Ilaria Cozzi, Giovanni Rossi, Emma Rullo and Valeria Ascoli.
Department of Radiological, Oncological and Pathological Sciences, Sapienza University, Rome.
Correspondence to:
Valeria Ascoli - Dipartimento di Scienze Radiologiche, Oncologiche e
Anatomo-Patologiche, Università Sapienza, Viale Regina Elena, 324 -
00161, Roma. Tel: +39-06-49973397. E-mail:
valeria.ascoli@uniroma1.it
Published: March 1, 2022
Received: October 22, 2021
Accepted: February 8, 2022
Mediterr J Hematol Infect Dis 2022, 14(1): e2022020 DOI
10.4084/MJHID.2022.020
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Primary
effusion lymphoma (PEL) is a large B-cell lymphoma growing within
body-cavities caused by the Kaposi sarcoma-associated herpesvirus
(KSHV)/human herpesvirus-8 (KSHV/HHV-8). It is mainly reported in
HIV-infected patients. The uncommon occurrence in the elderly supports
a form paralleling classic Kaposi sarcoma (KS), i.e. classic PEL, whose
characteristics are relatively underexplored. To better understand the
diagnostic modalities and clinical-epidemiological features of classic
PEL, articles reporting cases of PEL were identified through
MEDLINE/EMBASE databases (January 1998-July 2020) and screened
according to PRISMA guidelines to extract individual-level data. A
comparison was also performed between classic PEL and classic KS to
evaluate similarities and differences. We identified 105 subjects
(median age 77 years; 86% males), mainly from Mediterranean countries
(52%, first Italy) and Eastern Europe (7%). Common comorbidities were
heart failure (32%), cirrhosis (16%), and malignancy (20%) including
lymphoid neoplasms. Pleural cavity was the commonest site (67%). PEL
diagnosis was based on cytomorphology (89%), evidence of KSHV/HHV-8
infection (94%), EBV co-infection (28%) and clonality of IGH (59%), IGK
(14%), TRG (9%) alone or in multiple combinations. Compared to KS, age
(P<.001), gender-ratio (P=.08) and mortality (P<.001) were
significantly higher in PEL, whereas the frequency of PEL as a second
primary was similar (P=.44).
This is the first systematic review
of classic PEL case reports highlighting heterogeneity and lack of a
uniform multidisciplinary approach at diagnosis, in the absence of
specific guidelines as it happens for rare cancers. It is conceivable
that classic PEL is still underdiagnosed in Mediterranean countries
wherein KSHV/HHV-8 is endemic.
|
Introduction
Primary
effusion lymphoma (PEL) is a rare form of large B-cell lymphoma growing
in liquid phase within body cavities covered by the serous membranes
(pleura, pericardium, peritoneum), typically without solid tumor
lesions. PEL cells lack B-cell markers, present Ig gene rearrangements,
and a gene expression profile consistent with an
immunoblastic/plasmablastic derivation. The tumor clone is always
infected by the Kaposi sarcoma-associated herpesvirus (KSHV)/human
herpesvirus-8 (KSHV/HHV-8, gamma-herpesvirus) and may be coinfected
with Epstein-Barr virus (EBV). KSHV/HHV-8 was first detected in
AIDS-related Kaposi sarcoma (KS)[1] and found
thereafter in the other forms of KS: sporadic/classic KS,
endemic/sub-Saharan African KS, and iatrogenic KS. KSHV/HHV-8 was then
discovered in AIDS-related body cavity-based lymphoma,[2] designated as a distinct clinicopathologic entity as PEL in 1996[3]
and then included in the WHO classification of neoplasms of the
hematopoietic and lymphoid tissues published in 2001 and updated in
2008 (ICD-O-3 code 9678/3).[4] As KS, PEL has
been described in HIV-infected individuals with severe immunodeficiency
(AIDS-related PEL) and HIV-uninfected patients such as solid organ
transplant recipients (iatrogenic PEL) and elderly patients from areas
with moderate/high prevalence for KSHV/HHV-8 infection. The latter
supports a distinct clinico-epidemiological form of PEL resembling
classic KS, i.e., classic PEL.[5,6] As of yet, cases of PEL outside HIV infection in Africa have not been formally reported.
Given
the rarity of KSHV/HHV-8-positive PEL, the epidemiology is poorly
defined. Available Cancer Registry data such as the SEER database in
the United States refer to PEL in the HIV-positive setting,[7]
as most published studies. PEL cases in the HIV-negative are mainly
presented as isolated case reports supplemented by nonsystematic
reviews of the literature, and more rarely in small case series.
However, no studies are investigating the diagnostic work-up flow or
providing specific strategies to reach a final diagnosis, considering
the challenge of a lymphoma growing in effusions outside the HIV
infection. In addition, data regarding the patient origin,
comorbidities associated with the clinical presentation, other primary
neoplasms, laboratory results have been scarce.
We have performed
a systematic review of the case reports of classic PEL to analyze all
the available data recorded in the literature. The aims include
providing insights into clinicopathological and epidemiological
features of classic PEL and determining the diagnostic modalities,
focusing on KSHV/HHV-8 status ascertainment and clonality analysis. For
the purpose of this study, we defined ‘classic PEL’ all the
KSHV/HHV-8-positive PEL outside HIV-infection that were also
non-iatrogenic and non-associated with primary immunodeficiency. To
better understand this form of PEL, we compared classic PEL with
classic KS, the most common and better studied HHV-8-associated
disease.[8]
Methods
Data sources and search strategy.
We carried out a computerized search of the Ovid MEDLINE (PubMed) and
EMBASE (Ovid) databases for potentially relevant studies in the English
language published from January 1995 to July 2020: full articles with
abstract, letters, and brief articles as images. Given the rarity of
the disease, we also considered congress abstract indexed in search
databases and non-English studies with at least an English abstract
providing sufficient information for inclusion. The electronic search
strategy was as follows: KSHV AND lymphoma* [title] or PEL [title];
KSHV AND PEL [title]; KSHV/HHV8 AND lymphoma* [title]; KSHV-positive
AND lymphoma*[title]; KSHV-associated AND lymphoma*[title]; Primary AND
Effusion AND lymphoma* [title]; Body AND Cavity AND lymphoma* [title];
KSHV/HHV-8-associated AND lymphoma* [title]; KSHV/HHV-8-related AND
lymphoma* [title]; Body AND cavity-based AND lymphoma*[title]; Primary
AND Effusion AND lymphoma* [title] or PEL [title](((lymphoma, primary
effusion[MeSH Terms]) OR (primary effusion lymphomas[MeSH Terms])) OR
(primary effusion lymphoma[MeSH Terms])) OR (lymphomas, primary
effusion[MeSH Terms]) OR (KSHV AND primary effusion lymphoma* [MeSH
Terms])).
Inclusion and exclusion criteria.
The eligibility criteria were: i) primary localization of PEL in a
serous cavity covered by mesothelium (effusion-based disease without
solid nodal/extranodal tissue involvement); ii) morphologic features
and immunophenotype consistent with PEL; iii) demonstration of
KSHV/HHV-8 infection of the tumor clone. For inclusion, the published
cases must have documentation of diagnosis method(s) and individual
patient data. Criteria for exclusion were: i) negative/unrelated or
unknown KSHV/HHV-8 status; ii) HIV-infection; iii) solid organ
transplant or immunosuppressive therapy; iv) primary extracavitary
localization of PEL; v) primary immunodeficiency or idiopathic
lymphocytopenia. Further exclusion criteria were either non-relevant
titles or biomolecular and preclinical studies related to KSHV/HHV-8,
and narrative reviews. Case series that had their analysis pooled
without the description of individual patient data were also excluded.
Screening of literature.
The screening of eligible publications was carried out independently by
three reviewers (ER, GR and VA), first by screening titles and
abstracts and then reviewing the full text. All references meeting the
inclusion criteria, including those with scarce details, were
considered. Disagreements were resolved by consensus, and the final
decision was made by the author VA. In addition, IC undertook an
extensive updated literature search. All authors have contributed to
the data presentation and manuscript text. The whole review process was
performed according to PRISMA guidelines.[9]
Data extraction.
We extracted data on patient and PEL characteristics. Briefly, the
following aspects were considered: age, gender, country-of-origin, the
institution of diagnosis, comorbidities [Multicentric Castleman
Disease (MCD), KS, heart failure, cirrhosis and cause, other primary
cancers], laboratory data including blood count, LDH, markers of
inflammation, site of PEL, immunophenotype, immunoglobulins and T cell
receptor genes clonality evaluation, search for KSHV/HHV-8 and EBV
viral genomes, and outcomes.
Data synthesis and analysis.
Descriptive statistics were used to summarize data, with median and
interquartile range for continuous variables and frequencies and
percentages for dichotomous variables. The nonparametric Wilcoxon
signed-rank test was used to compare the median, the t-test to compare
the mean and the Fisher’s exact test to determine nonrandom
associations between two categorical variables. Stata version 14.2
(StataCorp, College Station, TX, USA). P <0.05 was considered
statistically significant.
Comparison between classic PEL and classic KS.
A PubMed search strategy including the terms Kapos*[Title] AND
classic*[Title] OR Mediterranean [Title] was carried out (January
1994-June 2020) to perform a comparison of the clinic-epidemiological
characteristics between classic PEL and classic KS. Since the classic
variant of KS is described as primarily occurring in individuals in
ethnic groups from Middle East, Eastern Europe, and the Mediterranean
regions,[8] the comparison was limited to classic PEL and classic KS
cases from these geographical areas. We decided arbitrarily to focus on
large case series/studies reporting at least 30 cases of classic KS who
have presented to referral centers for KS over a certain period, from
countries that in turn had reported cases of PEL. For inclusion, the
published cases must have had reviewers (VA, GR) screened all titles
and abstracts for eligibility.
Results
Selection and description of studies. The database searches identified 2214 studies (Figure 1).
After removing duplicate publications and papers that did not meet our
inclusion criteria, the remaining 121 studies were assessed for
eligibility. Forty-six studies were excluded following a more detailed
review. We included 74 studies in the final analysis, accounting for
105 individual patient cases. Over the 23-year period of publications,
32 studies were published during the first 12 years (1995-2008)[3,6,10-39] and 42 during the second 11 years (2009-2020)[40-81]
as full articles (n=58), letters (n=7), images (n=5), and conference
papers/abstracts (n=4). Sixty-eight (91.9%) articles reported one or
two cases.
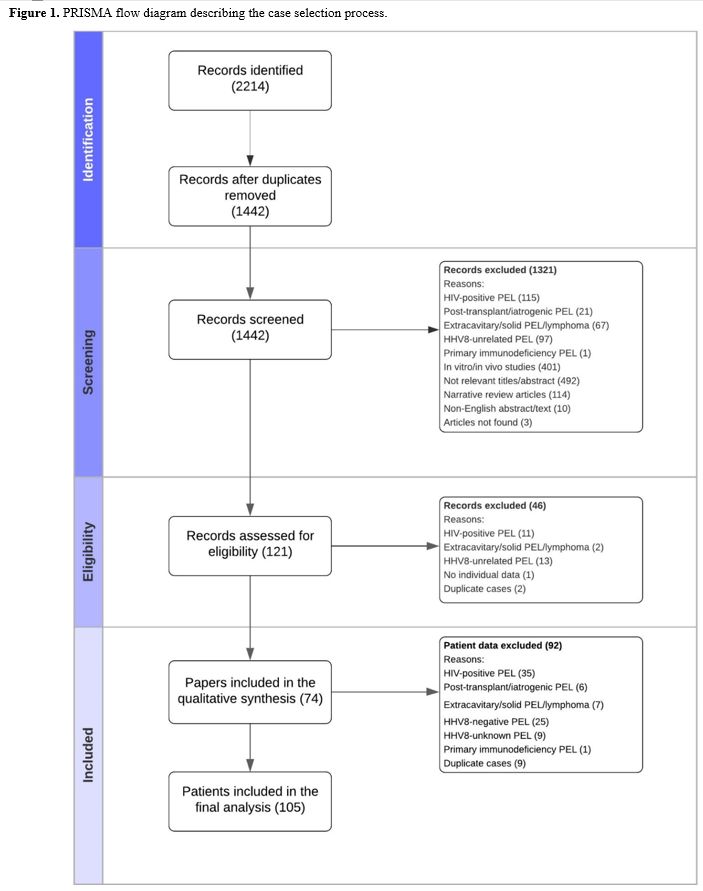 |
Figure
1. PRISMA flow diagram describing the case selection process.
|
Patient demographics, comorbidities and PEL site. Demographics for 105 patients and clinical characteristics are listed in Table 1.
The median (interquartile range, IQR) age was 77 (69-85) with no gender
difference (P>.05). The male-to-female ratio was 6:1. The
country-of-origin was documented in 46 cases. For publications by
institutes and centers where this information was unreported (42
cases), we used the country affiliation of co-authors as a substitute
for patients' country-of-origin; however, this strategy did not apply
to 16 studies (17 cases) because of countries with a high rate of
immigration (USA, Canada, England). Combining reported and presumed
country-of-origin, the majority of cases were from the Mediterranean
basin (52.3%) and Eastern Europe (6.9%), followed by East Asia (35.2%).
The most represented countries were Italy (21 cases), Taiwan (16
cases), Japan (8 cases), South Korea (6 cases), and Greece (6 cases).
 |
Table
1. Demographic and clinical characteristics of 105 patients with classic KSHV/HHV-positive PEL
|
The
clinical data were not consistently described. Of the underlying
conditions known in 95 patients (90.5%), heart failure was the most
common (31.5%); comparing patients with heart failure versus those
without, there was no difference in terms of age and sex (P>.05).
Fifteen patients (15.8%) had liver cirrhosis related to HBV and/or HCV
(n=9), alcohol (n=3), cryptogenic (n=3); the median age of cirrhotic
patients was significantly lower with respect to that of non-cirrhotic
patients (P<.001); there were also two HBV and two HCV mono-infected
patients without clinical evidence of cirrhosis. Overall, a history of
prior or concomitant malignancy was reported in 19/93 cases (20%), for
a total of 23 cancers. Three subjects had triple multiple primary
cancers. The co-occurrence of other KSHV/HHV-8-related neoplasms was
reported in 11 cases: KS (n=6), MCD (n=3), KS and MCD (n=2). Laboratory
parameters were described in a minority of the cases reviewed (49
patients, data not shown in Tables), of which 35 (71.4%) were reported
anemic, 3 (6.1%) leukopenic, and 14 (28.6%) as thrombocytopenic. The
median (IQR) hemoglobin count was 11 (9.4;12.4) in 36 patients whose
absolute value was reported. The CD4 count resulted below the normal
range in 10 out of 14 cases (71.4%), with a median (IQR) value of 241.5
(240;540). LDH and markers of inflammation (ESR and/or CRP) were
elevated in >70% of the patients tested (23/32 and 22/28,
respectively). Elevated serum beta2-microglobulin was found in 8 out of
9 patients tested.
Effusion in a single cavity was the most
commonly observed manifestation. Except for three cases involving both
the peritoneal and pericardial cavities, effusions in multiple sites
constantly involved the pleural cavity as either bilateral effusion
[pleural only (n=15), plus pericardial (n=5), plus peritoneal (n=3),
plus pericardial & peritoneal (n=3)] or unilateral effusion [plus
peritoneal (n=11), plus pericardial (n=4)]. The investigation of a
possible association between underlying pathologies and site of PEL
revealed statistically significant relationships between peritoneal
site and cirrhosis (P<.001) and bilateral pleural site and heart
failure (P<.001).
Methods of diagnosis, the sample of diagnosis, immunophenotype, virology, and clonality. Table 2
summarizes the integration of morphology with immunophenotype and
molecular techniques employed to diagnose PEL. Pathologic diagnosis was
performed primarily by cytology of effusions. The immunophenotype was
determined by flow cytometry (n=7) and immunohistochemistry (IHC) on
smears and/or cell blocks obtained from effusions (n=85) or other
specimens (n=4) for a total of 96 cases. The majority of the cases
lacked B/T cell antigens; yet, there were both CD3 positive (n=6) and
CD20 positive PELs (n=8). Details of morphology and immunophenotype
were not specified in 9 cases. The majority of the cases reviewed
(99/105; 94.3%) had proven KSHV/HHV-8 infection within PEL cells via
IHC and/or molecular analysis; expression of LANA-1 alone was the most
common approach (53/99; 53.5%), followed by DNA extraction and PCR
amplification of viral sequences alone (32/99; 32.3%), or through both
methods (14/99, 14.1%). Serum antibodies against KSHV/HHV-8[33,61] serum/effusion fluid[48] and lymph node KSHV/HHV-8 DNA[19] and unspecified method/sample[72]
were used as evidence of KSHV/HHV-8 etiologic involvement in a marginal
fraction of cases (6/105; 5.7%). Tumor EBER status (EBV coinfection)
was determined in 46/105 (43.8%) cases revealing mostly EBV-negative
tumors (71.7%).
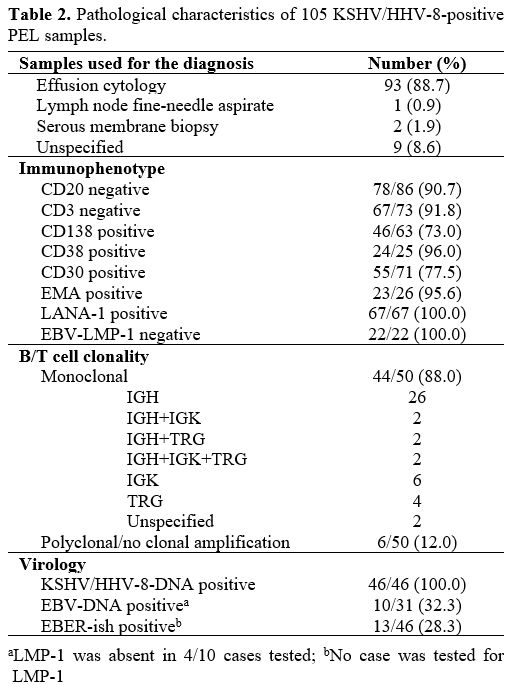 |
Table 2. Pathological characteristics of 105 KSHV/HHV-8-positive PEL samples.
|
Clonality
investigation was performed in 50 cases (47.6%); yet, the technique of
clonality detection was unspecified for 5 and reported for 45 samples,
respectively: PCR (n=27), Southern blot (n=8), FISH (n=7),
immunonephelometry, Northern blot, and chromosomal aberrations study
(n=1 for each method). The tracking of antigen-receptor gene
rearrangements for clonality analysis was successful in 44 out of 50
cases. Most PELs harbored rearrangements of the IGH locus alone or in
multiple combinations with IGK and/or TRG and of the IGK locus alone,
identifying a B-cell lineage; some cases had rearrangement of TRG alone
(genotypic infidelity); the residual cases were reported as clonal
without any reference to a specific gene rearrangement. Some cases were
described as ‘polyclonal’ or ‘negative for clonal rearrangement of the
IGH genes’. Clonality was unavailable, not ascertained, or ‘failed’ in
the residual 55 cases (52.4%).
Finally, considering two periods in
comparison (1995-2009 vs. 2010-2020), the analysis of clonality and
proof of KSHV/HHV-8 infection based on the search of the viral genome
were reported more often in articles published during the first phase
(P=0.009 and P<.001, respectively). On the other hand, LANA
immunostaining was performed more frequently in the subsequent ten-year
interval (P<.001).
Comparison between classic PEL and classic KS.
The database search for classic KS identified 348 articles and a single
systematic review referring to the treatment of classic KS.[82]
Overall, 308 studies did not meet the inclusion criteria for the
following reasons: case reports or series less than 30 cases (n=208);
unavailable clinical/demographical data (n=24); narrative reviews
(n=6); data from non-Mediterranean areas (n=20); registry data (n=13);
non-English language studies (n=27); other studies (n=10). After
excluding articles with duplicate data (n=14) and lower-quality studies
with insufficient information (n=14), the remaining 12 publications
were used for the comparison.[83-94] Table 3
shows a concise comparison of demographics, clinical and laboratory
characteristics between classic variant of PEL and classic variant of
KS from countries of the Mediterranean regions, Middle East, and
eastern Europe. In the group of patients with PEL as compared to KS,
the mean age was significantly higher (P<.001), and the
male-to-female ratio was higher, though non-significant (P=.08). The
percentage of patients with malignancy was similar in the two cohorts
(P=.44). The number of deaths for the disease was significantly higher
in PEL (P<.001). Only 2 KS studies reported detailed laboratory
data;[93–94] compared to KS, PEL cases had
significantly lower hemoglobin level (P<.001), lymphocyte and CD4
counts (P<.001) while there was no significant difference in the
white blood cell counts (P=.08).
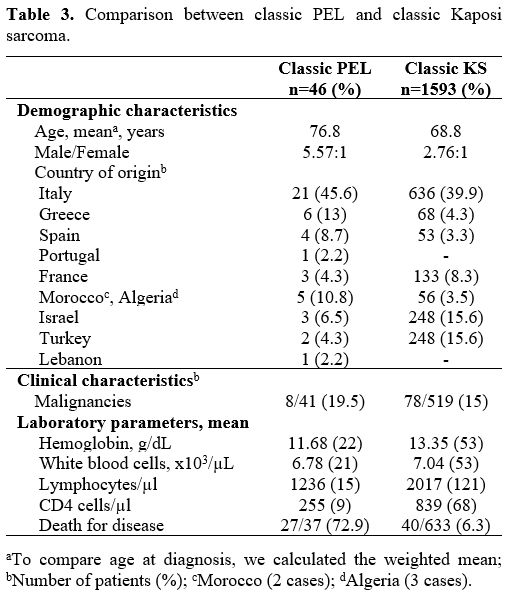 |
Table 3. Comparison between classic PEL and classic Kaposi sarcoma.
|
.
Discussion
There
was high heterogeneity in describing patient and PEL data across
different studies. We present here a systematic review of published
cases of classic PEL providing clinical and pathological insight into
rare disease, excluding cases developing in the HIV setting or any
immunosuppressive state (post-transplant, iatrogenic, and rare cases
associated with primitive immunodeficiencies).[79,95]
The review was undertaken and reported using the PRISMA guidelines,
including only cases of PEL caused by KSHV/HHV-8. The similar
epidemiological background shared by classic PEL and classic KS is why
we compared the two diseases.
One major finding is that a
significant proportion of the cases were observed in the elderly (more
men than women) over age 75 from Mediterranean countries (Italy first)
and Eastern Europe, and East Asia (Taiwan, Japan). Asia and Europe are
home to some of the world’s oldest populations (ages 65 and above). At
the top is Japan at 28 percent, followed by Italy at 23 percent.[96]
We
observed three recurring underlying conditions in comorbidity with
classic PEL: heart failure, cirrhosis, and malignancy. Bilateral
pleural effusions are commonly seen in patients with congestive heart
failure; ascites is the most common complication of liver cirrhosis.
Considering the great exchange of lymphocytes between pleural and
peritoneal cavities and secondary lymphoid organs, the presence of
cirrhosis and heart failure in PEL patients raises interest in the
potential pathogenetic role of associated effusions as an exogenous
stimulus for local clonal expansion of a subset of KSHV/HHV-8-infected
B-cells homing to body cavities.[97] It is
conceivable that these conditions play a role in the development of
PEL. Furthermore, patients with heart failure have an increased
significant risk of hematologic malignancies, with an incidence rate
ratio of 1.45 (95% CI 1.14–1.85, P=0.0027).[98] Liver
cirrhosis might be considered a condition with immunological
disturbances associated with a higher risk of developing
nodal/extranodal lymphoproliferative disorders, even as primary
effusion lymphoma in a body cavity.[99]
Interestingly, we found a statistical association between the bilateral
pleural site of PEL and congestive heart failure, and the peritoneal
site of PEL and cirrhosis. Regarding malignancy, the 20% frequency of
multiple primaries among PEL patients is slightly higher than expected
in literature (in the range of 2-17%).[100]
Interestingly, PEL patients aged >or= 70 years versus younger ones
have the same frequency of multiple primaries (19.1% vs. 20.5%,
respectively) at variance with the described higher prevalence in the
elderly compared with younger patients (15% vs. 6%).[101]
It is impossible to further comment on classic PEL and associated
malignancies for the small number of cancers and the lack and/or
incompleteness of oncological data in several articles. However, the
finding of prior Hodgkin’s lymphoma in three males (2 HCV-positive;
ages 43-44; 1 with colon cancer: age 68) is somewhat peculiar for the
rarity of this cancer. A further intriguing result is that of squamous
cell carcinomas of the oral cavity in two subjects (a man, age 69, from
Taiwan where the oral cancer incidence rate is the highest in the
world; a woman, age 77, from Italy with also breast cancer and
alcohol-related liver cirrhosis). We are no aware of data about the
risk of PEL secondary to a primary cancer; yet, a cancer registry study
has reported a significantly elevated risk of classic KS as a second
primary neoplasm following Hodgkin’s lymphoma with an odds ratio (OR)
of 7.5, chronic leukemia (OR=10), and breast cancer (OR=2.2) and a
non-significant risk after oral cavity malignancy (OR=1.9).[102]
In more than half of the PEL cases, no laboratory data were reported
thus preventing the description of shared clinical features, with the
exception of anemia (low hemoglobin), decreased immunity (low CD4
count) together with low-grade chronic inflammation (elevated markers
of inflammation) in the few patients who were tested; the latter can be
referred to as inflammaging,[103] which contributes to the pathogenesis of age-related PEL.
In
the current review, heterogeneity emerges in the diagnostic process of
PEL. Cytological examination of effusions and identification of
KSHV/HHV-8 infection in tumor cells by LANA positive immunostaining
and/or by molecular search of KSHV/HHV-8 genome contributed to the
diagnosis in 100% of instances where the testing was performed.
However, sporadic cases were diagnosed by histology of the serosal
membranes rather than by cytology of the intracavitary fluid,[77] with proof of KSHV/HHV-8 involvement by finding viral genome in extra-cavitary tissue samples[19] or by serology testing.[33,48,61]
The presence of KSHV/HHV-8 in lymphoma cells should be considered an
absolute requirement for diagnosing KSHV/HHV-8-associated PEL, whereas
the detection of serum antibodies against KSHV/HHV-8 is only a marker
of infection, not of disease.
Genetics of PEL typically include
finding clonal rearrangements of IG genes or, more rarely, of T-cell
receptor genes. Indeed, most reported cases of PEL harbor
rearrangements of the IGH locus alone or in multiple combinations with
IGK and/or TRG, but also of the IGK locus alone (identifying a B-cell
lineage); some cases had rearrangement of TRG alone (genotypic
infidelity). Nevertheless, clonality is not being systematically
investigated in PEL (50 cases, 47.61%), and six cases resulted
polyclonal (n=2) or ‘negative for clonal rearrangement of the IGH
genes’ (n=4), all of them tested only for IGH locus by PCR. Therefore,
considering the heterogeneity of rearrangements, the best way to
ascertain clonality in PEL would be to investigate IGH and IGK and TCR.
Failure to demonstrate clonality could be ascribed to: (i) impaired
primers annealing; (ii) genetic alterations involving the IG loci;
(iii) lack to investigation of IGK locus or TRG rearrangements. A
polyclonal pattern may be the expression of an effusion that mimics PEL
described as pseudo-PEL;[104] alternatively, it may suggest the possibility of polyclonal PEL or emerging PEL.[105]
The question of why clonality assessments are not available in many
cases (>50%) is not commented on by different authors, and
regrettably, these rare PELs have not been better analyzed. Clonality
testing represents a qualitative improvement to characterize
lymphoproliferative diseases and clarify the lineage of a
null-phenotype lymphoma such as PEL.
Although the epidemic
(HIV-related) and iatrogenic (post-transplant) variants of PEL easily
find a match regarding KS in these settings, less known is the
comparison between classic PEL in the elderly and classic KS for the
lack of ad hoc studies. The comparison between the two diseases was not
straightforward. We considered only 12 studies out of more than three
hundred, to collect fragmentary data; excluded articles focused on the
pathogenesis and the molecular landscape of KS, and therapeutic
options. Nevertheless, we attempted to build up a control group of KS
patients to compare it with classic PEL patients. It must be pointed
out that this comparison has limits linked to the difference in the
size of the two groups, that may affect the statistical power. Our
comparison indicates remarkable similarities between PEL and KS in the
elderly population, supporting a clinical variant of PEL paralleling
classic KS but has put in light some differences. In classic-PEL there
is a slightly higher male prevalence, and patients are significantly
older. Other important differences are found comparing the laboratory
data: PEL patients have lower lymphocytes count, CD4 count, and
hemoglobin levels. Classic PEL is far more lethal compared to classic
KS. These divergent aspects raise up some questions: (i) if the classic
variants of PEL and KS have a distinct biologic behavior (very
aggressive, PEL; indolent, KS) that would explain the clinical and
laboratory differences or (ii) if these diseases provoke a different
outcome because occurring in two different groups of hosts. Many
preclinical studies support the first hypothesis. KS spindle cells do
not behave like typical cancer cells; they do not form tumors in nude
mice, and the majority of KS tumors are polyclonal; all KS
tumor-derived cells to date have lost viral genomes upon ex vivo
cultivation. By contrast, PEL cell lines exhibit monoclonality, easily
grow when implanted in nude mice and maintain KSHV/HHV-8 indefinitely.
A few clinical observations support the second hypothesis: the host
status may influence the different clinical course between classic KS
and classic PEL. PEL patients are older than KS patients; their
cellular immunity decrease because of the immunosenescence process that
leads to reduced lymphocytes and CD4 counts, thus favouring KSHV/HHV-8
virulence, PEL cells survival, and worse prognosis and lethality. To
note, a minority of very elderly PEL patients (median age=85) had
concomitant KS in advanced stages with progressive disease.
Nevertheless, the finding of indolent cases of classic PEL and
aggressive and lethal classic KS cases may also be related to the host.
Finally, the worse prognosis of PEL patients may be explained because
this lymphoma is hidden in a body cavity effusion, differently from KS
lesions that arise on the skin and can be detected in their very early
phases.
The present review carries inherent limits associated with
the quality and completeness of the published studies. Considering the
rare occurrence of PEL outside immunodeficiency settings, we preferred
to include rather than exclude all available cases reported as
abstracts, short reports, images, in addition to full articles. Another
controversial issue might be the a priori
exclusion of extracavitary PEL cases. KSHV/HHV-8 has been associated
with a wide and heterogeneous group of lymphoproliferative processes
including liquid-based PEL and tissue-based extracavitary/solid
lymphomas with significant clinicopathological overlap.[106]
Although extracavitary/solid HHV8-positive lymphoma can precede or
follow a typical case of cavity effusion-based PEL, our study aimed
indeed to focus on the effusion-only PEL subgroup. This choice was
based on our case-series study on PEL with effusion-only disease in the
elderly, in the HIV-negative context.[79] Remarkably,
a recent multi-institutional case-series study has reported that 7 out
of 8 HIV-negative PEL patients (median age >75 years) had
effusion-only disease; by contrast, patients with extracavitary PEL
were younger and more likely HIV-positive.[107] The
comparison between classic PEL and classic KS was hampered by the lack
of uniformity in KS studies that often report aggregated data rather
than highlighting individual patient’s profile, so data availability
bias is a potential concern of our analysis. A higher power study with
more cases is warranted to further investigate the differences between
classic PEL and classic KS.
Conclusions
Our
study is the first systematic review of the literature focusing on
classic PEL, a clinicopathological variant of KSHV/HHV-8-related
effusion-only PEL probably underrecognized in the elderly population of
KSHV/HHV-8 endemic areas.
Acknowledgments
This study is dedicated to the memory of Francesco Lo Coco, Professor of Hematology (1955-2019).
References
- Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J,
Knowles DM, Moore PS. Identification of herpesvirus-like DNA sequences
in AIDS-associated Kaposi’s sarcoma. Science. 1994;266: 1865–1869. https://doi.org/10.1126/science.7997879
- Cesarman
E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi’s Sarcoma–Associated
Herpesvirus-Like DNA Sequences in AIDS-Related Body-Cavity–Based
Lymphomas. New England Journal of Medicine. 1995;332: 1186–1191. https://doi.org/10.1056/NEJM199505043321802
- Nador
RG, Cesarman E, Chadburn A, Dawson DB, Ansari MQ, Said J, Knowles DM.
Primary Effusion Lymphoma: A Distinct Clinicopathologic Entity
Associated With the Kaposi’s Sarcoma-Associated Herpes Virus. Blood.
1996;88(2):645-6. https://doi.org/10.1182/blood.V88.2.645.bloodjournal882645
- Said
J, Cesarman E. Primary effusion lymphoma. In: Swerdlow SH, Campo E,
Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, eds. WHO
Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th
ed. IARC Press; 2017:323-324
- Calabrò ML,
Sarid R. Human herpesvirus 8 and lymphoproliferative disorders.
Mediterranean Journal of Hematology and Infectious Diseases.
2018;10(1). https://doi.org/10.4084/mjhid.2018.061
- Ascoli
V, lo Coco F, Torelli G, Vallisa D, Cavanna L, Bergonzi C, et al. Human
herpesvirus 8-associated primary effusion lymphoma in human
immunodeficiency virus-negative patients: A clinico-epidemiologic
variant resembling classic Kaposi’s sarcoma. Haematologica.
2002;87:339–43. https://moh-it.pure.elsevier.com/en/publications/human-herpesvirus-8-associated-primary-effusion-lymphoma-in-human
- Khoury
J, Willner CA, Gbadamosi B, Gaikazian S, Jaiyesimi I. Demographics and
Survival in Primary Effusion Lymphoma: SEER Database Analysis. Blood.
2018;132: 5397–5397. https://doi.org/10.1182/blood-2018-99-115473
- Cesarman E, Damania B, Krown SE, Martin J, Bower M, Whitby D. Kaposi sarcoma. Nature Reviews Disease Primers. 2019;5. https://doi.org/10.1038/s41572-019-0060-9
- Stewart
LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, Tierney JF.
Preferred reporting items for a systematic review and meta-analysis of
individual participant data: The PRISMA-IPD statement. JAMA - Journal
of the American Medical Association. 2015; 313: 1657–1665. https://doi.org/10.1001/jama.2015.3656
- Nador
RG, Cesarman E, Knowles DM, Said JW. Herpes-Like DNA Sequences in a
Body-Cavity–Based Lymphoma in an HIV-Negative Patient. New England
Journal of Medicine. 1995;333: 943–943. https://www.nejm.org/doi/full/10.1056/NEJM199510053331417
- Strauchen
JA, Hauser AD, Burstein D, Jimenez R, Moore PS, Chang Y. Body Cavity -
Based Malignant Lymphoma Containing Kaposi Sarcoma - Associated
Herpesvirus in an HIV-Negative Man with Previous Kaposi Sarcoma. Annals
of Internal Medicine. 1996;125: 822–825. https://doi.org/10.7326/0003-4819-125-10-199611150-00006
- Carbone
A, Gloghini A, Vaccher E, Zagonel V, Pastore C, Palma PD, Branz F,
Saglio G, Volpe R, Tirelli U, Gaidano G. Kaposi’s sarcoma-associated
herpesvirus DNA sequences in AIDS-related and AIDS-unrelated
lymphomatous effusions. British Journal of Haematology.
1996;94(3):533-43. https://doi.org/10.1046/j.1365-2141.1996.d01-1826.x
- Said
JW, Tasaka T, Takeuchi S, Asou H, de Vos S, Cesarman E, Knowles DM,
Koeffler HP. Primary effusion lymphoma in women: Report of two cases of
Kaposi’s sarcoma herpes virus-associated effusion-based lymphoma in
human immunodeficiency virus-negative women. Blood. 1996;88: 3124–3128.
https://doi.org/10.1182/blood.v88.8.3124.bloodjournal8883124
- Okada
T, Katano H, Tsutsumi H, Kumakawa T, Sawabe M, Arai T, et al.
Body-cavity-based lymphoma in an elderly AIDS-unrelated male.
International Journal of Hematology. 1998;67:417-422. https://doi.org/10.1016/s0925-5710(98)00014-0
- Teruya-Feldstein
J, Zauber P, Setsuda JE, Berman EL, Sorbara L, Raffeld M, Tosato G,
Jaffe ES. Expression of human herpesvirus-8 oncogene and cytokine
homologues in an HIV-seronegative patient with multicentric Castleman's
disease and primary effusion lymphoma. Lab Invest. 1998
Dec;78(12):1637-42. Erratum in: Lab Invest 1999;79(7):835.
- Vu
HN, Jenkins FW, Swerdlow SH, Locker J, Lotze MT. Pleural effusion as
the presentation for primary effusion lymphoma. Surgery. 1998;123:
589–591. https://doi.org/10.1067/msy.1998.88087
- Ascoli
V, Scalzo CC, Danese C, Vacca K, Pistilli A, lo Coco F. Human herpes
virus-8 associated primary effusion lymphoma of the pleural cavity in
HIV-negative elderly men. Eur Respir J. 1999 Nov;14:1231-1234. https://erj.ersjournals.com/content/14/5/1231
- San
Miguel P, Manzanal A, García González R, Bellas C. Association of body
cavity-based lymphoma and human herpesvirus 8 in an HIV-seronegative
male: Report of a case with immunocytochemical and molecular studies.
Acta Cytologica. 1999;43: 299–302. https://doi.org/10.1159/000330998
- Ariad
S, Benharroch D, Lupu L, Davidovici B, Dupin N, Boshoff C. Early
peripheral lymph node involvement of human herpesvirus 8- associated,
body cavity-based lymphoma in a human immunodeficiency virus- negative
patient. Archives of Pathology and Laboratory Medicine. 2000;124:
753–755. https://doi.org/10.5858/2000-124-0753-eplnio
- Codish
S, Abu-Shakra M, Ariad S, Zirkin HJ, Yermiyahu T, Dupin N, Boshoff C,
Sukenik S. Manifestations of three KSHV/HHV-8-related diseases in an
HIV-negative patient: Immunoblastic variant multicentric Castleman’s
disease, primary effusion lymphoma, and Kaposi’s sarcoma. American
Journal of Hematology. 2000;65: 310–314. https://doi.org/10.1002/1096-8652(200012)65:4<310::AID-AJH11>3.0.CO;2-G
- Polskj
JM, Evans HL, Grosso LE, Popovic WJ, Taylor L, Dunphy CH. CD7 and
CD56-Positive Primary Effusion Lymphoma in a Human Immunodeficiency
Virus-Negative Host. Leukemia and Lymphoma. 2000;39: 633–639. https://doi.org/10.3109/10428190009113394
- Lechapt-Zalcman
E, Challine D, Ne Delfau-Larue M-H, Haioun C, Desvaux D, Gaulard P.
Association of Primary Pleural Effusion Lymphoma of T-Cell Origin and
Human Herpesvirus 8 in a Human Immunodeficiency Virus-Seronegative Man.
Arch Pathol Lab Med. 2001. https://doi.org/10.5858/2001-125-1246-AOPPEL
- Pérez
CL, Rudoy S. Anti-CD20 monoclonal antibody treatment of human
herpesvirus 8-associated, body cavity-based lymphoma with an unusual
phenotype in a human immunodeficiency virus-negative patient. Clinical
and Diagnostic Laboratory Immunology. 2001;8: 993–996. https://:10.1128/CDLI.8.5.993-996.2001
- Klepfish
A, Sarid R, Shtalrid M, Shvidel L, Berrebi A, Schattner A. Primary
effusion lymphoma (PEL) in HIV-negative patients - A distinct clinical
entity. Leukemia and Lymphoma. 2001;41: 439–443. https://doi.org/10.3109/10428190109058002
- Boulanger
E, Hermine O, Fermand JP, Radford-Weiss I, Brousse N, Meignin V,
Gessain A. Human Herpesvirus 8 (KSHV/HHV-8)-Associated Peritoneal
Primary Effusion Lymphoma (PEL) in Two HIV-Negative Elderly Patients.
American Journal of Hematology. 2004;76: 88–91. https://doi.org/10.1002/ajh.20048
- Munichor
M, Cohen H, Sarid R, Manov I, Iancu TC. Human herpesvirus 8 in primary
effusion lymphoma in an HIV-seronegative male: A case report. Acta
Cytologica. 2004;48: 425–430. https://doi.org/10.1159/000326398
- Luppi
M, Trovato R, Barozzi P, Vallisa D, Rossi G, Re A, Ravazzini L, Potenza
L, Riva G, Morselli M, Longo G, Cavanna L, Roncaglia R, Torelli G.
Treatment of herpesvirus associated primary effusion lymphoma with
intracavity cidofovir. Leukemia. Nature Publishing Group; 2005. pp.
473–476. https://doi.org/10.1038/sj.leu.2403646
- Halfdanarson
TR, Markovic SN, Kalokhe U, Luppi M. A non-chemotherapy treatment of a
primary effusion lymphoma: Durable remission after intracavitary
cidofovir in HIV negative PEL refractory to chemotherapy. Annals of
Oncology. 2006;17:1849–1850. https://doi.org/10.1093/annonc/mdl139
- Won
JH, Han SH, Bae SB, Kim CK, Lee NS, Lee KT, Park SK, Hong DS, Lee DW,
Park HS. Successful eradication of relapsed primary effusion lymphoma
with high-dose chemotherapy and autologous stem cell transplantation in
a patient seronegative for human immunodeficiency virus. International
Journal of Hematology. 2006;83: 328–330. https://doi.org/10.1532/IJH97.A30510
- Hsieh
PY, Huang SI, Li DK, Mao TL, Sheu JC, Chen CH. Primary effusion
lymphoma involving both pleural and abdominal cavities in a patient
with hepatitis B virus-related liver cirrhosis. Journal of the Formosan
Medical Association. 2007;106: 504–508. https://doi.org/10.1016/S0929-6646(09)60302-8
- Siddiqi
T, Joyce RM. A case of HIV-negative primary effusion lymphoma treated
with bortezomib, pegylated liposomal doxorubicin, and rituximab.
Clinical Lymphoma and Myeloma. 2008;8: 300–304. https://doi.org/10.3816/CLM.2008.n.042
- Cobo
F, Hernández S, Hernández L, Pinyol M, Bosch F, Esteve J,
López-Guillermo A, Palacín A, Raffeld M, Montserrat E, Jaffe ES, Campo
E. Expression of potentially oncogenic KSHV/HHV-8 genes in an
EBV-negative primary effusion lymphoma occurring in an HIV-seronegative
patient. Journal of Pathology. 1999;189: 288–293. https://doi.org/10.1002/(SICI)1096-9896(199910)189:2<288::AID-PATH419>3.0.CO;2-F
- Niitsu
N, Chizuka A, Sasaki K, Umeda M. Human herpes virus-8 associated with
two eases of primary-effusion lymphoma. Annals of Hematology. 2000;79:
336–339. https://doi.org/10.1007/s002779900145
- Buonaiuto
D, Rossi D, Guidetti F, Vivenza D, Berra E, Deambrogi C, Ariatti C,
Franceschetti S, Conconi A, Ronco M, Valente G, Colombi S. Human
herpesvirus type 8-associated primary lymphomatous effusion in an
elderly HIV-negative patient: clinical and molecular characterization.
Annali italiani di medicina interna: organo ufficiale della Società Italiana di Medicina Interna. 2002;17: 54–59.
- Wakely
PE, Menezes G, Nuovo GJ. Primary Effusion Lymphoma: Cytopathologic
Diagnosis Using In Situ Molecular Genetic Analysis for Human
Herpesvirus 8. Modern Pathology. 2002;15: 944–950. https://doi.org/10.1038/modpathol.3880635
- Metaxa-Mariatou
V, Papaioannou D, Loli A, Papadopoulou I, Gazouli M, Mavroudis P,
Nasioulas G. Subtype C1 persistent infection of KSHV/HHV-8 in a PEL
patient. Leukemia and Lymphoma. 2005;46: 1507–1512. https://doi.org/10.1080/10428190500161965
- Shirokov
D, Kadyrova E, Anokhina M, Kondratyeva T, Gourtsevich V, Tupitsyn N. A
case of KSHV/HHV-8-associated HIV-negative primary effusion lymphoma in
moscow. Journal of Medical Virology. 2007;79: 270–277.
https://doi.org/10.1002/jmv.20795
- Dargent
JL, Kains JP, Verhest A. Primary effusion lymphoma presenting as
Richter’s syndrome [1]. Cytopathology. 2007. pp. 319–321. https://doi.org/10.1111/j.1365-2303.2007.00465.x
- Su
YC, Chai CY, Chuang SS, Liao YL, Kang WY. Cytologic diagnosis of
primary effusion lymphoma in an HIV-negative patient. Kaohsiung Journal
of Medical Sciences. 2008;24: 548–552. https://doi.org/10.1016/S1607-551X(09)70015-4
- Brimo F, Popradi G, Michel R, Auger M. Primary effusion lymphoma involving three body cavities. CytoJournal. 2009;6. https://doi.org/10.4103/1742-6413.56361
- de
Filippi R, Iaccarino G, Frigeri F, di Francia R, Crisci S, Capobianco
G, Arcamone M, Becchimanzi C, Amoroso B, de Chiara A, Corazzelli G,
Pinto A. Elevation of clonal serum free light chains in patients with
HIV-negative primary effusion lymphoma (PEL) and PEL-like lymphoma.
British Journal of Haematology. 2009. pp. 405–408. https://doi.org/10.1111/j.1365-2141.2009.07846.x
- Wu
SJ, Hung CC, Chen CH, Tien HF. Primary effusion lymphoma in three
patients with chronic hepatitis B infection. Journal of Clinical
Virology. 2009;44: 81–83. https://doi.org/10.1016/j.jcv.2008.08.015
- Kishimoto
K, Kitamura T, Hirayama Y, Tate G, Mitsuya T. Cytologic and
immunocytochemical features of EBV negative primary effusion lymphoma:
Report on seven Japanese cases. Diagnostic Cytopathology. 2009;37:
293–298. https://doi.org/10.1002/dc.21022
- Honda
M, Morikawa T, Yamaguchi Y, Tani M, Yamaguchi K, Iijima K, et al. A
case of a primary effusion lymphoma in the elderly. Japanese Journal of
Geriatrics. 2009;46: 551–556. https://doi.org/10.3143/geriatrics.46.551
- Wang
HY, Fuda FS, Chen W, Karandikar NJ. Notch1 in primary effusion
lymphoma: A clinicopathological study. Modern Pathology. 2010;23:
773–780. https://doi.org/10.1038/modpathol.2010.67
- Makis
W, Stern J. Hepatitis C-related primary effusion lymphoma of the pleura
and peritoneum, imaged with F-18 FDG PET/CT. Clinical Nuclear Medicine.
2010;35: 797–799. https://doi.org/10.1097/RLU.0b013e3181ef09b1
- Stingaciu
S, Ticchioni M, Sudaka I, Haudebourg J, Mounier N. Intracavitary
cidofovir for human herpes virus-8-associated primary effusion lymphoma
in an HIV-negative patient. Clinical Advances in Hematology and
Oncology. 2010;8: 367–372.
- Yiakoumis
X, Pangalis GA, Kyrtsonis MC, Vassilakopoulos TP, Kontopidou FN,
Kalpadakis C, et al. Primary effusion lymphoma in two HIV-negative
patients successfully treated with pleurodesis as first-line therapy.
Anticancer Research. 2010;30: 271–276. https://ar.iiarjournals.org/content/30/1/271/tab-article-info
- Gandhi
SA, Mufti G, Devereux S, Ireland R. Primary effusion lymphoma in an
HIV-negative man. British Journal of Haematology. 2011;155:411. https://doi.org/10.1111/j.1365-2141.2011.08778.x
- Ganzel
C, Rowe JM, Ruchlemer R. Primary effusion lymphoma in a HIV-negative
patient associated with hypogammaglobulinemia. American Journal of
Hematology. 2011;86:777–781. https://doi.org/10.1002/ajh.22068
- Kumar
T, Rosen M, Breuer F. Primary Effusion Lymphoma Involving Pleural and
Peritoneal Cavities in An HIV Negative Patient.
2011.183.1_meetingabstracts.a2953. https://doi.org/10.1164/ajrccm-conference
- Nakayama-Ichiyama
S, Yokote T, Kobayashi K, Hirata Y, Hiraoka N, Iwaki K, Takayama A,
Akioka T, Oka S, Miyoshi T, Fukui H, Tsuda Y, Takubo T, Tsuji M,
Higuchi K, Hanafusa T. Primary effusion lymphoma of T-cell origin with
t(7;8)(q32;q13) in an HIV-negative patient with HCV-related liver
cirrhosis and hepatocellular carcinoma positive for HHV6 and
KSHV/HHV-8. Annals of Hematology. 2011;90:1229–1231. https://doi.org/10.1007/s00277-011-1165-8
- Zacharis
E, Ntoula E, Sevastiadou M, Fragkopoulos C, Gklisti E, Alexiadou A,
Plyta S, Panousi A. Paramount role of ancillary techniques in
diagnosing a rare incident of primary effusion lymphoma by fine needle
aspiration cytology. Cytopathology. 2011;22(s1):150. Available: https://www.embase.com/search/results?subaction=viewrecord&id=L70678203&from=export%0Ahttp://dx.doi.org/10.1111/j.1365-2303.2011.00911.x
- Kabiawu-Ajise
OE, Harris J, Ismaili N, Amorosi E, Ibrahim S. Primary effusion
lymphoma with central nervous system involvement in an HIV-negative
homosexual male. Acta Haematologica. 2012;128: 77–82. https://doi.org/10.1159/000338183
- Lobo
C, Amin S, Ramsay A, Diss T, Kocjan G. Serous fluid cytology of
multicentric Castleman’s disease and other lymphoproliferative
disorders associated with Kaposi sarcoma-associated herpes virus: A
review with case reports. Cytopathology. 2012;55: 76–85. https://doi.org/10.1111/j.1365-2303.2011.00868.x
- Nepka
C, Kanakis D, Samara M, Kapsoritakis A, Potamianos S, Karantana M, et
al. An unusual case of Primary Effusion Lymphoma with aberrant T-cell
phenotype in a HIV-negative, HBV-positive, cirrhotic patient, and
review of the literature. CytoJournal. 2012;9:16. https://doi.org/10.4103/1742-6413.97766
- Karataş
SG, Bayrak R, Balçk ÖŞ, Yalçn KS, Atici E, Akyildiz Ü, Koşar A. Human
Immunodeficiency Virus (HIV)-Negative and Human Herpes Virus-8
(KSHV/HHV-8)-Positive Primary Effusion Lymphoma: A Case Report and
Review of the Literature. Turkish Journal of Hematology. 2013;30:
67–71. https://doi.org/10.4274/tjh.53215
- Kumode
T, Ohyama Y, Kawauchi M, Yamaguchi T, Miyatake JI, Hoshida Y, Tatsumi
Y, Matsumura I, Maeda Y. Clinical importance of human herpes virus-8
and human immunodeficiency virus infection in primary effusion
lymphoma. Leukemia and Lymphoma. 2013;54: 1947–1952. https://doi.org/10.3109/10428194.2012.763122
- Ozbalak
M, Tokatli I, Özdemirli M, Tecimer T, Ar MC, Örnek S, et al. Is
valganciclovir really effective in primary effusion lymphoma: Case
report of an HIV(-) EBV(-) KSHV/HHV-8(+) patient. European Journal of
Haematology. 2013;91: 467–469. https://doi.org/10.1111/ejh.12174
- Song JY, Jaffe ES. KSHV/HHV-8-Positive but EBV-Negative primary effusion lymphoma. Blood. 2013. 3712. https://doi.org/10.1182/blood-2013-07-515882
- Antar
A, Hajj H el, Jabbour M, Khalifeh I, EL-Merhi F, Mahfouz R, et al.
Primary effusion lymphoma in an elderly patient effectively treated by
lenalidomide: Case report and review of literature. Blood Cancer
Journal. 2014;4:e190. https://doi.org/10.1038/bcj.2014.6
- Sasaki
Y, Isegawa T, Shimabukuro A, Yonaha T, Yonaha H. Primary Effusion
Lymphoma in an Elderly HIV-Negative Patient with Hemodialysis:
Importance of Evaluation for Pleural Effusion in Patients Receiving
Hemodialysis. Case Reports in Nephrology and Urology. 2014;4: 95–102. https://doi.org/10.1159/000363223
- Wang
HY, Thorson JA. T-cell primary effusion lymphoma with pseudo-monoclonal
rearrangements for immunoglobulin heavy chain. Blood. American Society
of Hematology. 2015;126: 1856. https://doi.org/10.1182/blood-2015-06-648923
- Klepfish
A, Zuckermann B, Schattner A. Primary effusion lymphoma in the absence
of HIV infection-clinical presentation and management. QJM. 2015;108:
481–488. https://doi.org/10.1093/qjmed/hcu232
- Nicola
M, Onorati M, Bianchi CL, Pepe G, Bellone S, di Nuovo F. Primary
Effusion Lymphoma: Cytological Diagnosis of a Rare Entity-Report of Two
Cases in HIV-Uninfected Patients from a Single Institution. Acta
Cytologica. 2015;59: 425–428. https://doi.org/10.1159/000441938
- Birsen
R, Boutboul D, Crestani B, Seguin-Givelet A, Fieschi C, Bertinchamp R,
Giol M, Malphettes M, Oksenhendler E, Galicier L. Talc pleurodesis
allows long-term remission in HIV-unrelated Human Herpesvirus
8-associated primary effusion lymphoma. Leukemia and Lymphoma.
2017;58:1993-1998. https://doi.org/10.1080/10428194.2016.1271947
- Gonzalez-Farre
B, Martinez D, Lopez-Guerra M, Xipell M, Monclus E, Rovira J, Garcia F,
Lopez-Guillermo A, Colomo L, Campo E, Martinez A. HHV8-related lymphoid
proliferations: A broad spectrum of lesions from reactive lymphoid
hyperplasia to overt lymphoma. Modern Pathology. 2017;30: 745–760. https://doi.org/10.1038/modpathol.2016.233
- Chan
TSY, Mak V, Kwong YL. Complete radiologic and molecular response of
HIV-negative primary effusion lymphoma with short-course lenalidomide.
Annals of Hematology. 2017;96: 1211–1213. https://doi.org/10.1007/s00277-017-2995-9
- Yamani
F, Chen W. Cytokeratin-positive primary effusion lymphoma: a diagnostic
challenge. British Journal of Haematology. 2018;180: 9. https://doi.org/10.1111/bjh.14904
- Galán
J, Martin I, Carmona I, Rodriguez-Barbero JM, Cuadrado E, García-Alonso
L, García-Vela JA. The utility of multiparametric flow cytometry in the
detection of primary effusion lymphoma (PEL). Cytometry Part B -
Clinical Cytometry. 2019;96: 375–378. https://doi.org/10.1002/cyto.b.21637
- Li
Y, Henn P, Zhang Y, Sands A, Zhang N. 160 Case Report: Primary Effusion
Lymphoma in an 88-Year-Old Man. American Journal of Clinical Pathology.
2018;149: S68–S69. https://doi.org/10.1093/ajcp/aqx121.159
- Mirza
AS, Dholaria BR, Hussaini M, Mushtaq S, Horna P, Ravindran A, Kumar A,
Ayala E, Kharfan-Dabaja MA, Bello C, Chavez JC, Sokol L. High-dose
Therapy and Autologous Hematopoietic Cell Transplantation as
Consolidation Treatment for Primary Effusion Lymphoma. Clinical
Lymphoma, Myeloma and Leukemia. 2019;19: e513–e520. https://doi.org/10.1016/j.clml.2019.03.021
- Shin
J, Ko YH, Oh SY, Yoon DH, Lee JO, Kim JS, Park Y, Shin HJ, Kim SJ, Won
JH, Yoon SS, Kim WS, Koh Y. Body Cavity-Based Lymphoma in a Country
with Low Human Immunodeficiency Virus Prevalence: A Series of 17 Cases
from the Consortium for Improving Survival of Lymphoma. Cancer Research
and Treatment. 2019;51:1302–12. https://doi.org/10.4143/crt.2018.555
- Aguilar
C, Laberiano C, Beltran B, Diaz C, Taype-Rondan A, Castillo JJ.
Clinicopathologic characteristics and survival of patients with primary
effusion lymphoma. Leukemia and Lymphoma. 2020;61: 2093–2102. https://doi.org/10.1080/10428194.2020.1762881
- Kropf
J, Gerges M, Perez AP, Ellis A, Mathew M, Ayesu K, Ge L, Carlan SJ. T
Cell Primary Effusion Lymphoma in an HIV-Negative Man with Liver
Cirrhosis. Am J Case Rep. 2020;21:e919032. https://doi.org/10.12659/AJCR.919032
- Kuo
HI, Yu YT, Chang KC, Su PL, Li SS, Huang TH. Human herpesvirus
8-related primary effusion lymphoma in four HIV-uninfected patients
without organ transplantation. Respirology Case Reports. 2020;8:e00508.
https://doi.org/10.1002/rcr2.508
- Parente
P, Zanelli M, Zizzo M, Covelli C, Carosi I, Ascani S, Graziano P.
Primary effusion lymphoma metachronous to multicentric Castleman
disease in an immunocompetent patient. Pathology Research and Practice.
2020;216:153024. https://doi.org/10.1016/j.prp.2020.153024
- Yuan
L, Cook JR, Elsheikh TM. Primary effusion lymphoma in human immune
deficiency (HIV)-negative non-organ transplant immunocompetent
patients. Diagnostic Cytopathology. 2020;48: 380–385. https://doi.org/10.1002/dc.24371
- Rossi
G, Cozzi I, della Starza I, de Novi LA, de Propris MS, Gaeta A,
Petrucci L, Pulsoni A, Pulvirenti F, Ascoli V. Human
herpesvirus-8–positive primary effusion lymphoma in HIV-negative
patients: Single institution case series with a multidisciplinary
characterization. Cancer Cytopathology. 2021;129: 62–74. https://doi.org/10.1002/cncy.22344
- Chen
BJ, Wang RC, Ho CH, Yuan CT, Huang WT, Yang SF, Hsieh PP, Yung YC, Lin
SY, Hsu CF, Su YZ, Kuo CC, Chuang SS. Primary effusion lymphoma in
Taiwan shows two distinctive clinicopathological subtypes with rare
human immunodeficiency virus association. Histopathology. 2018;72:
930–944. https://doi.org/10.1111/his.13449
- Fernández-Trujillo
L, Bolaños JE, Velásquez M, García C, Sua LF. Primary effusion lymphoma
in a human immunodeficiency virus-negative patient with unexpected
unusual complications: A case report. Journal of Medical Case Reports.
2019;23:13. https://doi.org/10.1186/s13256-019-2221-6
- Régnier-Rosencher
E, Guillot B, Dupin N. Treatments for classic Kaposi sarcoma: A
systematic review of the literature. Journal of the American Academy of
Dermatology. 2013;68: 313–331. https://doi.org/10.1016/j.jaad.2012.04.018
- Lospalluti
M, Mastrolonardo M, Loconsole F, Conte A, Rantuccio F. Classical
Kaposi’s sarcoma: A survey of 163 cases observed in Bari, South Italy.
Dermatology. 1995;191: 104–108. https://doi.org/10.1159/000246525
- Goedert
JJ, Vitale F, Lauria C, Serraino D, Tamburini M, Montella M, Messina A,
Brown EE, Rezza G, Gafà L, Romano N. Risk factors for classical
Kaposi’s sarcoma. Journal of the National Cancer Institute. 2002;94:
1712–1718. https://doi.org/10.1093/jnci/94.22.1712
- Brenner
B, Weissmann-Brenner A, Rakowsky E, Weltfriend S, Fenig E,
Friedman-Birnbaum R, Sulkes A, Linn S. Classical Kaposi sarcoma:
Prognostic factor analysis of 248 patients. Cancer. 2002;95: 1982–1987.
https://doi.org/10.1002/cncr.10907
- Stratigos
AJ, Malanos D, Touloumi G, Antoniou A, Potouridou I, Polydorou D,
Katsambas AD, Whitby D, Mueller N, Stratigos JD, Hatzakis A.
Association of clinical progression in classic Kaposi’s sarcoma with
reduction of peripheral B lymphocytes and partial increase in serum
immune activation markers. Archives of Dermatology. 2005;141:
1421–1426. https://doi.org/10.1001/archderm.141.11.1421
- Cottoni
F, Masala MV, Pattaro C, Pirodda C, Montesu MA, Satta R, Cerimele D, de
Marco R. Classic Kaposi sarcoma in northern Sardinia: A prospective
epidemiologic overview (1977-2003) correlated with malaria prevalence
(1934). Journal of the American Academy of Dermatology. 2006;55:
990–995. https://doi.org/10.1016/j.jaad.2006.03.007
- Brambilla
L, Bellinvia M, Tourlaki A, Scoppio B, Gaiani F, Boneschi V.
Intralesional vincristine as first-line therapy for nodular lesions in
classic Kaposi sarcoma: A prospective study in 151 patients. British
Journal of Dermatology. 2010;162: 854–859. https://doi.org/10.1111/j.1365-2133.2009.09601.x
- Errihani
H, Berrada N, Raissouni S, Rais F, Mrabti H, Rais G. Classic Kaposi’s
sarcoma in Morocco: Clinico-epidemiological study at the national
institute of oncology. BMC Dermatology. 2011;11:15. https://doi.org/10.1186/1471-5945-11-15
- Régnier-Rosencher
E, Boutron I, Avril MF, Dupin N. Do anti-hypertensive renin-angiotensin
system inhibitors contribute to the development of classical Kaposi
sarcoma? Journal of the European Academy of Dermatology and
Venereology. 2016;30: 1199–1201. https://doi.org/10.1111/jdv.13120
- Cetin
B, Aktas B, Bal O, Algin E, Akman T, Koral L, Kaplan MA, Demirci U,
Uncu D, Ozet A. Classic Kaposi’s sarcoma: A review of 156 cases.
Dermatologica Sinica. 2018;36: 185–189. https://doi.org/10.1016/j.dsi.2018.06.005
- Kandaz
M, Bahat Z, Guler OC, Canyilmaz E, Melikoglu M, Yoney A. Radiotherapy
in the management of classic Kaposi’s sarcoma: A single institution
experience from Northeast Turkey. Dermatologic Therapy. 2018;31:e12605.
https://doi.org/10.1111/dth.12605
- Marcoval
J, Bonfill-Ortí M, Martínez-Molina L, Valentí-Medina F, Penín RM,
Servitje O. Evolution of Kaposi sarcoma in the past 30 years in a
tertiary hospital of the European Mediterranean basin. Clinical and
Experimental Dermatology. 2019;44: 32–39. https://doi.org/10.1111/ced.13605
- Denis
D, Seta V, Regnier-Rosencher E, Kramkimel N, Chanal J, Avril MF, Dupin
N. A fifth subtype of Kaposi’s sarcoma, classic Kaposi’s sarcoma in men
who have sex with men: a cohort study in Paris. Journal of the European
Academy of Dermatology and Venereology. 2018;32: 1377–1384. https://doi.org/10.1111/jdv.14831
- Richetta
A, Amoruso GF, Ascoli V, Natale ME, Carboni V, Carlomagno V, Pezza M,
Cimillo M, Maiani E, Mattozzi C, Calvieri S. PEL, Kaposi’s sarcoma
KSHV/HHV-8+ and idiopathic T-lymphocitopenia CD4+. Clinica Terapeutica.
2007;158: 151–155.
- Countries with the oldest population in the world. [cited 13 Sep 2021]. https://www.prb.org/resources/countries-with-the-oldest-populations-in-the-world/
- Berberich
S, Förster R, Pabst O. The peritoneal micromilieu commits B cells to
home to body cavities and the small intestine. Blood.
2007;109:4627-4634. https://doi.org/10.1182/blood-2006-12-064345
- Banke
A, Schou M, Videbæk L, Møller JE, Torp-Pedersen C, Gustafsson F, Dahl
JS, Køber L, Hildebrandt PR, Gislason GH. Incidence of cancer in
patients with chronic heart failure: A long-term follow-up study.
European Journal of Heart Failure. 2016;18: 260–266. https://doi.org/10.1002/ejhf.472
- Ascoli
V, lo Coco F, Artini M, Levrero M, Fruscalzo A, Mecucci C. Primary
effusion Burkitt’s lymphoma with t(8;22) in a patient with hepatitis C
virus related cirrhosis. Human Pathology. 1997;28: 101–104. https://doi.org/10.1016/S0046-8177(97)90287-2
- Vogt
A, Schmid S, Heinimann K, Frick H, Herrmann C, Cerny T, Omlin A.
Multiple primary tumours: Challenges and approaches, a review. ESMO
Open. 2017;2:e000172 https://doi.org/10.1136/esmoopen-2017-000172
- Luciani
A, Ascione G, Marussi D, Oldani S, Caldiera S, Bozzoni S, Codecà C,
Zonato S, Ferrari D, Foa P. Clinical analysis of multiple primary
malignancies in the elderly. Medical Oncology. 2009;26: 27–31. https://doi.org/10.1007/s12032-008-9075-x
- Iscovich
J, Boffetta P, Franceschi S, Azizi E, Sarid R. Classic Kaposi sarcoma:
Epidemiology and risk factors. Cancer. 2000;88: 500–517. https://doi.org/10.1002/(SICI)1097-0142(20000201)88:3<500::AID-CNCR3>3.0.CO;2-9
- Franceschi
C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new
immune–metabolic viewpoint for age-related diseases. Nature Reviews
Endocrinology. 2018;14:576-590. https://doi.org/10.1038/s41574-018-0059-4
- Ascoli
V, Calabrò ML, Giannakakis K, Barbierato M, Chieco-Bianchi L, Gastaldi
R, Narciso P, Gaidano G, Capello D. Kaposi’s sarcoma-associated
herpesvirus/human herpesvirus 8-associated polyclonal body cavity
effusions that mimic primary effusion lymphomas. International Journal
of Cancer. 2006;119: 1746–1748. https://doi.org/10.1002/ijc.21965
- Boulanger
E, Gérard L, Gabarre J, Molina JM, Rapp C, Abino JF, Cadranel J,
Chevret S, Oksenhendler E. Prognostic factors and outcome of human
herpesvirus 8-associated primary effusion lymphoma in patients with
AIDS. Journal of Clinical Oncology. 2005;23: 4372–4380. https://doi.org/10.1200/JCO.2005.07.084
- Vega
F, Miranda RN, Medeiros LJ. KSHV/HHV8-positive large B-cell lymphomas
and associated diseases: a heterogeneous group of lymphoproliferative
processes with significant clinicopathological overlap. Mod Pathol.
2020 Jan;33(1):18-28. https://doi.org/10.1038/s41379-019-0365-y
- Hu
Z, Pan Z, Chen W, Shi Y, Wang W, Yuan J, Wang E, Zhang S, Kurt H, Mai
B, Zhang X, Liu H, Rios AA, Ma HY, Nguyen ND, Medeiros LJ, Hu S.
Primary Effusion Lymphoma: A Clinicopathological Study of 70 Cases.
Cancers (Basel). 2021 Feb 19;13(4):878. https://doi.org/10.3390/cancers13040878
[TOP]



