Farah Qasem1, A'sem Abu-Qamar1, Batool Aqel1, Rand Aladayleh1, Ilham Alteerah R.1, Ahmad Magableh4, Hisham Bawa" neh4, Feras Al-fararjeh1,2 and Abdalla Awidi1,2,3.
1 Medical School, The University of Jordan, Amman, Jordan.
2 Jordan University Hospital, Amman, Jordan.
3 Cell Therapy Center, The University of Jordan, Amman, Jordan.
4 Al-Basheer Hospital, Ministry of Health, Amman, Jordan.
Published: May 1, 2022
Received: October 9, 2021
Accepted: April 4, 2022
Mediterr J Hematol Infect Dis 2022, 14(1): e2022031 DOI
10.4084/MJHID.2022.031
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background and objective:
Very scanty reports from the middle east and north Africa (MENA) region
have been published on multiple myeloma (MM). Multiple myeloma registry
has been established at Jordan University Hospital (JUH) since 2009. In
this work we aim to review Multiple Myeloma registry with data from 113
patients who were diagnosed with MM at JUH and analyze their management
and course.
Methods: This
is a non-interventional, and retrospective analysis of MM registry from
2009-2016 involving 113 patients at JUH. Statistical analysis was done
using the Statistical Package for the Social Sciences (SPSS). Overall
survival (OS) was analyzed with the Kaplan-Meier method. P value was
considered significant if it was (<0.05).
Results:
We found no gender difference in this registry. The median age is 62
years. Most patients are ISS stage II and III (36.28% for each).
Immunoglobulin type G Kappa is the dominant subtype. Bone pain is the
most common presenting symptom. The most common laboratory finding is
anemia (45.6%). Most of our patients (85.2%) had received thalidomide
and dexamethasone, while only 14.8% received bortezomib, thalidomide,
and dexamethasone.
The
mean overall survival (OS) in our patients was 74 months, and the
median survival was 38 months. Median OS for ISS stage I, II, and III
were 96, 46, and 16 months respectively.
Conclusion:
MM in a developing country presents a challenging disease compared with
that in industrial countries in both the epidemiology and management.
An improved road map in the care of MM in these countries is needed.
The use of three or four drug combination upfront is warranted.
However, this is limited because of the high cost of these drugs. We
expect the following decade to show better survival and quality of life
for MM patients once these drugs are widely used.
|
Introduction
Multiple myeloma accounts for 13-15% of all hematological malignancies, the second most common after non-Hodgkin lymphoma.[1]
It is usually a disease of older people, and its incidence is variable
by geographic area, being most common in developed countries where the
incidence is increasing with age. USA data show that it is more
frequent in males and black people.[2] In industrial
countries, the median patient's age at diagnosis is approximately 66–70
years old, with 37% of patients younger than 65 years of age.[3] Symptomatic multiple myeloma is associated with significant morbidity and mortality, especially with end-organ failure.[4]
There
have been significant improvements in the treatment of multiple myeloma
in the last few years. The introduction of autologous hematopoietic
stem cell transplantation has positively impacted overall survival. In
addition, novel agents such as proteasome inhibitors, immunomodulatory
drugs, monoclonal antibodies, and CAR-T cells are changing the history
of the disease, producing higher progression-free survival and overall
survival.[4]
Few studies have been published from developing countries describing the epidemiology of the disease,[5-8] with very scanty reports from the middle east and north Africa (MENA) region.[9-12]
Since
the population in the MENA region is expected to have a more aging
population in the coming decades, the incidence is expected to
increase, as shown in a recent publication from Lebanon.[12] We are not aware of any published work on a large cohort of patients with multiple myeloma from Jordan.
The
aim of this study is to review the department registry of multiple
myeloma patients at Jordan University Hospital (JUH), which is
representative of the whole country, over 8 years with patient
characteristics and disease patterns, survival, and therapy used in a
real-world experience.
Methods
Patients.
This non-interventional, single-center, retrospective study analyzes
the registry of patients with newly diagnosed multiple myeloma (MM)
between 01/2009 and the end of 2016.
A total of 128 patients were
reviewed, representing (27.6%) of all MM patients in the country during
the study period, which is estimated to be around 464 patients as
reported by the Jordanian National cancer registry.[13]
The registry captures data prospectively. The data include patients'
particulars, medical history, and diagnostic tests, including imaging
tests, stage, pathology, laboratory findings, follow-up, therapy, and
cytogenetics. As for the imaging tests, an x-ray of the skull and
skeletal survey of the long bones and spine were done. In selected
patients and in patients not showing abnormal findings on x-ray images,
MRI images of the spine or the suspected affected area were done. In
some patients, PET scans were carried out. No patient had a low dose
whole body ct scan.
Patients who entered the registry between the beginning of 2009 and the end of 2016 are the subject of this report.
This
study was approved by the Institutional Review Board committee of the
Cell Therapy Center and JUH, Amman, Jordan. Written informed consent
that adhered to the declaration of Helsinki was obtained from all
participants. The patient must be 18 years old or older.
Diagnosis of multiple myeloma, staging, and risk classification was made in accordance with ESMO guidelines.[14]
As
for the treatment, only two different reimbursable regiments were used
as first-line; a combination of dexamethasone and thalidomide (DT),
which was dominantly used in the first few years of the registry, and a
combination of Bortezomib (Velcade), dexamethasone and thalidomide
(VDT) which was used during the later years of the registry or for
patients considered candidates for single autologous bone marrow
transplantation (ABMT) with high dose melphalan (180 mg/m2).
If patient progressed or failed first-line therapy, the reimbursed
drugs were: dexamethasone with melphalan, or either drug alone with or
without palliative radiation. Subjects who had autologous bone marrow
transplantation were given monthly bortezomib post-ABMT maintenance for
one year. If they relapse, there was no specific reimbursable
combination. Renal insufficiency was defined as per ESMO guidelines in
the CRAB criteria (serum creatinine > 177 mol/L (> 2 mg/dL).
The
Statistical analysis was done using the Statistical Package for the
Social Sciences (SPSS). Overall survival (OS) was analyzed with the
Kaplan-Meier method. P-value was considered significant if it was
< 0.05.
Results
One
hundred twenty-eight patients with MM were diagnosed and treated
between 2009 and 2016 at JUH. Demographic and laboratory data are shown
in table 1. The mean age was
63.3 years. Again, the distribution of ages was found to be equal in
both genders, with 50% of each gender. Regarding the presenting
symptomatology and findings, 61.1% complained of bone pain, with 70.5%
of patients having lytic bone lesions on x-ray imaging or MRI imaging,
45.6% had symptoms of anemia, and 20% had renal insufficiency as
defined by ESMO guidelines (serum creatinine > 177 μmol/L (> 2
mg/dL). Concerning the immunoglobulin subtypes of multiple myeloma in
the entire population, 79.1% of the patients had the IgG monoclonal
band, of which 79.2% had IgG Kappa myeloma, and 20.8% had IgG lambda
myeloma 13.9% of the patients had the IgA monoclonal band, of which
56.25% had IgA kappa myeloma, and 43.75% had IgA lambda myeloma. In
addition, 7% of the patients had light chain myeloma. As for the ISS
stage, 27.4% were stage I, 36.28% were stage II, and 36.28 were stage
III. The mean and median survival for each type is shown in table 2.
Survival analysis of the non-transplant population (113) patients was
as follows: Median overall survival (95% confidence interval [CI]) was
38.00 months (Range: 23.133-52.867). Stage 1 had a median survival of
96 months. Stage II had a median survival of 46. months and stage III
had a median survival of 16 months (Table 2). Figure 1
demonstrates the survival difference between the ISS stage and the
different immunoglobulin types of myelomas. There was a statistical
significance concerning ISS stages (p < 0.05). However, the survival
difference between the types of myelomas was statistically
insignificant (p>0.05), as shown in table 2. 85.2% of the patients were treated using DT, while 14.8% were treated using VDT (Table 2). Figure 2
demonstrates the survival difference between treatment regimens used.
The survival analysis was not statistically significant (p > 0.05) (Table 2). Survival curves are shown in figures 1 and 2. As for the overall survival (OS) and progression-free survival (PFS) in the ABMT population, details are shown in figure 2.
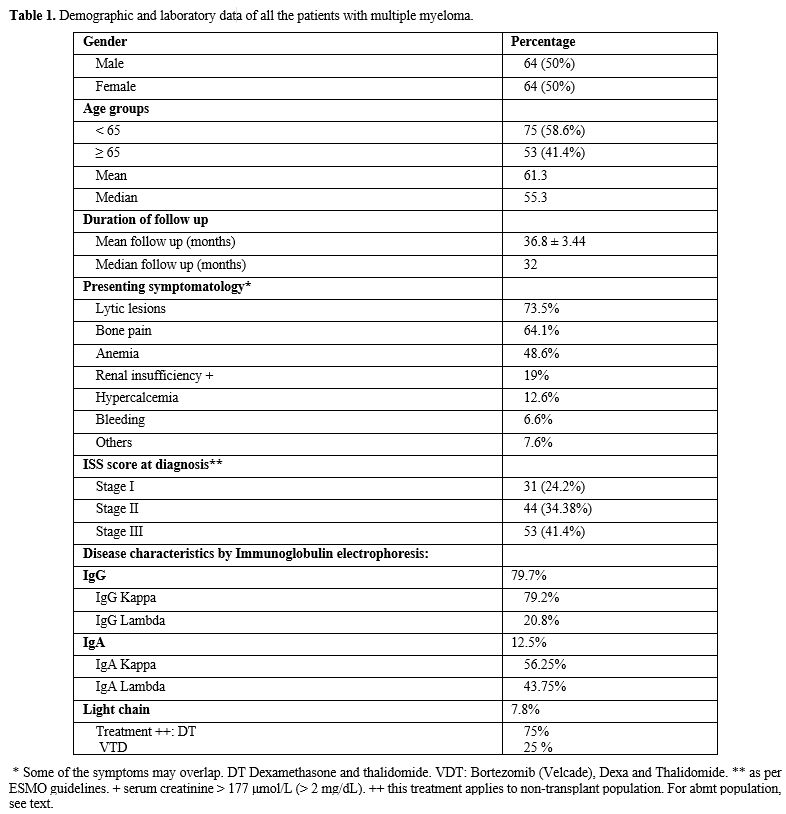 |
Table
1. Demographic and laboratory data of all the patients with multiple myeloma. |
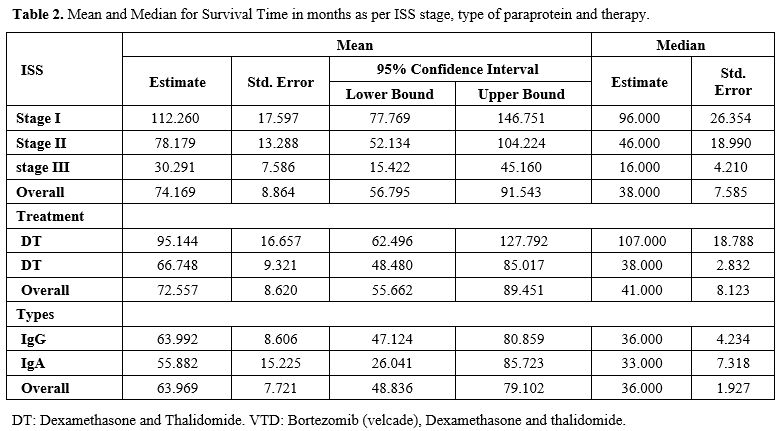 |
Table 2. Mean and Median for Survival Time in months as per ISS stage, type of paraprotein and therapy. |
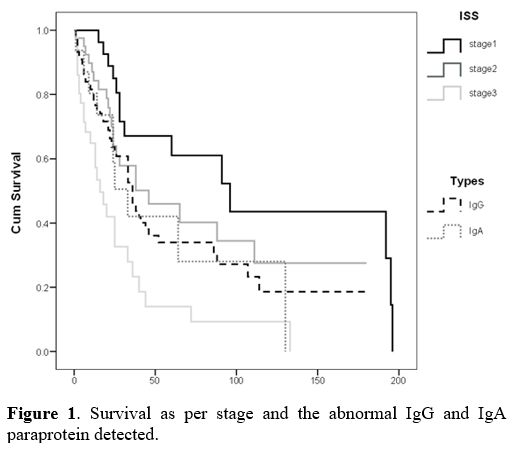 |
Figure 1. Survival as per stage and the abnormal IgG and IgA paraprotein detected. |
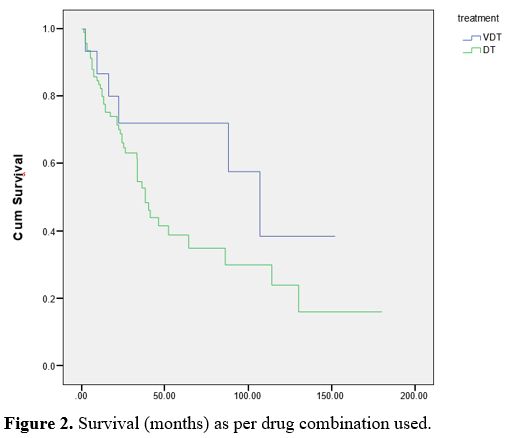 |
Figure 2. Survival (months) as per drug combination used.
|
Discussion
Our
study involves 128 Multiple Myeloma patients who were followed at the
Hematology department of JUH and was the first conducted in Jordan.
The yearly number of new myeloma cases in Jordan during the registry period was 58 cases per year.[13]
During the period 2009 to 2016, it is estimated that 464 new cases were
diagnosed with myeloma in the whole country. With 128 cases in our
registry during the same period, it seems that it captured (27.6%) of
all myeloma cases in Jordan, which makes it very representative of the
disease in the country. There was no difference in gender distribution,
as found in the Jordan cancer registry in 2012.[13] The gender distribution is similar to that reported from Latin America[5] but was significantly different from Iran.[8]
The
median age of our patients was 55.3, and the mean age ± SD was
61.30±10.8, which is comparable to the Iranian study's mean age±SD of
61.98±11.44 years.[8] Other studies from the MENA
region showed an even younger median age, such as the study from
Algeria that showed a median age of 53 years.[10] The age of our patients is not close to the age of 66 years reported by the Mayo clinic series.[15]
We
found out that 58.6% of our patients were younger than 65 years,
comparable to the Iranian study. In contrast, a Swedish study showed
that 72% of their patients were older than 65 years of age.[16] In a real-world study conducted in Europe, the Middle East, and Africa, 75% of patients were older than 65.[17]
The
most common presenting symptom in our patients was bone pain (61.1%),
similarly to the studies from Belgium, France, Germany, Italy, Spain,
Switzerland, and the UK,[18] as well as the study reported from Beijing/China.[19] However, the registry did not capture skeletal-related events (SRE) in the disease course other than at presentation.
The
prevalence of anemia at any time during the disease is reported to be
about 45.2% in our study, which is close to the prevalence reported in
a recent registry study in the USA (45%).[20] The Swedish study showed a comparable percentage of patients with anemia (49%).[16]
The
prevalence of renal dysfunction as defined by ESMO guidelines in our
study was 20%, similar to that reported in European countries (20%)[17] and in the Swedish study (18%).[16] The Iran study reported that 33.8% of their patients had a creatinine higher than 2.[8]
Hypercalcemia was seen in 11.6% of our patients, similar to that reported in the Mayo clinic[15] and the Swedish studies,[16] 13% in both. Hypercalcemia was slightly higher in the European countries study, which was reported in 19% of patients.[18]
73.5% of our patients had osteolytic lesions by imaging (shown in table 1), comparable to the Swedish study (77%).[16]
Figures 3 and 4 show
the mean overall survival (OS) and progression-free survival in
transplant patients, which was not reached during the follow-up period.
We noticed certain important findings when comparing survival results in this study with other reported studies.[21-26]
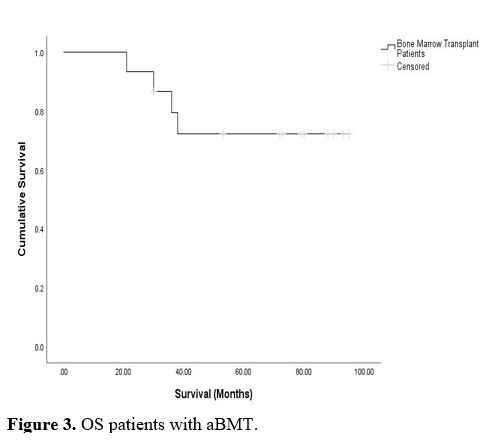 |
Figure 3. OS patients with aBMT. |
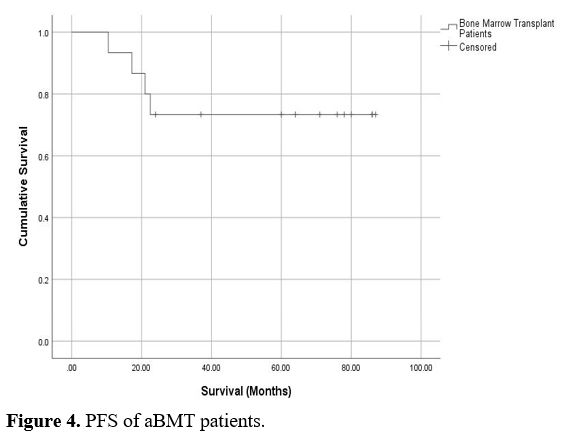 |
Figure 4. PFS of aBMT patients.
|
Median
OS for ISS stages I, II, and III were 96, 46, and 16 months
respectively. Comparing this with the RS (rescaled range) in the
Swedish study, it shows a similar median survival for ISS stage I,
which was 8.2 years, and lower median survival for stage II and III;
5.6 and 3.2 years, respectively.[16]
Since most
of our patients represent a typical Jordanian population in which most
people are working class, lower and middle-income class, it may be
possible that the poor outcome might be explained, in part, by the poor
economic status of these patients. Socioeconomic status is reported to
be a prognostic factor for the overall survival of multiple myeloma
patients, as shown in recent publications.[27] In the
Middle East and North Africa, extreme poverty rates nearly doubled
between 2015 and 2018, from 3.8% to 7.2%, according to the world bank
report 2020.[28]
Based on new data, on July 1, 2017, the World Bank classified Jordan as a lower-middle-income country.[29]
Given all these socioeconomic factors in the middle east, it is only
expected to find lower survival related to multiple myeloma compared to
European or industrial countries.
Since most Jordanians are ethnic
Arabs, the ethnicity probably does not play a role in our study despite
being reported in other countries to be of importance.[30]
Most of our patients at the time of diagnosis had ISS stage II (34.38%)
and stage III (41.4%), and only 24.2% with stage I. The fact that the
patients present in the advanced stage indicates a lack of proper
awareness and patient education.
A study from South Korea reported
that 48.8% of Multiple Myeloma patients were ISS stage II and 40.2%
were ISS stage III, while only 11% were stage I.[31] In the Swedish study, 44% of the patients were reported to be at ISS stage II at diagnosis and 33% at stage III.[16]
These findings suggest that patients in Jordan and internationally have
significant MM-related organ damage at diagnosis, so initiatives
facilitating earlier diagnosis are warranted.
VDT (Bortezomib,
Dexamethasone, Thalidomide) was used in 14.8% of our patients, DT
(Dexamethasone and Thalidomide) in 85.2%. The mean survival time for
each regimen was: 95 and 66 months, respectively. Unfortunately, our
institution at the time of the study did not have the approval to
reimburse bortezomib until late in the registry; hence VDT was not
widely used. In addition, we had no access to second-line new drugs for
the treatment of MM.
As for the types of proteins secreted by the
malignant plasma cells, the most common type was IgG (79.1%), of which
79.2% had IgG Kappa myeloma, and 20.8% had IgG lambda myeloma. 13.9% of
our patients had IgA with equal IgA Kappa and Lambda distribution. Only
a small number of patients (7%) had light-chain myeloma. In the Mayo
Clinic study, the immunoglobulin distribution for IgG, IgA, and light
chain were 52%, 21%, and 16%, respectively.[32]
In
our study, the mean survival time for IgG, IgA, and light chain myeloma
was 64 months, 56 months, and 34 months. Mean survival for myeloma
Lambda light chains (for IgA and IgG combined) was 73 months, while for
Kappa light chains (for IgA and IgG combined) was 58 months.
We
believe that reporting our findings will help revisit the management
pathways of multiple myeloma in a developing country with limited
financial resources. We realize the lack of cytogenetic and molecular
data because of non-availability at our institution. Since 2016, we
have access to cytogenetics and more recent access to newer agents such
as ad lenalidomide, carfilzomib, and daratumumab. However, we still
have no access to CAR-T cells.
We need to bridge gaps with
institutions in the industrial world to help the patients with mm.
Therefore, we welcome establishing more organized collaboration and
participation in clinical trials and studies with these institutions.
We believe the decade from 2017 to 2027 will show far better molecular, cytogenetic data and better survival rates.
References
- Raab MS, Podar K, Breitkreutz I, Richardson PG, Anderson KC. Multiple myeloma. Lancet. 2009;374(9686):324-39. https://doi.org/10.1016/S0140-6736(09)60221-X
- Becker N. Epidemiology of multiple myeloma. Recent Results Cancer Res. 2011;183: 25-35. https://doi.org/10.1007/978-3-540-85772-3_2 PMid:21509679
- Palumbo A, Anderson K. Multiple Myeloma. New England Journal of Medicine. 2011; 364:1046-1060. https://doi.org/10.1056/NEJMra1011442 PMid:21410373
- Kazandjian D. Multiple myeloma epidemiology and survival, a unique malignancy. Semin Oncol. 2016; 43(6): 676-681. https://doi.org/10.1053/j.seminoncol.2016.11.004 PMid:28061985 PMCid:PMC5283695
- Hungria
VT, Maiolino A, Martinez G, Duarte GO, Bittencourt R, Peters L,
Colleoni G, Oliveira LC, Crusoé E, Coelho ÉO, Pasquini R, Magalhães SM,
Nunes R, Neto JV, Faria RM, Souza M, Hamerschlak N, Flantl D, Navarro
JR, Conte G, Gomez-Almaguer D, Ruiz-Argüelles G, Durie BG;
International Myeloma Working Group Latin America. Observational study
of multiple myeloma in Latin America. Ann Hematol. 2017
Jan;96(1):65-72. doi: 10.1007/s00277-016-2866-9. Epub 2016 Nov 5. https://doi.org/10.1007/s00277-016-2866-9 PMid:27815724
- Nnonyelum
ON, Anazoeze MJ, Eunice NO, Emmanuel OO, Stella AT, Marcus AI, Taiwo
BM, Olufela KO, Chinawaeze AJ, Orkuma JA, Dalhat GG, Otobo UI. Multiple
myeloma in Nigeria: a multi-centre epidemiological and biomedical
study. Pan Afr Med J. 2015 Nov 24;22:292. doi:
10.11604/pamj.2015.22.292.7774. https://doi.org/10.11604/pamj.2015.22.292.7774 PMid:26966488 PMCid:PMC4769058
- Sadia
S, Syed M, Saira P, Ali H, Basharat M, Multiple Myeloma: a
Retrospective Analysis of 61 Patients from a Tertiary Care Center.
Asian Pac J Cancer Prev, 2016; 17 (4), 1833-1835. https://doi.org/10.7314/APJCP.2016.17.4.1833 PMid:27221861
- Jalaeikhoo
H, Sharifzadeh M, Rajaeinejad M, Keyhani M, Zokaasadi M. Retrospective
Analysis of 345 Multiple Myeloma Cases: An Investigation from 2
Institutions. Arch Iran Med. 2018 Sep 1;21(9):412-417
- El
Husseiny NM, Kasem N, El Azeeim HA, Mattar MW. Multiple myeloma: a
descriptive study of 217 Egyptian patients. Ann Hematol.
2014;93(1):141-5. https://doi.org/10.1007/s00277-013-1849-3 PMid:23892925
- Mohammadi
L, Harir N, Brahimi M, Moulessehoul S, Bekadja MA. Epidemiological,
clinical and pronostic aspects of multiple myeloma eligible for
therapeutic intensification followed by autologous hematopoietic stem
cell in the Algerian West: report of 147 cases. Tunis Med. 2017
Jun;95(6):415-421.
- Mseddi-Hdiji S,
Haddouk S, Ben Ayed M, Tahri N, Elloumi M, Baklouti S, Hachicha J,
Krichen MS, Bahloul Z, Masmoudi H. Gammapathies monoclonales en
Tunisie: analyse épidémiologique, immunochimique et étiologique d'une
série de 288 cas [Monoclonal gammapathies in Tunisia: epidemiological,
immunochemical and etiological analysis of 288 cases]. Pathol Biol
(Paris). 2005 Feb;53(1):19-25. French. doi:
10.1016/j.patbio.2004.01.014. https://doi.org/10.1016/j.patbio.2004.01.014 PMid:15620605
- Jalloul
M, Sater AA, Ballout I, Annan K, Mokdad T, Lakis ZA, Khachfe HH.
Multiple myeloma in Lebanon: Trend analysis, 10- year projections and
comparisons to other countries. Cancer Treat Res Commun. 2022 Jan 7;
30:100513. Doi: 10.1016/j.ctarc.2022.100513. https://doi.org/10.1016/j.ctarc.2022.100513 PMid:35026534
- Al-Sayaideh,
Ayoub & Nimri, Omar & Arqoub, Kamal & Al-Zaghal, Marwan
& Halasa, Wafa. (2016). Cancer Incidence In Jordan - 2012. https://doi.org/10.13140/RG.2.1.1251.6246
- P.
Moreau, J. San Miguel, P. Sonneveld, M. V. Mateos, E. Zamagni, H.
Avet-Loiseau, R. Hajek, M. A. Dimopoulos, H. Ludwig, H. Einsele, S.
Zweegman, T. Facon, M. Cavo, E. Terpos, H. Goldschmidt, M. Attal &
C. Buske. Multiple Myeloma: ESMO Clinical Practice Guidelines Ann Oncol
(2017) 28 (suppl 4): iv52-iv61. https://doi.org/10.1093/annonc/mdx096 PMid:28453614
- Kyle
RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, Fonseca R,
Rajkumar SV, Offord JR, Larson DR, Plevak ME, Therneau TM, Greipp PR.
Review of 1027 patients with newly diagnosed multiple myeloma. Mayo
Clin Proc. 2003 Jan;78(1):21-33. doi: 10.4065/78.1.21. https://doi.org/10.4065/78.1.21 PMid:12528874
- Blimark
CH, Turesson I, Genell A, Ahlberg L, Björkstrand B, Carlson K, Forsberg
K, Juliusson G, Linder O, Mellqvist UH, Nahi H, Kristinsson SY; Swedish
Myeloma Registry. Outcome and survival of myeloma patients diagnosed
2008-2015. Real-world data on 4904 patients from the Swedish Myeloma
Registry. Haematologica. 2018 Mar;103(3):506-513. doi:
10.3324/haematol.2017.178103. Epub 2017 Dec 7. https://doi.org/10.3324/haematol.2017.178103 PMid:29217784 PMCid:PMC5830385
- Mohty
M, Terpos E, Mateos MV, Cavo M, Lejniece S, Beksac M, Bekadja MA,
Legiec W, Dimopoulos M, Stankovic S, Durán MS, De Stefano V, Corso A,
Kochkareva Y, Laane E, Berthou C, Salwender H, Masliak Z, Pečeliūnas V,
Willenbacher W, Silva J, Louw V, Nemet D, Borbényi Z, Abadi U, Pedersen
RS, Černelč P, Potamianou A, Couturier C, Feys C, Thoret-Bauchet F,
Boccadoro M; EMMOS Investigators. Multiple Myeloma Treatment in
Real-world Clinical Practice: Results of a Prospective, Multinational,
Noninterventional Study. Clin Lymphoma Myeloma Leuk. 2018
Oct;18(10):e401-e419. doi: 10.1016/j.clml.2018.06.018. Epub 2018 Jun
25. https://doi.org/10.1016/j.clml.2018.06.018 PMid:30030033
- Yong
K, Delforge M, Driessen C, Fink L, Flinois A, Gonzalez-McQuire S,
Safaei R, Karlin L, Mateos MV, Raab MS, Schoen P, Cavo M. Multiple
myeloma: patient outcomes in real-world practice. Br J Haematol. 2016
Oct;175(2):252-264. doi: 10.1111/bjh.14213. Epub 2016 Jul 13. https://doi.org/10.1111/bjh.14213 PMid:27411022 PMCid:PMC5096152
- Wang
L, Jin FY, Li Y, Sun JN, Zhang JJ, Tang R, Zhong YP. IgA Type Multiple
Myeloma, Clinical Features, and Prognosis. Chin Med J (Engl). 2018 May
20;131(10):1249-1250. doi: 10.4103/0366-6999.231513. https://doi.org/10.4103/0366-6999.231513 PMid:29722346 PMCid:PMC5956780
- Rifkin
RM, Abonour R, Terebelo H, Shah JJ, Gasparetto C, Hardin J, Srinivasan
S, Ricafort R, Nagarwala Y, Durie BG. Connect MM Registry: The
Importance of Establishing Baseline Disease Characteristics. Clin
Lymphoma Myeloma Leuk. 2015 Jun;15(6):368-76. doi:
10.1016/j.clml.2014.12.002. Epub 2014 Dec 11. https://doi.org/10.1016/j.clml.2014.12.002 PMid:25617035
- Kumar
SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S, Kapoor P,
Dingli D, Hayman SR, Leung N, Lust J, McCurdy A, Russell SJ, Zeldenrust
SR, Kyle RA, Rajkumar SV. Continued improvement in survival in multiple
myeloma: changes in early mortality and outcomes in older patients.
Leukemia. 2014 May;28(5):1122-8. doi: 10.1038/leu.2013.313. Epub 2013
Oct 25. https://doi.org/10.1038/leu.2013.313 PMid:24157580 PMCid:PMC4000285
- Riva
E, Bove V, Villano F, Mori M, Córdoba C, Noria A, Petruskevicius P,
Cardeza A, Díaz L. From guidelines to real world: results from the
National Multiple Myeloma Registry in Uruguay on 222 newly diagnosed
multiple myeloma patients from 2012 to 2015. Curr Med Res Opin. 2019
Jul;35(7):1197-1203. doi: 10.1080/03007995.2019.1568091. Epub 2019 Feb
8. https://doi.org/10.1080/03007995.2019.1568091 PMid:30621522
- Remes
K, Anttila P, Silvennoinen R, Putkonen M, Ollikainen H, Terävä V,
Sinisalo M, Kananen K, Schain F, Castren-Kortegangas P, Järvinen TM,
Pisini M, Wahl F, Dixon T, Leval A. Real-world treatment outcomes in
multiple myeloma: Multicenter registry results from Finland 2009-2013.
PLoS One. 2018 Dec 5;13(12):e0208507. doi:
10.1371/journal.pone.0208507. https://doi.org/10.1371/journal.pone.0208507 PMid:30517181 PMCid:PMC6281251
- Liwing
J, Uttervall K, Lund J, Aldrin A, Blimark C, Carlson K, Enestig J,
Flogegård M, Forsberg K, Gruber A, Haglöf Kviele H, Johansson P, Lauri
B, Mellqvist UH, Swedin A, Svensson M, Näsman P, Alici E, Gahrton G,
Aschan J, Nahi H. Improved survival in myeloma patients: starting to
close in on the gap between elderly patients and a matched normal
population. Br J Haematol. 2014 Mar;164(5):684-93. doi:
10.1111/bjh.12685. Epub 2013 Dec 9. https://doi.org/10.1111/bjh.12685 PMid:24313224
- Shustik,
C., Bergsagel, D. E., & Pruzanski, W. (1976). Kappa and lambda
light chain disease: survival rates and clinical manifestations. Blood 1976 Jul;48(1):41-51. https://doi.org/10.1182/blood.V48.1.41.41 PMid:820387
- Nair
B, Waheed S, Szymonifka J, Shaughnessy JD Jr, Crowley J, Barlogie B.
Immunoglobulin isotypes in multiple myeloma: laboratory correlates and
prognostic implications in total therapy protocols. Br J Haematol. 2009
Apr;145(1):134-7. doi: 10.1111/j.1365-2141.2008.07547.x. Epub 2008 Dec
20. https://doi.org/10.1111/j.1365-2141.2008.07547.x PMid:19120351 PMCid:PMC3644943
- Intzes
S, Symeonidou M, Zagoridis K, Bezirgianidou Z, Vrachiolias G,
Spanoudaki A, Spanoudakis E. Socioeconomic Status is Globally a
Prognostic Factor for Overall Survival of Multiple Myeloma Patients:
Synthesis of Studies and Review of the Literature. Mediterr J Hematol
Infect Dis. 2021 Jan 1;13(1):e2021006. doi:10.4084/MJHID.2021.006 https://doi.org/10.4084/mjhid.2021.006 PMid:33489045 PMCid:PMC7813274
- https://openknowledge.worldbank.org/bitstream/handle/10986/34496/9781464816024.pdf
- https://www.worldbank.org/en/country/jordan/brief/qa-jordan-country-reclassification
- Marinac
CR, Ghobrial IM, Birmann BM, Soiffer J, Rebbeck TR. Dissecting racial
disparities in multiple myeloma. Blood Cancer J. 2020 Feb 17;10(2):19.
doi: 10.1038/s41408-020-0284-7 https://doi.org/10.1038/s41408-020-0284-7 PMid:32066732 PMCid:PMC7026439
- Drayson
M, Begum G, Basu S, Makkuni S, Dunn J, Barth N, Child JA. Effects of
paraprotein heavy and light chain types and free light chain load on
survival in myeloma: an analysis of patients receiving
conventional-dose chemotherapy in Medical Research Council UK multiple
myeloma trials. Blood. 2006 Sep 15;108(6):2013-9. doi:
10.1182/blood-2006-03-008953. Epub 2006 May 25. https://doi.org/10.1182/blood-2006-03-008953 PMid:16728700
- Woo
YR, Kim JS, Lim JH, Hwang S, Kim M, Bae JM, Park YM, Min CK, Kim DW,
Park HJ. Prevalence and clinicopathologic characteristics of multiple
myeloma with cutaneous involvement: A case series from Korea. J Am Acad
Dermatol. 2018 Mar;78(3):471-478.e4. doi: 10.1016/j.jaad.2017.08.054.
Epub 2017 Nov 6. https://doi.org/10.1016/j.jaad.2017.08.054 PMid:29107338
[TOP]





