycles.
Primary
Granulocyte Colony Stimulating Factor (G-CSF) prophylaxis was used in
patients with age > 65 years and/or renal/liver dysfunctions and/or
open wounds and/or bone marrow involvement and/or with high infection
risk.
HP eradication treatment was given to all patients positive for HP infection as determined by the 14C-Urea breath test or histological features.
Treatment response was assessed according to Lugano criteria.[12]
Results
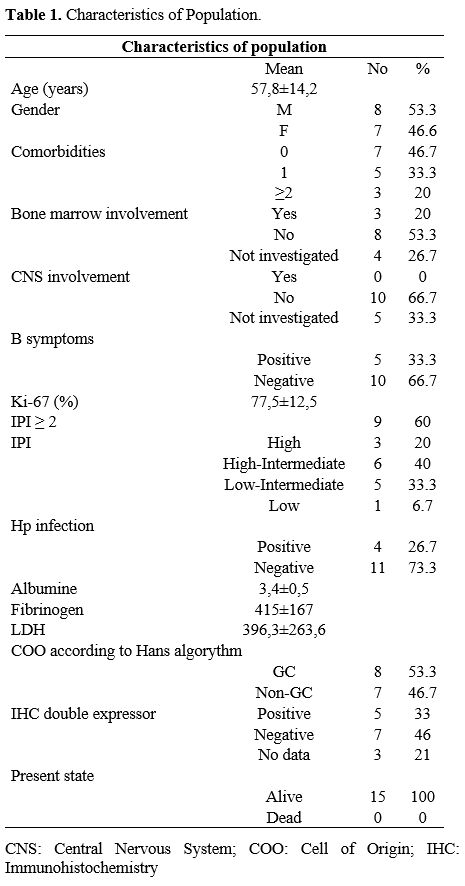
Demographics/Staging. Major patient characteristics are shown in table 1.
The population consisted of 15 patients. The mean age was 57.8 years
(range 27-75 years), 53.3% male, 46.6% female. Seven patients did not
have any comorbidity, five patients had one comorbidity, three patients
had at least two comorbidities. B symptoms were experienced by 33.3% of
patients. Mean Ki67 expression was 77.5% (range 60%-95%). All patients
presented with stage IV of Ann-Arbor classification (Gastrointestinal
involvement al staging PET-TC scan). No patients had a history of prior
anti-lymphoma therapies or hematological diagnoses (including low-grade
lymphomas and subsequent transformation). International Prognostic
Index (IPI) score was high in 20% of patients, high-intermediate in 40%
of patients, low-intermediate in 33.3%, low in 6.6%.[13]
Central Nervous System-IPI (CNS-IPI) was also calculated in every
patient: 10 of them had high risk CNS-IPI. Diagnostic Lumbar Puncture
was performed in every high-risk patient and resulted in negative in
all cases.
 |
Table
1. Characteristics of Population.
|
Eleven patients received bone marrow biopsy; in 3 (20%), bone marrow involvement was documented.
Mean
LDH was 396.3 UI/L (range 98-910 UI/L). Serum albumin was 3.4 g/dL
(range 2.2-4.38 g/dL). 4 patients were positive for HP infection.
Imaging studies. Staging CT-PET was performed in all patients. Results are summarized in table 2.
All patients presented at least another site of pathologic uptake and
GI involvement; uptaking tissues, in the absence of other possible
causes, were considered to be referred to DLBCL involvement even in the
absence of histological exam. The most common sites were
lymphadenopathies, detected in all patients.
Pathology findings.
8 patients presented Germinal Centre DLBCL (GC-DLBCL) (53.3%), and 7
presented non-Germinal Centre DLBCL (46.6%) (NGC-DLBCL), by Hans'
algorithm.[14]
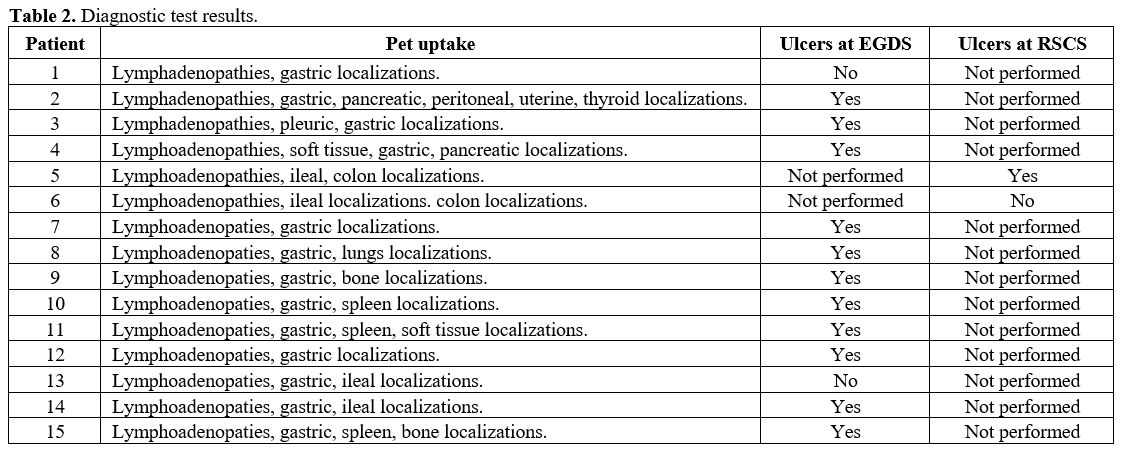 |
Table 2. Diagnostic test results.
|
Six
patients (40%) were affected by "double expressor" lymphoma (Positive
Immunohistochemistry for MYC and BCL2). BCL2 was expressed in 9 cases
(60%), whereas MYC was expressed in 7 patients (46.6%).
Complete
immunohistochemistry data were not available in 4 of 15 patients. In 7
out of 15, FISH was performed searching BCL2, BCL6, and MYC
translocation. None of them resulted translocated. In cases where FISH
was not performed, three patients were classified as GC-DLBCL by Hans'
algorithm and MYC by immunohistochemistry was performed in 4 of them (3
positives, 75%).
Endoscopic findings. Esophagogastroduodenoscopy (EGDS) was performed in all patients and detected ulcers in 11 of them (73.3%) (Figure 1). In patients who did not have ulcers, gastric involvement was supposed by PET positivity and confirmed by biopsy (Table 2).
In these patients (2), EGDS showed gastric erosions, and in 1 of them,
mucosal thickening. Rectocolonsigmoidoscopy (RSCS) was performed in 2
patients due to PET positivity and detected colon ulcers in 1 one of
them (6.6%); performed biopsies confirmed the lesions as lymphoma
localizations (Table 2).
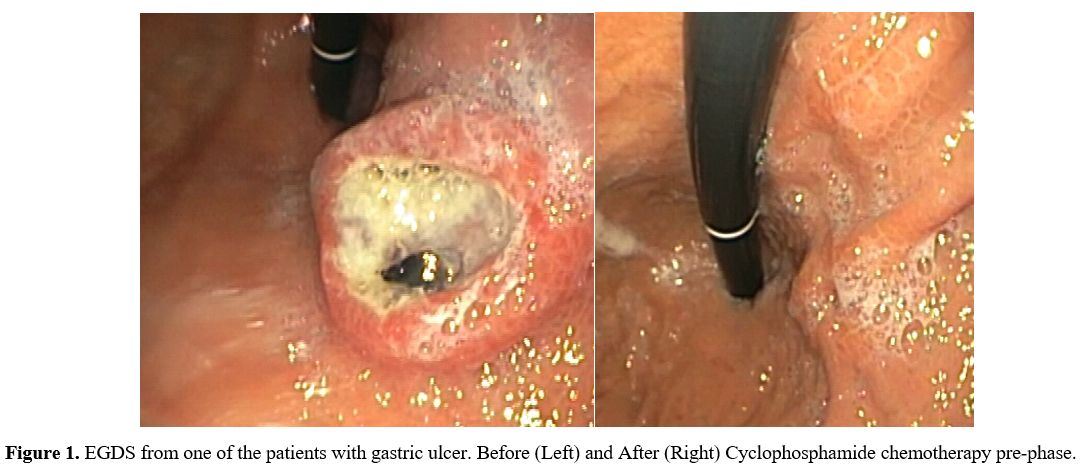 |
Figure 1. EGDS from one of
the patients with gastric ulcer. Before (Left) and After (Right)
Cyclophosphamide chemotherapy pre-phase.
|
After low-dose pre-phase chemotherapy, GI ulcers healed in 91.6% of cases (1 ulcer detected) (Table 3).
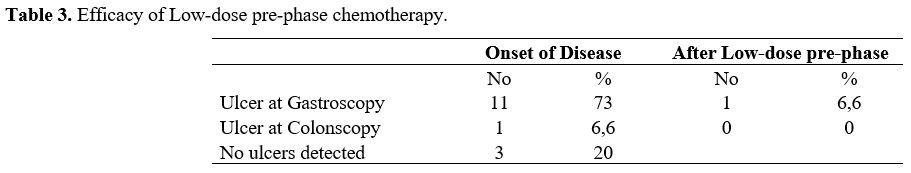 |
Table 3. Efficacy of Low-dose pre-phase chemotherapy.
|
During low-dose pre-phase chemotherapy, one patient, the only one with colon ulcers, presented rectorrhagia.
Therapy. Response rates to the whole treatment (Low-dose pre-phase + R-CHOP) are shown in Table 4.
9 patients (60%) achieved complete response, 4 patient (26.6%) partial
response, 2 (13,3%) patients presented progression of the disease.
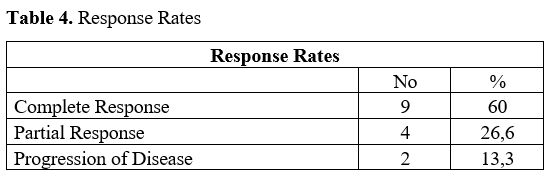 |
Table 4. Response Rates.
|
Endoscopies
were repeated at the end of R-CHOP treatment. All resulted negative for
DLBCL involvement. Non/partial responders' GI mucosal involvement was
not detected.
There were no significant differences between
complete responders and non/partial responders for age (p=0.5), cell of
origin according to Hans algorithm (p=0.5), fibrinogen at onset
(p=0.7), LDH at onset (p=1), albumin at onset (p=0.6), ki67 (p=0.8),
IPI score (p=0.8) and number of involved sites (p=0.8).
No adverse effects or particular toxicity were observed during the cyclophosphamide low-dose pre-phase. Table 5
summarizes the whole pre-phase and chemotherapy-related toxicities. No
treatment was delayed because of toxicities. The most frequent adverse
event was neutropenia (73.3%); the most frequent non-hematological
event adverse was transaminases elevation (20%).
All patients are still alive. The mean time of follow-up was 35.6 months (median 31 months).
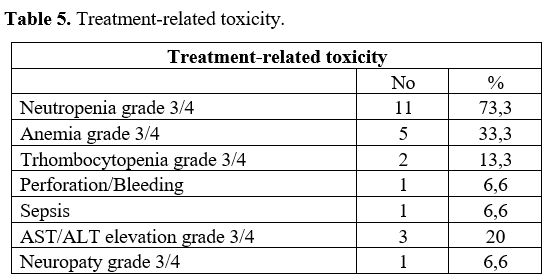 |
Table 5. Treatment-related toxicity.
|
Discussion
GI
tract is the most common extranodal site involved in NHL, especially in
DLBCL, the most frequent histotype among aggressive hematological
malignancies of the gastrointestinal tract.[15]
GI
involvement in systemic DLBCL is a negative prognostic factor for the
risk of bleeding, perforation, or stenosis (risk reported between 6.2%
and 43% by different authors) and for the patient's impossibility to
feed effectively, resulting in defedation and worsening of performance
status.[16,17]
Therefore, it is evident the importance of treating these forms safely, rapidly, and effectively.
Cui
et al. described a treatment strategy using a low-dose pre-phase
chemotherapy (Cyclophosphamide 0.2 g and Vincristine 1 mg intravenously
twice a week for 2-4 weeks) in patients with PG-DLBCL and a stomach
ulcer. After the pre-phase, the patients underwent conventional-dose
chemotherapy. Compared to cases treated in the same center with only
conventional-dose chemotherapy, this strategy proved safer, effective
on the ulcers (85.7% of ulcer healing), and better response and overall
survival outcomes.[8]
Even if the experience
described by Cui et al. is the only pre-phase chemotherapy strategy we
found in the literature, it is not easy to compare it with ours.
Indeed, we utilized only Cyclophophamide with a different schedule, the
patients treated in our institution were all stage IV of Ann Arbor
classification with a likely higher IPI score (Median IPI 2.6 ± 1 vs.
IPI ≤ 2 in 71.4% of patients), and not all the patients presented ulcer
at the diagnosis (Pre-phase chemotherapy was also performed in a
patient with gastrointestinal involvement at PET-TC scan without mucosa
lesions).
Ann Arbor stage is an independent prognostic factor and
is included in IPI as a prognostic index of outcome and, as Cui et al.
highlighted, of bleeding and/or perforation in gastric lymphomas
treated with chemotherapy.[8,9]
Despite the high
mean IPI (2.6), only one of our patients experienced mild, non-lethal
rectorrhagia, and the ulcer healing rate was 91.6%.
Furthermore, the complete response rate was 60%, with an overall response of 86.6% (Table 4).
These data are in line with response rates reported by the
International Non-Hodgkin's Lymphoma Prognostic Factors Project (67%
complete response rate in IPI 2, 55% in IPI 3. Mean IPI of our
populations (2.6) and, more recently, by Nowakowski and Czuczman, who
report 40% of patients with refractory disease or disease relapsing
after an initial response, with important differences between DLBCL
molecular subtypes.[9,18] In this
regard, about half of our patients presented GC-DLBCL (53.3%) while the
other half NGC-DLBCL (46.6%), in line with the findings of Nagakita et
al. who examined 49 primary gastrointestinal DLBCL, half of which (49
%) was non‐GCB‐like phenotype by Hans' algorithm.[19]
The
most frequent treatment-related toxicity in the present study was
neutropenia, which occurred more frequently than in the study of Cui et
al. (73.3% vs. 60.7%). The most frequent non-hematological adverse
event was transaminases elevation, which occurred more frequently than
in the study of Cui et al. (20% vs. 7.1%) (Table 5).[8]
Myelosuppression, and in particular neutropenia, is a common adverse effect in patients treated with R-CHOP.[20]
The higher incidence of neutropenia in our study probably relies on the
different number of cycles of conventional chemotherapy after the
low-dose pre-phase (six to eight in our protocol vs. four to six). The
high incidence in both the studies of transaminases elevation (20% and
7.1%) is probably due to the hepatotoxic effect of Cyclophosphamide,
used in more massive dosages than conventional chemotherapy.[21]
Conclusions
Our
experience gives evidence that low-dose pre-phase chemotherapy could be
a safe and effective way to prevent adverse events not only in G-DLBCL,
as Cui et al. highlighted, but also in systemic DLBCL with
gastrointestinal involvement.[8]
High IPI confirms to be a useful tool to predict adverse events in DLBCL gastrointestinal involvement.
Issues
still pending are the best drug or combination of drugs to use in
pre-phase chemotherapy, the most appropriate schedule, and if PET-TC
uptake without mucosa lesions constitutes a real risk of adverse events.
Thus, more studies on a larger scale are necessary to clarify these aspects.
Compliance with Ethical Standards
Disclosure of potential conflicts of interest:
The authors did not receive support from any organization for the
submitted work. The authors have no relevant financial or non-financial
interests to disclose.
Ethics Approval:
All procedures performed in studies involving human participants were
in accordance with the ethical standards of the institutional and/or
national research committee and with the 1964 Helsinki declaration and
its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Funding
"Volontari per Policlinico Tor Vergata" Association.
Acknowledgement
The authors thank "Volontari per Policlinico Tor Vergata" Association.
References
- Morton, L. M. et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood (2006). https://doi.org/10.1182/blood-2005-06-2508 PMid:16150940 PMCid:PMC1895348
- Ghimire, P., Wu, G. Y. & Zhu, L. Primary gastrointestinal lymphoma. World J. Gastroenterol. (2011). https://doi.org/10.3748/wjg.v17.i6.697 PMid:21390139 PMCid:PMC3042647
- Al-Akwaa, A. M., Siddiqui, N. & Al-Mofleh, I. A. Primary gastric lymphoma. World Journal of Gastroenterology (2004). https://doi.org/10.3748/wjg.v10.i1.5 PMid:14695759 PMCid:PMC4717077
- Herrmann,
R., Panahon, A. M., Barcos, M. P., Walsh, D. & Stutzman, L.
Gastrointestinal involvement in non-Hodgkin's lymphoma. Cancer (1980). https://doi.org/10.1002/1097-0142(19800701)46:1<215::AID-CNCR2820460136>3.0.CO;2-6
- Coiffier,
B. et al. Long-term outcome of patients in the LNH-98.5 trial, the
first randomized study comparing rituximab-CHOP to standard CHOP
chemotherapy in DLBCL patients: A study by the Groupe d'Etudes des
Lymphomes de l'Adulte. Blood (2010). https://doi.org/10.1182/blood-2010-03-276246 PMid:20548096 PMCid:PMC2951853
- Sohn,
B. S. et al. The comparison between CHOP and R-CHOP in primary gastric
diffuse large B cell lymphoma. Ann. Hematol. (2012). https://doi.org/10.1007/s00277-012-1512-4 PMid:22752193
- Spectre,
G. et al. Bleeding, obstruction, and perforation in a series of
patients with aggressive gastric lymphoma treated with primary
chemotherapy. Ann. Surg. Oncol. (2006). https://doi.org/10.1245/s10434-006-9069-x PMid:17009162
- Cui,
Y. et al. Safety and efficacy of low-dose pre-phase before
conventional-dose chemotherapy for ulcerative gastric diffuse large
B-cell lymphoma. Leuk. Lymphoma (2015). https://doi.org/10.3109/10428194.2015.1014366 PMid:25676238
- Liu
Y, Liu Y, Zhao P, et al. Switching Fractioned R-CHOP Cycles to Standard
R-CHOP Cycles Guided by Endoscopic Ultrasonography in Treating Patients
with Primary Gastric Diffuse Large B-Cell Lymphoma. Cancer Manag Res.
2020;12:5041-5048. https://doi.org/10.2147/CMAR.S260974 PMid:32612391 PMCid:PMC7323805
- Magnoli
F, Bernasconi B, Vivian L et al. Primary extranodal diffuse large
B-cell lymphomas: Many sites, many entities? Clinico-pathological,
immunohistochemical and cytogenetic study of 106 cases. Cancer Genet.
2018 Dec;228-229:28-40. https://doi.org/10.1016/j.cancergen.2018.08.001 PMid:30553470
- Ollila
TA, Olszewski AJ. Extranodal Diffuse Large B Cell Lymphoma: Molecular
Features, Prognosis, and Risk of Central Nervous System Recurrence.
Curr Treat Options Oncol. 2018 Jun 21;19(8):38. https://doi.org/10.1007/s11864-018-0555-8 PMid:29931605 PMCid:PMC6294323
- Cheson
BD, Fisher RI, Barrington SF et al.; Recommendations for initial
evaluation, staging, and response assessment of Hodgkin and non-Hodgkin
lymphoma: the Lugano classification. J Clin Oncol. 2014 Sep
20;32(27):3059-68. https://doi.org/10.1200/JCO.2013.54.8800 PMid:25113753 PMCid:PMC4979083
- International
Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model
for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993 Sep
30;329(14):987-94. https://doi.org/10.1056/NEJM199309303291402 PMid:8141877
- Hans
CP, Weisenburger DD, Greiner TC et al. Confirmation of the molecular
classification of diffuse large B-cell lymphoma by immunohistochemistry
using a tissue microarray. Blood. 2004 Jan 1;103(1):275-82. https://doi.org/10.1182/blood-2003-05-1545 PMid:14504078
- Bautista-Quach
MA, Ake CD, Chen M, Wang J. Gastrointestinal lymphomas: Morphology,
immunophenotype and molecular features. J Gastrointest Oncol.
2012;3(3):209-225.
- List AF, Greer JP,
Cousar JC et al. Non-Hodgkin's lymphoma of the gastrointestinal tract:
an analysis of clinical and pathologic features affecting outcome. J
Clin Oncol. 1988 Jul;6(7):1125-33. https://doi.org/10.1200/JCO.1988.6.7.1125 PMid:3392561
- Spectre
G, Libster D, Grisariu S, Da'as N, Yehuda DB, Gimmon Z, Paltiel O.
Bleeding, obstruction, and perforation in a series of patients with
aggressive gastric lymphoma treated with primary chemotherapy. Ann Surg
Oncol. 2006 Nov;13(11):1372-8. https://doi.org/10.1245/s10434-006-9069-x PMid:17009162
- Nowakowski
GS, Czuczman MS. ABC, GCB, and Double-Hit Diffuse Large B-Cell
Lymphoma: Does Subtype Make a Difference in Therapy Selection? Am Soc
Clin Oncol Educ Book. 2015:e449-57. https://doi.org/10.14694/EdBook_AM.2015.35.e449 PMid:25993209
- Nagakita
K, Takata K, Taniguchi K et al. Clinicopathological features of 49
primary gastrointestinal diffuse large B-cell lymphoma cases;
comparison with location, cell-of-origin, and frequency of MYD88 L265P.
Pathol Int. 2016 Aug;66(8):444-52. https://doi.org/10.1111/pin.12439 PMid:27439595
- Sehn
LH, Martelli M, Trněný M et al. U. A randomized, open-label, Phase III
study of obinutuzumab or rituximab plus CHOP in patients with
previously untreated diffuse large B-Cell lymphoma: final analysis of
GOYA. J Hematol Oncol. 2020 Jun 6;13(1):71. https://doi.org/10.1186/s13045-020-00900-7 PMid:32505213 PMCid:PMC7276080
- Cengiz
M, Cetik Yildiz S, Demir C, Şahin İK, Teksoy Ö, Ayhanci A.
Hepato-preventive and anti-apoptotic role of boric acid against liver
injury induced by Cyclophosphamide. J Trace Elem Med Biol. 2019
May;53:1-7. doi: 10.1016/j.jtemb.2019.01.013. Epub 2019 Jan 23. https://doi.org/10.1016/j.jtemb.2019.01.013 PMid:30910191
[TOP]





