Claudio Ucciferri1,2, Jacopo Vecchiet1, Antonio Auricchio1 and Katia Falasca1.
1 Clinic of Infectious Diseases – Department of Medicine and Science of Aging, University "G. d'Annunzio" Chieti-Pescara– Italy.
2 Department of Medicine and Health Sciences, University of Molise – Campobasso – Italy.
Correspondence to:
Falasca Katia. Clinic of Infectious Diseases, Dept. of Medicine
and Science of Aging, University “G. D’Annunzio” School of Medicine,
Via dei Vestini, 66100 Chieti – Italy. Tel. +39-0871-358595. E-mail:
k.falasca@unich.it
Published: March 1, 2022
Received: November 1, 2021
Accepted: February 11, 2022
Mediterr J Hematol Infect Dis 2022, 14(1): e2022023 DOI
10.4084/MJHID.2022.023
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
To the editor
A
new pandemic emerged last year for the healthcare community worldwide:
Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-Cov2).
Coronavirus disease 2019 (Covid-19) has affected hundreds of millions
of people globally since it was declared.[1] Different studies on Covid-19 try to find an effective therapy for the virological phase[2,3] and the immunological phase.[4-8]
Several vaccines have been developed to stop the spread of the virus
and gain mass immunity. BNT162b2 mRNA vaccine is largely effective and
is widely administered in high-risk populations.[9]
However, despite the high effectiveness, vaccination can be associated
with grade 1-2 local reactions (pain at the injection, injection-site
redness, or swelling) and systemic reaction (fatigue, fever, headache).[10]
These reactions discourage vaccination in some people. The use of drugs
capable of rebalancing the activity of the immune system against
infections, such as pidotimod, could reduce the adverse effects and get
the immunological vaccine response.[5,11]
Based on these premises, a study was designed to verify whether the use
of pidotimod mediated the immune response linked to the second dose of
Covid-19 vaccination, evaluating as an endpoint the adverse effects and
immunological response associated with injection of the second dose of
BNT162b2 mRNA vaccine into a healthy population in subjects taking
Pidotimod versus a control group taking no therapy.
We designed a
single-center cohort study to test this hypothesis by enrolling
healthcare workers (nurses and doctors working at the Infectious
Diseases Clinic, University' G. D'Annunzio', SS Annunziata Hospital of
Chieti Italy), undergoing the BNT162b2 mRNA vaccination from January to
February 2021. Clinical and demographical data were collected for each
participant. All nurses and doctors working in the Infectious Diseases
Clinic (Covid-19 unit) who had carried out the first dose of the
BNT162b2 mRNA vaccine were proposed to participate, up to the
enrollment of 30 participants (12 of which were male (40%), and all of
them were of Caucasian ethnicity with a median age of 48 years),
excluding all healthcare workers with a paste or present diagnosis of
COVID-19. All the participants were negative for the SARS-CoV-2
molecular swab at the enrollment. All participants were randomized to
receive Pidotimod or not. A total of 10 participants took Pidotimod 800
mg bid orally fasting from the fourth day before the second dose of the
BNT162b2 mRNA vaccine for six days. The remaining 20 participants did
not take any therapy. Demographic, clinical, and adverse event data
were collected one week after the vaccination. The two groups of
subjects, with and without supplementation with pidotimod, were
homogeneous for age and sex.
All participants filled out a
questionnaire investigating the following effects: pain, redness,
swelling and pain in the injection site, headache, fatigue,
musculoskeletal pain, fever, gastrointestinal symptoms, itching,
lymphadenopathy, difficulty falling asleep/insomnia, agitation, skin
rash, anaphylaxis, and others.
A plasma sample was collected in
all participants five days before the second vaccination dose and seven
days after the second dose to measure the SARS-CoV-2 IgM and IgG levels
developed.
All participants vaccinated had no adverse events
immediately (within an hour) after vaccine administration. No
significant differences were found between the anti-SARS-Cov2 IgM and
IgG levels before vaccination of the two groups. Likewise, we found no
significant differences between the two groups comparing the
anti-SARS-CoV2 IgM levels post-vaccination. All the components of the
"pidotimod group" increased their IgM value versus the 65% of the
control group (p<0.05). The SARS-Cov2 IgG levels were statistically
increased after vaccination in both groups, but we have not found
significant differences between the groups (Table 1).
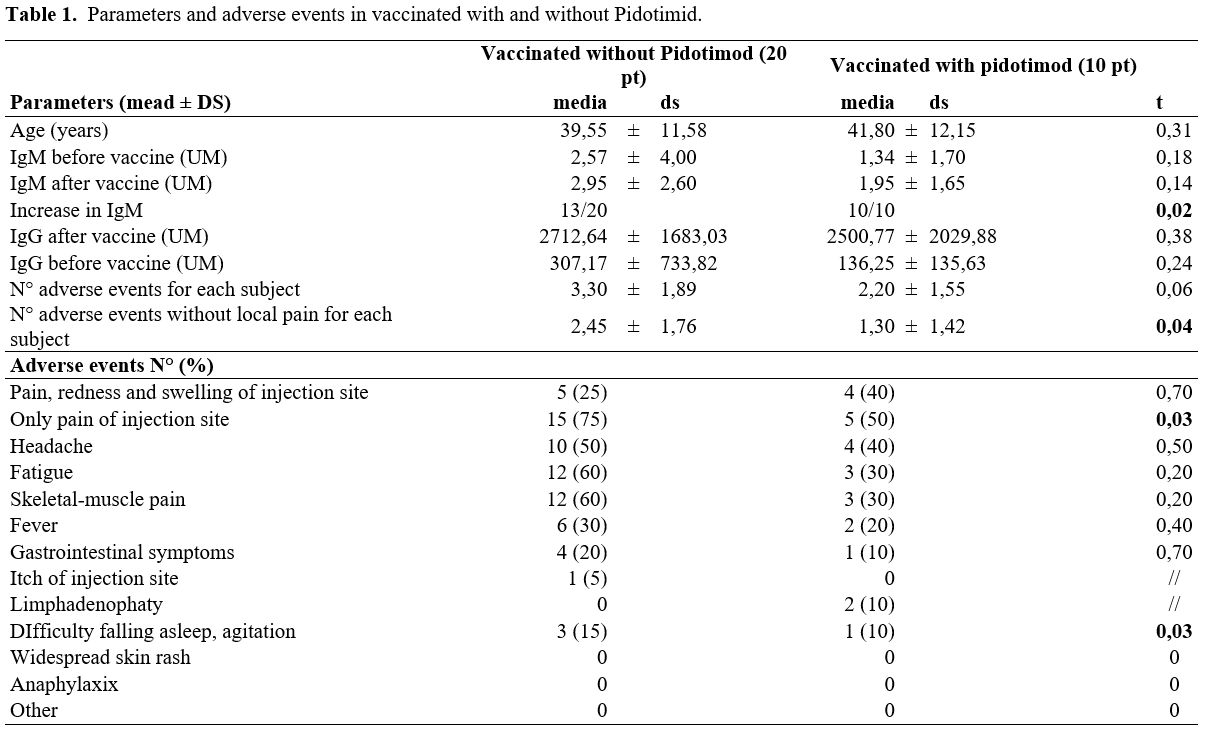 |
Table
1. Parameters and adverse events in vaccinated with and without Pidotimid. |
The
number of total adverse events described in the control group (without
supplementation) was higher than in the group with pidotimod
supplementation, and the difference is significant (p< 0.05) if we
compare the number of adverse events described, excluding the injection
site pain that is the most common and expected event between the two
groups (Table 1).
Analyzing
the adverse events in detail, in the group of subjects supplemented
with pidotimod, fewer cases of pain at the injection site of the
vaccine are described, and fewer cases of difficulty falling asleep and
agitation than in the control group (Table 1).
This
study describes a strategy to reduce the adverse events without
reducing the immunologic response to SARS-CoV-2 vaccination.
The study's main finding is the evidence of reduction of vaccination-related adverse events by using pidotimod.
BNT162b2
vaccine is a nucleoside-modified mRNA vaccine developed by Pfizer and
BioNTech to prevent COVID-19. BNT162b2, like gene-based vaccines,
carries genetic instructions for producing an antigen by the vaccine
recipient cells; specifically, the target is the antigen of the surface
spike protein, which is used by the virus to bind and fuse with host
cells.[12,13] BNT162b2 administered as two 30 µg doses 21 days apart was generally well tolerated in the studies.[9]
The registration study showed reactogenicity in 8183/21720
participants. BNT162b2 vaccine provoked local reactions, mainly
mild-to-moderate pain at the injection site (more than 80%). A
noticeably lower percentage of participants reported injection-site
redness or swelling. Systemic events were reported more often in
younger than the older population. Systemic reactions are often
described more after the second dose than after the first. The
most-reported systemic events were fatigue and headache (more than
50%). After the second dose, more than 15% of participants reported
fever. Severe systemic events were reported in less than 2% of vaccine
recipients.[9]
These reactions are linked to the immune response established in the patient.[14]
These effects are often disabling, leading vaccinators to take time off
work or the need to take medications. Therefore, it may discourage some
candidates from vaccinating.
Therefore, it is useful to search
for a substance that reduces adverse events without altering, but
possibly improving, the immune response to the vaccine. The
immunomodulating molecule pidotimod appeared an ideal candidate.
Pidotimod is a dipeptide able to act on immune activities, as
demonstrated in previous studies, by improving macrophages' function
and increasing the secretion of certain cytokines.[15,16]
The effect of Pidotimod was previously analyzed in the elderly,
demonstrating its immunostimulatory effect, able to improve T cells
proliferation;[17-19] this finding has recently also been demonstrated for the HIV positive population.[11]
From
the clinical point of view, pidotimod in coadministration with
influenza vaccination, in a chronic obstructive pulmonary disease adult
study, showed a lower exacerbation in patients to the placebo.[20]
In
a recent study in outpatient populations affected by SARS-CoV2
infection, pidotimod appeared to be a valid option to reduce the
duration of symptoms in patients, as an earlier defervescence could
prevent the indolent course of cytokine cascade activation.[5]
Starting
from these assumptions, the data of our work in the healthy population
has shown that it is possible to reduce the rate of events related to
the reactogenicity of the vaccine.
An interesting remark is an
increase in IgM levels in all the subjects of the pidotimod group,
which could represent a booster effect on the subsequent immunological
memory developed by the subjects.
On the other hand, there were no
significant differences in IgG levels, probably due to a limited period
of observation and the small size of the sample.
This work
demonstrates how pidotimod improves tolerability, not interfering with
the production of antibodies in subjects. The findings described in
this paper could encourage more doctors and people to get vaccinated,
allowing them to gain the mass immunity needed to end this pandemic
first.
The study's main limitation is the small number of people and the limited observation time.
Acknowledgments: Thanks to all healthcare workers who participated in the study.
References
- Hamed SM, Elkhatib WF, Khairalla AS, Noreddin AM.
Global dynamics of SARS-CoV-2 clades and their relation to COVID-19
epidemiology. Sci Rep. 2021;11(1). https://doi.org/10.1038/s41598-021-87713-x PMid:33875719 PMCid:PMC8055906
- Lai
CC, Chen CH, Wang CY, Chen KH, Wang YH, Hsueh PR. Clinical efficacy and
safety of remdesivir in patients with COVID-19: A systematic review and
network meta-analysis of randomized controlled trials. J Antimicrob
Chemother. 2021;76(8). https://doi.org/10.1093/jac/dkab093 PMid:33758946 PMCid:PMC8083728
- Goldberg
E, Ben Zvi H, Sheena L, Sofer S, Krause I, Sklan EH, et al. A real-life
setting evaluation of the effect of remdesivir on viral load in
COVID-19 patients admitted to a large tertiary centre in Israel. Clin
Microbiol Infect. 2021;27(6). https://doi.org/10.1016/j.cmi.2021.02.029 PMid:33705849 PMCid:PMC7939997
- Katia
F, Myriam DP, Ucciferri C, Auricchio A, Di Nicola M, Marchioni M,
Eleonora C, Emanuela S, Cipollone F, Vecchiet J. Efficacy of
canakinumab in mild or severe COVID-19 pneumonia. Immun Inflamm Dis.
2021 Jun;9(2):399-405. https://doi.org/10.1002/iid3.400 PMid:33465283 PMCid:PMC8013503
- Ucciferri
C, Barone M, Vecchiet J, Falasca K. Pidotimod in Paucisymptomatic
SARS-CoV2 Infected Patients. Mediterr J Hematol Infect Dis. 2020 Jul
1;12(1):e2020048. https://doi.org/10.4084/mjhid.2020.048 PMid:32670526 PMCid:PMC7340237
- Ucciferri
C, Vecchiet J, Falasca K. Role of monoclonal antibody drugs in the
treatment of COVID-19. World J Clin Cases. 2020 Oct 6;8(19):4280-4285. https://doi.org/10.12998/wjcc.v8.i19.4280 PMid:33083387 PMCid:PMC7559676
- D'Ardes
D, Pontolillo M, Esposito L, Masciarelli M, Boccatonda A, Rossi I,
Bucci M, Guagnano MT, Ucciferri C, Santilli F, Di Nicola M, Falasca K,
Vecchiet J, Schael T, Cipollone F. Duration of COVID-19: Data from an
Italian Cohort and Potential Role for Steroids. Microorganisms. 2020
Aug 31;8(9):1327. https://doi.org/10.3390/microorganisms8091327 PMid:32878286 PMCid:PMC7564504
- Ucciferri
C, Auricchio A, Di Nicola M, Potere N, Abbate A, Cipollone F, Vecchiet
J, Falasca K. Canakinumab in a subgroup of patients with COVID-19.
Lancet Rheumatol. 2020 Aug;2(8):e457-ee458. https://doi.org/10.1016/S2665-9913(20)30167-3
- Polack
FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL,
Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury
S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci
Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR,
Şahin U, Jansen KU, Gruber WC; C4591001 Clinical Trial Group. Safety
and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med.
2020;383(27):2603-2615. https://doi.org/10.1056/NEJMoa2034577 PMid:33301246 PMCid:PMC7745181
- Fabiani
M, Ramigni M, Gobbetto V, Mateo-Urdiales A, Pezzotti P, Piovesan C.
Effectiveness of the comirnaty (BNT162b2, BioNTech/Pfizer) vaccine in
preventing SARS-CoV-2 infection among healthcare workers, Treviso
province, Veneto region, Italy, 27 December 2020 to 24 March 2021.
Eurosurveillance. 2021;26(17). https://doi.org/10.2807/1560-7917.ES.2021.26.17.2100420
- Ucciferri
C, Falasca K, Reale M, Tamburro M, Auricchio A, Vignale F, Vecchiet J.
Pidotimod and Immunological Activation in Individuals Infected with
HIV. Curr HIV Res. 2021;19(3):260-268. https://doi.org/10.2174/1570162X18666210111102046 PMid:33430735
- Abbasi J. COVID-19 and mRNA Vaccines-First Large Test for a New Approach. JAMA. 2020 Sep 22;324(12):1125-1127. https://doi.org/10.1001/jama.2020.16866 PMid:32880613
- Li
J, Huang DQ, Zou B, Yang H, Hui WZ, Rui F, Yee NTS, Liu C, Nerurkar SN,
Kai JCY, Teng MLP, Li X, Zeng H, Borghi JA, Henry L, Cheung R, Nguyen
MH. Epidemiology of COVID-19: A systematic review and meta-analysis of
clinical characteristics, risk factors, and outcomes. J Med Virol. 2021
Mar;93(3):1449-1458. https://doi.org/10.1002/jmv.26424 PMid:32790106 PMCid:PMC7436673
- Lamb YN. BNT162b2 mRNA COVID-19 Vaccine: First Approval. Vol. 81, Drugs. 2021. https://doi.org/10.1007/s40265-021-01480-7 PMid:33683637 PMCid:PMC7938284
- Giagulli
C, Noerder M, Avolio M, Becker PD, Fiorentini S, Guzman CA, Caruso A.
Pidotimod promotes functional maturation of dendritic cells and
displays adjuvant properties at the nasal mucosa level. Int
Immunopharmacol. 2009 Nov;9(12):1366-73. https://doi.org/10.1016/j.intimp.2009.08.010 PMid:19712757
- Ferrario BE, Garuti S, Braido F, Canonica GW. Pidotimod: the state of art. Clin Mol Allergy. 2015 May 21;13(1):8. https://doi.org/10.1186/s12948-015-0012-1 PMid:25999796 PMCid:PMC4440502
- Weinberger B. Vaccines for the elderly: current use and future challenges. Immun Ageing. 2018 Jan 22;15:3. https://doi.org/10.1186/s12979-017-0107-2 PMid:29387135 PMCid:PMC5778733
- Tang
MLK, Hsiao KC, Ponsonby AL, Donath S, Orsini F, Axelrad C, Pitkin S.
Probiotics and oral immunotherapy for peanut allergy - Authors' reply.
Lancet Child Adolesc Health. 2017 Nov;1(3):e1-e2. https://doi.org/10.1016/S2352-4642(17)30101-3
- Burgio
GR, Marseglia GL, Severi F, De Benedetti F, Masarone M, Ottolenghi A,
et al. Immunoactivation by pidotimod in children with recurrent
respiratory infections. Arzneimittel-Forschung/Drug Res. 1994;44(12 A).
- Hamed
SM, Elkhatib WF, Khairalla AS, Noreddin AM. Global dynamics of
SARS-CoV-2 clades and their relation to COVID-19 epidemiology. Sci Rep.
2021 Apr 19;11(1):8435. https://doi.org/10.1038/s41598-021-87713-x PMid:33875719 PMCid:PMC8055906
[TOP]
