Viviane Lamim Lovatel1, Luize Otero1, Ercole Pietro Orlando2,3, Claudia Diniz4, Monica Kopischitz Praxedes Lusis3 and Teresa de Souza Fernandez1.
1 Cytogenetic Laboratory, Bone Marrow Transplantation Center (CEMO), National Cancer Institute (INCA), Rio de Janeiro, Brazil.
2 Outpatient Department, Bone Marrow Transplantation Center (CEMO), National Cancer Institute (INCA), Rio de Janeiro, RJ, Brazil.
3 Hematology Department, Antônio Pedro University Hospital (HUAP), RJ, Brazil.
4 Immunology Laboratory, Bone Marrow Transplantation Center (CEMO), National Cancer Institute (INCA), Rio de Janeiro, Brazil.
Correspondence to:
Teresa de Souza Fernandez. Instituto Nacional de Câncer (INCA). Centro
de Transplante de Medula Óssea (CEMO), Laboratório de Citogenética.
Praça Cruz Vermelha no23, 6º andar. Centro. Rio de Janeiro. RJ. Brasil.
CEP: 20230-130. Phone: +55 21 3207-1701. e-mail:
teresafernandez@inca.gov.br
Published: January 1, 2022
Received: September 29, 2021
Accepted: December 18, 2021
Mediterr J Hematol Infect Dis 2022, 14(1): e2022013 DOI
10.4084/MJHID.2022.013
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
To the editor
Myelodysplastic
syndrome (MDS) comprises a heterogeneous group of clonal stem cell
disorders, characterized by ineffective hematopoiesis, bone marrow (BM)
dysplasias, peripheral blood (PB) cytopenias and increased risk of
evolution to acute myeloid leukemia (AML). MDS occurs mainly in elderly
patients. Reviewing the literature, we notice that chromosomal
translocations are rare cytogenetic abnormalities in de novo
MDS. Therefore, their reporting is essential for identifying new
cytogenetic prognostic risk groups and pointing out genes possibly
involved in the leukemic transformation.[1-3] Here, we describe a yet unreported t(11;16)(q23;q24) with KMT2A rearrangement (KMT2A-r) in a young adult patient with de novo MDS associated with evolution to AML and a poor prognosis.
A
34-year-old male was admitted to National Cancer Institute, Rio de
Janeiro in October 2017 with pancytopenia. The myelogram and BM biopsy
showed hypercellularity, myeloid hyperplasia with dysplastic cells and
10% of blasts. The diagnosis was MDS with an excess of blasts-2
(MDS-EB-2) according to WHO classification.[3] The
patient was indicated to the treatment with a hypomethylating agent,
but this drug was not available at the institution at that time. The
patient had blood transfusion support and initiated treatment with
recombinant erythropoietin (EPO) combined with folic acid, with no
response. In May 2018, the myelogram and BM biopsy showed dysplastic
cells and maintained 10% of myeloblasts (Figure 1A-C).
Immunophenotyping showed 7.7% of myeloid blast cells expressing medium
intensity CD45 and CD117+/HLA-DR+/CD34+/CD38+/CD13+ and dysplastic
cells (Figure 1D-H). These results confirmed the previous diagnosis of MDS-EB-2.[3] Cytogenetic analysis of BM cells by G-banding showed: 46,XY,t(11;16)(q23;q24)[5]/46,XY[20] (Figure 1I).
Fluorescence in situ hybridization (FISH) was performed using LSI MLL
dual color, break apart rearrangement probe (Vysis, Abbott, USA). FISH
analysis showed one allele with a split signal indicating the KMT2A-r (Figure 1J).
Additionally, the reciprocal translocation was confirmed by using a
whole chromosome painting (WCP) probe for chromosome 16 (Vysis, Abbott,
USA). No DNMT3A mutations were identified within exons 19, 20, 21, 22, and 23.[4]
Allogeneic hematopoietic stem cell transplantation (HSCT) was
indicated, but no donor was found. Decitabine was initiated with 20 mg/m2/day
intravenous in 1 hour for 5 consecutive days, with cycles of 28/28
days. In November 2018, two months after the fifth cycle of Decitabine,
the patient showed improved anemia and thrombocytopenia, although
severely neutropenic. In May 2019, the PB count showed: Hb 10 g/dL,
platelets 30.000/mm3, 2.000 WBC/mm3, 2% of blasts. The BM immunophenotype showed 5% of myeloid blast cells. The FISH analysis demonstrated KMT2A-r
in BM cells. Three months later, immunophenotyping of PB showed 22% of
myeloid blast cells, characterizing the evolution from MDS to AML. The
patient was hospitalized and underwent remission-inducing chemotherapy
for AML (Cytarabine 100 mg/m2/EV/day in continuous infusion for seven consecutive days and Daunorubicin at a dose of 60 mg/m2/day EV on days D1/D2/D3). In February 2020, he was submitted to intensification chemotherapy [Cytarabine 1.5g/m2/EV/12/12-hours on days D1/D3 and D5, totaling six doses of Cytarabine (2nd
intensification cycle)]. No blast cells were observed at PB, but the
patient evolved with gradual severe cytopenias. Until April 2020, there
was no BM recovery. In June 2020, blood counts revealed an expansion of
the leukemic clone, presenting more than 50% of blasts in PB. Treatment
with subcutaneous Cytarabine was initiated (200 to 300 mg/week), with
no hematological response. Despite all efforts, the patient presented
bleeding from the central nervous system in August 2020, secondary to
treatment-refractory hyperleukocytosis and central leukocytosis,
evolving to death. The summary of the steps showing the evolution from
MDS to AML is described in Figure 2.
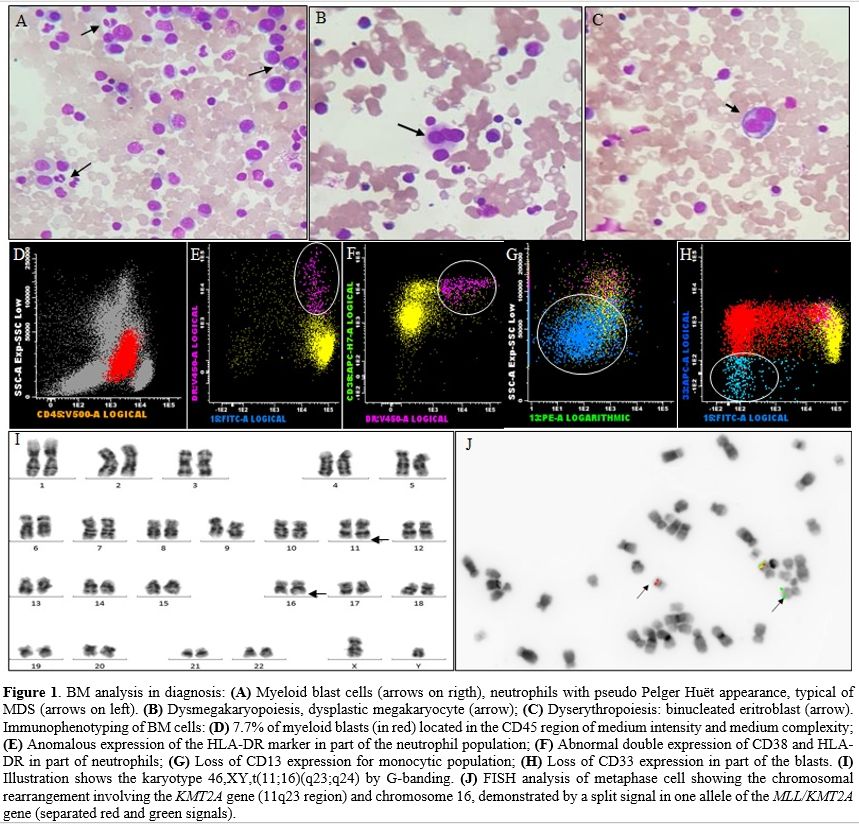 |
Figure 1. BM analysis in diagnosis: (A) Myeloid blast cells (arrows on rigth), neutrophils with pseudo Pelger Huët appearance, typical of MDS (arrows on left). (B) Dysmegakaryopoiesis, dysplastic megakaryocyte (arrow); (C) Dyserythropoiesis: binucleated eritroblast (arrow). Immunophenotyping of BM cells: (D) 7.7% of myeloid blasts (in red) located in the CD45 region of medium intensity and medium complexity; (E) Anomalous expression of the HLA-DR marker in part of the neutrophil population; (F) Abnormal double expression of CD38 and HLA-DR in part of neutrophils; (G) Loss of CD13 expression for monocytic population; (H) Loss of CD33 expression in part of the blasts. (I) Illustration shows the karyotype 46,XY,t(11;16)(q23;q24) by G-banding. (J) FISH analysis of metaphase cell showing the chromosomal rearrangement involving the KMT2A gene (11q23 region) and chromosome 16, demonstrated by a split signal in one allele of the MLL/KMT2A gene (separated red and green signals).
|
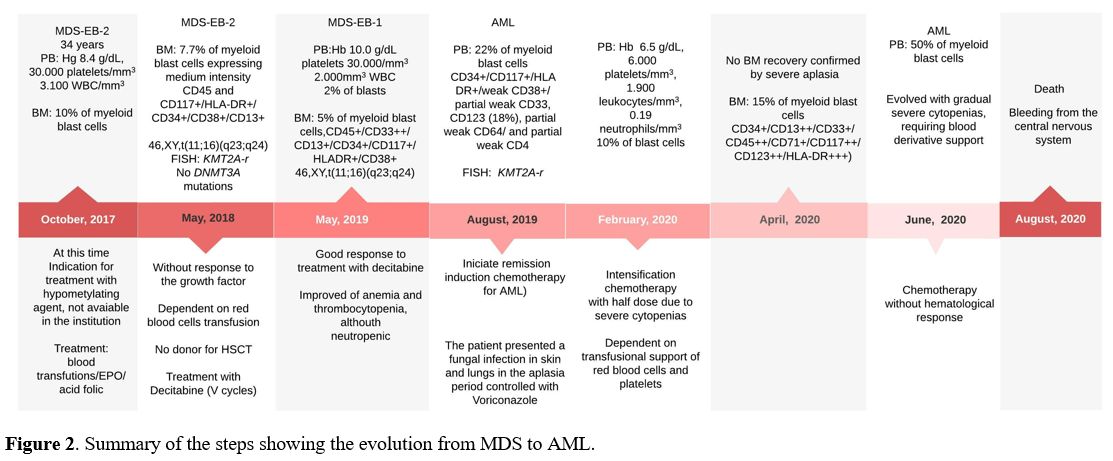 |
Figure 2. Summary of the steps showing the evolution from MDS to AML. |
In
MDS, chromosomal translocations as sole chromosomal abnormality
involving chromosome 11 occur in approximately 0.2% of patients.[1] Due to the low number of patients showing 11q23/KMT2A
translocations in MDS, their real prognostic impact is unknown, and
patients with this genetic alteration are assigned to the
intermediate-risk group in IPSS-R.[1,2] Our report describes the clinical outcome in a young adult patient with de novo MDS showing t(11;16)(q23;q24) with KMT2A-r.
Considering the age of our patient, some studies in MDS (mainly
associated with HSCT) had, in their cohort, patients between 18 and 55
years, therefore including young adult patients.[5] In our patient, the diagnosis of MDS-EB-2 was made according to the criteria of WHO classification.[3]
The immunophenotyping analysis showed 7.7% of blasts and dysplastic
features in the neutrophil population, monocytic and loss of CD33 in
blast cells, characteristics not observed in AML, even with a slower
dynamics course.[3] A broad scientific review showed that only four cases of acute leukemia with t(11;16)(q23;q24) had been described so far.[6-9]
The patients described with t(11;16)(q23;q24) were associated with
leukemia relapse and refractoriness to treatment. In the present study,
the patient also had a poor clinical outcome, progressing from MDS to
AML and refractoriness to treatment. It is important to note that the
hypomethylating agents(HMA) have been considered the standard of care
for MDS patients. However, HMA is not curative. Response to these drugs
occurs in approximately 50% of patients, and the duration of response
is transient because HMAs do not eradicate neoplastic clones.[10] HSCT remains the only possible curative option for MDS patients.[5,10] This study and literature review highlight the importance of cytogenetics, molecular tests, and clinical follow-up of de novo
MDS patients to identify new prognostic risk groups and research new
therapeutic drugs for these patients. Since this study was the
first that described the t(11;16) with KMT2A-r associated
with AML evolution and poor prognosis in a patient with MDS, it is
necessary new studies involving a more number of patients to provide a
better understanding of t(11;16) with KMT2A-r involved in MDS pathogenesis and its prognosis impact.
Acknowledgements
This
study was supported by the Brazilian Ministry of Health (National
Institute of Cancer/INCA, Brazil) and Conselho Nacional de
Desenvolvimento Científico e Tecnológico (CNPq). The authors thank
Eliana Abdelhay, Elaiza Kós, Filipe Vicente dos Santos-Bueno and Bruno
Almeida Lopes for their technical support.
Authorship and Disclosures
VLL
and TSF designed the study and wrote the paper; LO performed the
conventional cytogenetics, VLL performed the FISH analysis and the
molecular tests; EPO and MKPL attended the patient and collected
clinical data; CD performed the immunophenotyping; TSF supervised and
reviewed the manuscript. All authors contributed significantly to the
work, seeing and approving the manuscript and its submission. The
authors declare that they have no competing interests.
Ethics Approval and Consent to Participate
Informed
consent was obtained from the case in accordance with the Declaration
of Helsinki and this study was approved by the Ethics and Research
Committee of National Cancer Institute (reference number # 3401739).
References
- Bacher U, Schanz J, Braulke F, Haase D. Rare
cytogenetic abnormalities in myelodysplastic syndromes. Mediterr J
Hematol Infect Dis. 2015; 7: e2015034. https://doi.org/10.4084/MJHID.2015.034
- Greenberg
PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F, et al.
Revised international prognostic scoring system for myelodysplastic
syndromes. Blood 2012; 120:2454-2465. https://doi.org/10.1182/blood-2012-03-420489
- Arber
DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM,
Bloomfield CD, Cazzola M, Vardiman JW. The 2016 revision to the World
Health Organization classification of myeloid neoplasms and acute
leukemia. Blood 2016; 19:127:2391-405. https://doi.org/10.1182/blood-2016-03-643544
- Pezzi
A, Moraes L, Valim V, Amorin B, Melchiades G, Oliveira F, et al. DNMT3A
Mutations in Patients with Acute Myeloid Leukemia in South Brazil. Adv
Hematol. 2012; 2012: 697691. https://doi.org/10.1155/2012/697691
- Warlick
ED, Cioc A, Defor T, Dolan M, Weisdorf D. Allogeneic stem cell
transplantation for adults with myelodysplastic syndromes: importance
of pretransplant disease burden. Biol Blood Marrow Transplant. 2009;
15:30-8. https://doi.org/10.1016/j.bbmt.2008.10.012
- Kwong
YL, Tso SC, Wong KF, Tang KC, Chan TK. Translocational rearrangements
of 11q23 in acute monoblastic leukemia. Cancer Genet Cytogenet 1995;
82:76-79. https://doi.org/10.1016/0165-4608(94)00127-W
- Heerema
NA, Sather HN, Ge J, Arthur DC, Hilden JM, Trigg ME, et al. Cytogenetic
studies of infant acute lymphoblastic leukemia: poor prognosis of
infants with t(4;11) - a report of the Children's Cancer Group.
Leukemia 1999; 13:679-686. https://doi.org/10.1038/sj.leu.2401413
- Rubnitz
JE, Raimondi SC, Tong X, Srivastava DK, Razzouk BI, Shurtleff SA, et
al. Favorable impact of the t(9;11) in childhood acute myeloid
leukemia. J Clin Oncol 2002; 20:2302-2309. https://doi.org/10.1200/JCO.2002.08.023
- Zerkalenkova
E, Lebedeva S, Kazakova A, Baryshev P, Meyer C, Marschalek R, et al. A
case of pediatric acute myeloid leukemia with t(11;16)(q23;q24) leading
to a novel KMT2A-USP10 fusion gene. Genes, Chromosomes and Cancer 2018;
57:522-524. https://doi.org/10.1002/gcc.22646
- Santini V. How I treat MDS after hypomethylation agent failure. Blood 2019; 133:521-529. https://doi.org/10.1182/blood-2018-03-785915
[TOP]

