Karin Mayer1, Nicolaus Hegge1, Ernst Molitor2, Peter Brossart1 and Corinna Hahn-Ast1.
1 Medizinische Klinik III, Hämatologie/Onkologie, Universitätsklinikum Bonn, Bonn, Germany.
2 Institut für Medizinische Mikrobiologie, Immunologie und Parasitologie (IMMIP), Universitätsklinikum Bonn, Bonn, Germany.
Correspondence to: Dr.
Karin Mayer. Medizinische Klinik III Universitätsklinikum Bonn,
Venusberg – Campus 1, 53127 Bonn, Germany. Tel: +49-228-287-17231, Fax:
+49-228-287-9017231. E-mail:
Karin.mayer@ukbonn.de
Published: May 1, 2022
Received: November 8, 2021
Accepted: April 2, 2022
Mediterr J Hematol Infect Dis 2022, 14(1): e2022032 DOI
10.4084/MJHID.2022.032
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
In febrile neutropenia, either linezolid (LIN) or vancomycin (VAN) can
be used if a gram-positive infection is suspected. Interestingly there
is no literature in which both are compared in the setting of febrile
neutropenia. Therefore, we provide here the results of a retrospective
analysis of adding VAN versus LIN in patients with febrile neutropenia.
Methods: Patients with
haematological diseases and febrile neutropenia after myelosuppressive
chemotherapy and no clearance of infection after the first empiric
broad-spectrum antibiotic were escalated to VAN or LIN from 03/2010 to
03/2014 at the University Hospital Bonn were included in this
retrospective analysis.
Results:
Out of the 73 patients, 50 had received VAN and 23 LIN. The median
hospitalisation time in the LIN cohort was significantly shorter than
in the VAN cohort (LIN 16 days vs VAN 20 days p=0.046). Successful
defervescence with the escalation to VAN or LIN could be detected in
76% of the LIN cases and 50% in the VAN group (p=0.052). This trend to
better efficacy with LIN was also shown by a higher rate of
discontinuation of VAN and escalation to another antibiotic scheme
(54.2%) than in the LIN cohort (24%, p=0.052).
Conclusion: The
antibiotic therapy in febrile neutropenia with LIN showed a trend of
better efficacy than therapy with VAN. However, because of the small
sample size and the retrospective manner, VAN may still be considered a
reasonable option in neutropenic fever, and randomised studies are
needed in this field.
|
Introduction
Patients
undergoing myelosuppressive chemotherapy are at high risk for
infections, possibly leading to life-threatening complications and,
therefore, a major cause of morbidity and mortality.[1]
Leukocytes are an important aspect of bacterial clearance. The
bacterial clearance is disturbed during myelosuppression, which leads
to neutropenia, opening the door for severe bacterial infection.
Neutropenia is defined as absolute neutrophils (ANC) < 500/µl for 48h.[2]
It is important to consider severe neutropenia a medical emergency
because the severe immunocompromised patient is highly likely to
develop sepsis out of a simple infection. Neutropenia is classified
into different risk groups depending on how long the duration of
neutropenia is expected. With the increasing duration of neutropenia,
the risk of developing severe infections is also increasing.[3]
The
duration of neutropenia depends on the tumour type and the given
chemotherapy. For example, patients with haematological diseases have a
higher risk for prolonged neutropenia and risk of infections than
patients with solid tumours.[4]
If an infection
occurs with fever or other signs of infection during neutropenia, it is
called febrile neutropenia. The incidence of febrile neutropenia in
patients with haematological diseases is about 70-80%;[5] in patients with solid tumours, it is far below (10-50%).[2,6]
Febrile neutropenia needs immediate empirical antibiotic treatment because of the risk of mortality.[2,7]
The
ECIL guidelines recommend that if a gram-positive is likely the reason
to add an appropriate agent to the already empiric therapy, the AGIHO
(Infectious disease working party of the German Society of Haematology
and Medical Oncology (DGHO)) explicitly recommends the adding of
linezolid if there is a suspicion for a gram-positive infection (like
mucositis, or catheter-infection).[3,8]
If a gram-positive infection is suspected beneath linezolid, vancomycin
can also be a valid option, most commonly used over many years, but it
has to keep in mind that vancomycin is not working in VRE
(vancomycin-resistant enterococci).
One
important difference between the two antibiotic therapies is that
linezolid is only bacteriostatic, and vancomycin is a bactericide.
Therefore, it is very interesting if there is a difference in the
efficacy in a high risk setting like febrile neutropenia between these
two therapies. Interestingly there is no literature in which vancomycin
and linezolid are compared in the setting of febrile neutropenia in the
presence of a suspected gram-positive bacteria. Therefore, we provide
the results obtained from a retrospective analysis of the two regimes
adding VAN versus LIN in patients with febrile neutropenia and
suspected gram-positive infection. We compare the efficacy of the two
regimens with regard to the incidence of fever, microbiologically
documented infections, infection-related deaths and differences in the
bacterial species detected.
Materials and Methods.
Study population.
Patients with febrile neutropenia after myelosuppressive chemotherapy
because of a haematological disease and no clearance of infection after
the first empiric broad-spectrum antibiotic were escalated to VAN or
LIN from 03/2010 to 03/2014 at the University Hospital Bonn were
included in this retrospective cohort study. Neutropenia was defined as
leukocytes < 1G/l or neutrophile granulocytes < 0,5G/l. All
included patients had fever >38°C and age > 18 years. Patients
with known allergy to VAN or LIN were excluded.
The data
collection was done by a standard questionnaire, which contained
baseline characteristics like sex, age, disease status, type of
infection, days of fever, laboratory results, medication and other
variables and was already used in another publication.[9]
Treatment protocol.
All included patients had a fever in neutropenia and showed treatment
failure of the initial empiric therapy, e.g. ongoing fever after at
least three days of initial antibiotic treatment or clinical worsening
irrespective of the duration of first-line therapy with signs of a
suspected gram-positive infection. Therefore, they were escalated to
VAN or LIN either as monotherapy or in combination with another
broad-spectrum antibiotic. The judgment for VAN or LIN was done by the
physician's clinical decision.
Antibiotic prophylaxis was cotrimoxazole 960 mg twice weekly and ciprofloxacin 500mg twice daily.
If
fever in neutropenia occurred, the antibiotic prophylaxis was stopped,
and a broad-spectrum antibiotic, usually tazobactam/piperacillin 3x4,5g
daily or meropenem 3x1g daily, was initiated.
Patients who
switched from VAN to LIN or LIN to VAN during their treatment phase
were still registered in the antibiotic group, which they received as
the first escalation scheme.
VAN was given as a bolus infusion (1g
every 12h), and drug levels were monitored routinely at least every
second day. The dose of vancomycin was reduced or increased if
necessary to maintain drug trough concentrations between 5-15mg/l. If
oral administration was possible, LIN was also given as a bolus
infusion 2 times a day (600mg) or orally b.i.d. (600mg).
Definitions of endpoints.
Successful antibiotic therapy was defined as defervescence for at least
seven days without any sign of continuing infection. Treatment failure
was defined if there was fever persistence longer than 72-96h after
starting VAN or LIN.
Febrile episodes were classified as fever of
unknown origin (FUO), pneumonia (radiologically confirmed) and
non-pneumonic microbiologically documented infection (MDI) and/or
clinically documented infection (CDI).
Microbiologically
documented infections were defined as infections with the occurrence of
fever and evidence for bacteria or viral or fungal pathogens detected
in normally sterile body sites. Staphylococci or Micrococci were only classified as a cause of infection if detected at least two times in sterile body sites.
Clinically
documented infections such as venous line, soft tissue, and
gastrointestinal infections were assumed when the patient had typical
clinical infection symptoms but no proof of microbial pathogens in the
collected specimen. Additionally, in the absence of a positive
microbiological specimen, pneumonia was defined as fever with
infiltrates in radiologic imaging.
Side effects were classified
according to CTCAE-version 4.03. An adverse event of special interest
was nephrotoxicity, which was documented by monitoring the serum
creatinine and glomerular-filtration rates (GFR) before, during and
after the therapy with VAN or LIN.
Costs of treatment.
All course costs on the ward were obtained by analysing the different
therapies' costs based on the DRG, OPS. Also the costs of the
antibiotic treatment with VAN or LIN from the first day of VAN or LIN
were calculated using the prices of the hospital pharmacy, incl. VAT.
Ethical considerations.
All study investigators were staff of Department III of Internal
Medicine. Because of the retrospective manner, no interventions were
performed as part of the study. Instead, patient care, data collection
and analysis were performed by site personnel using current techniques
of privacy assurance. In Northrhine-Westphalia state, Germany, neither
an Ethics Committee's approval nor patient consent is necessary.
Statistical analysis.
Mann-Whitney U, Fisher's exact, Chi-Square tests were used to test for
differences as indicated in the results section. A two-sided p-value
below 0.05 was considered statistically significant. Statistical
analyses were performed by SPSS Statistics Version 21 (IBM Corp.,
Armonk, NY).
Results
Study population.
In the retrospective analysis, 84 episodes of fever in 73 patients
could be analysed. In all these cases, the patients had received
myelosuppressive chemotherapy because of haematological malignancy and
experienced fever in neutropenia, which was treated with a
broad-spectrum antibiotic. Because of treatment failure, the antibiotic
therapy was escalated to VAN or LIN because of suspected gram-positive
bacteria.
Out of the 73 patients, 50 had received VAN and 23 LIN.
In 2/3 of all patients, the underlying malignancy was AML (acute
myeloid leukaemia) (VAN 62%, LIN 70%), followed by a
Non-Hodgkin-Lymphoma (VAN 18%, LIN 9%). Also, patients with acute
lymphatic leukaemia (ALL) and multiple myeloma (MM) were included (Table 1).
About
half of the patients in both cohorts were female (VAN 40%, LIN 48%,
p=0.613), and the median age was 57 years (VAN IQR 49-62 years, LIN
33-69 years, p=0.512). Also, there was no difference in patients aged
>60 years in both treatment groups (40%). In addition, there were no
significant differences in cardiovascular or pulmonary comorbidities.
Patient characteristics are shown in Table 1.
The median duration
of neutropenia was not significantly different between the VAN and the
LIN group, but there was a trend to a slightly shorter duration in the
LIN group (LIN 15 days, VAN 17 days p=0.955).
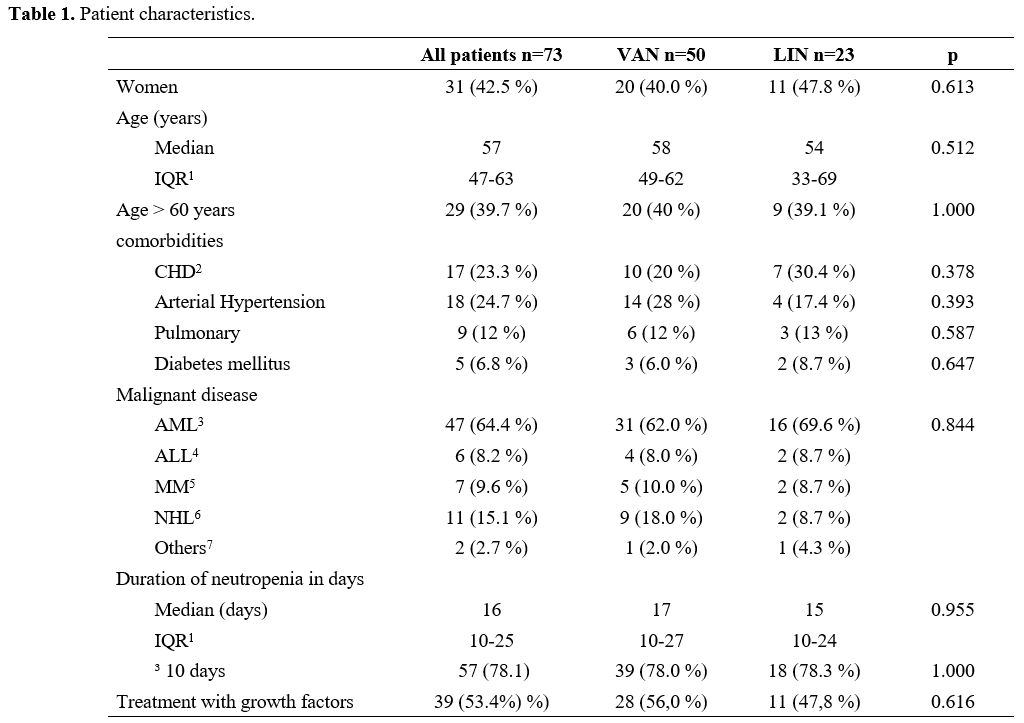 |
Table 1. Patient characteristics. |
Treatment cases. As
already described, 73 patients could be included in this retrospective
analysis. These 73 patients experienced 84 neutropenic fever events,
for which they received 59 VAN treatments and 25 LIN as escalation
therapy because of persistent infection.
Same as for patient
data, there were no significant differences in age, sex, comorbidities,
underlying disease, and remission status in the treatment cases between
the VAN and LIN groups (data not shown).
One significant
difference was that in the LIN group were significantly more treatment
cases with an elevated serum-creatinine (>1,3mg/dl) before the start
of the escalated antibiotic therapy (LIN n=4, VAN n=1, p=0.026).
In
nearly all cases in both groups, either VAN or LIN were given in
combination with another broad-spectrum antibiotic therapy (VAN n=53,
89,9%, LIN n=23, 92%, p=0.633). In almost all cases the combination
partner was meropenem (VAN=45, 84,7%, LIN n=19, 82,6%), followed by
fosfomycin as partner (VAN n=2, 3,8%, LIN n=2, 8,7%), further
antibiotics were metronidazole, tazobactam/piperacillin, fosfomycin and
clarithromycin in some cases (p=0.490).
In about 10% in both treatment groups, an antimycotic drug was added (VAN n=8, 10%, LIN n=3, 12%, p=1.0).
In most cases, VAN or LIN was added as the first escalation of antibiotic treatment (level 2, VAN n=53, 89,8%, LIN n=21, 84%).
Efficacy. Successful
defervescence with the escalation to VAN or LIN could be detected in
76% of the LIN cases and 50% in the VAN group (p=0.052). This trend to
better efficacy with LIN was also shown by a higher rate of the
discontinuation of VAN and escalation to another antibiotic scheme in
the VAN group (54.2%) than in the LIN cohort (24%, p=0.052).
Probably
because of the higher rate of further change in the antibiotic strategy
in the VAN group, the median duration of total antibiotic treatment was
significantly longer in the VAN than in the LIN cohort (VAN 9 days, LIN
7 days, p=0.029).
The median duration of the application of VAN or
LIN in the two cohorts is not significantly different (VAN 6 days, LIN
7 days, p=0.269).
The median time of hospitalisation in the LIN
cohort was significantly shorter than in the VAN cohort (LIN 16 days
(IQR 11-21) vs VAN 20 days (IQR13-28), p=0.046). When only the days
were counted since the antibiotic therapy with VAN or LIN was started,
there was still a trend to a shorter hospitalisation time in the LIN
treated patients, but this was not significant (LIN 12 days (IQR 8-18),
VAN 16 days (IQR 11-22), p=0.109). Also, in the duration of the whole
episode of fever (first day of fever until the 7th
fever-free day), there was a trend for a shorter median duration in the
LIN group than in the patients who received vancomycin as escalation,
but this was also not significant (LIN 11 days (IQR 9-16), VAN 13 days
(IQR10-19), p=0.113). The median of fever days after the escalation to
VAN or LIN was also not statistically different (LIN 2 days (IQR 1-5),
VAN 3 days (IQR 1-6), p=0.176 (Table 2).
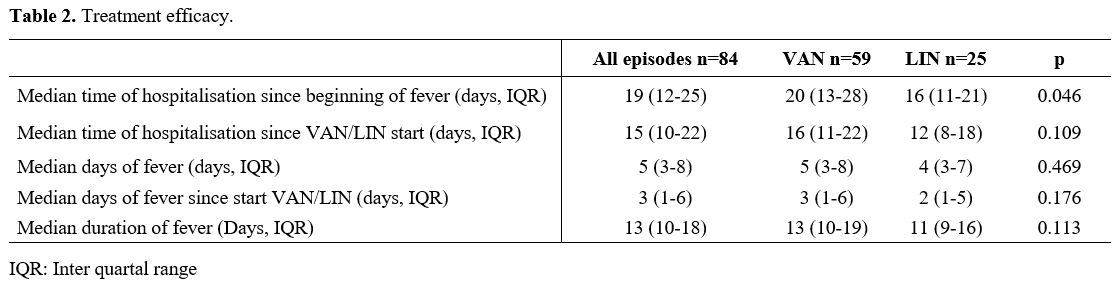 |
Table 2. Treatment efficacy. |
Microbiologically documented infections (MDI). A
bacterial pathogen could be found in about half of the fever episodes
(VAN n=31, 52.5%, LIN n=10, 40%, p=0.914). In the differentiation in
both treatment groups most of the bacterial cases were gram-positive
(VAN n=26, 83.9%, LIN n=10, 100%, p=1.0). Most of the gram-positive
detected species were Staphylococcus spp (VAN n=19, 32.2%, LIN n=5, 20%), followed by Streptococcus spp (VAN n=3, 5.1%, LIN n=2, 8%). In the VAN treatment group, one case was a vancomycin-resistant enterococcus.
There
were no significant differences in both treatment groups regarding the
bacterial species, Glycopeptide-sensibility or gram-differentiation (Table 3).
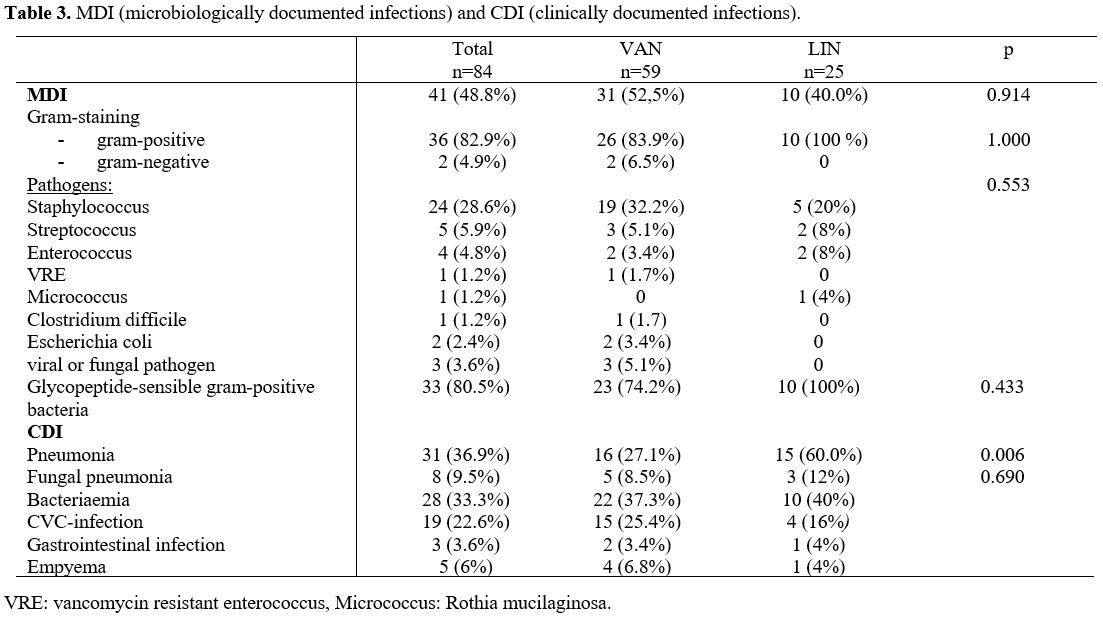 |
Table 3. MDI (microbiologically documented infections) and CDI (clinically documented infections). |
Clinically documented infections (CDI). Eleven fever episodes in the VAN and 2 in the LIN cohort showed no clinical infection focus (VAN 18.6%, LIN 8,0%, p=0.914).
About
40% in both groups showed bacteriaemia (VAN n=22, 37.3%, LIN n=10,
40%). In the LIN treated group there were significant more pneumonias
(VAN n=16, 27.1%, LIN n=15, 60%, p=0.006). Around 10% of the treated
cases had mould pneumonia (VAN n=5, 8.5%, LIN n=3, 12%). Clinical
central venous catheter infections in both treatment groups were
detected in around 20% of cases (VAN n=15, 25.4%, LIN n=4, 16%). Only a
few gastrointestinal infections could be detected in both groups (in
the VAN group 2 cases, one of these was a clostridium difficile
infection. In the LIN group, we found one gastrointestinal infection
without C. diff detection (Table 3).
Toxicity
Renal toxicity.
In the LIN group there were significant more cases with a serum
creatinine >1,3mg/dl before the start of VAN or LIN (VAN n=1, 1,7%,
LIN n=4, 16%, p=0.026). But there was no statistical difference in the
median GFR between both treatment groups (med. GFR VAN 98,15 ml/min,
LIN 105,8 ml/min, p=0.638). During VAN or LIN application in both
groups the serum creatinine level increased >0,5mg/dl in about 10%
(VAN n=9, 15,3%, LIN n=2, 8%, p=0.493). Nephrotoxicity was also not
significant different in the two treatment cohorts (grade 1 VAN n=5,
8,5%, LIN n=3, 12%, grade 2 n=0 in both groups and grade 3 VAN n=1,
1,7%, LIN n=0, p=0.707).
There were no significant differences in
other potentially nephrotoxic medications during the treatment in both
groups (liposomal amphotericin B or aminoglycoside therapy) (Table 4).
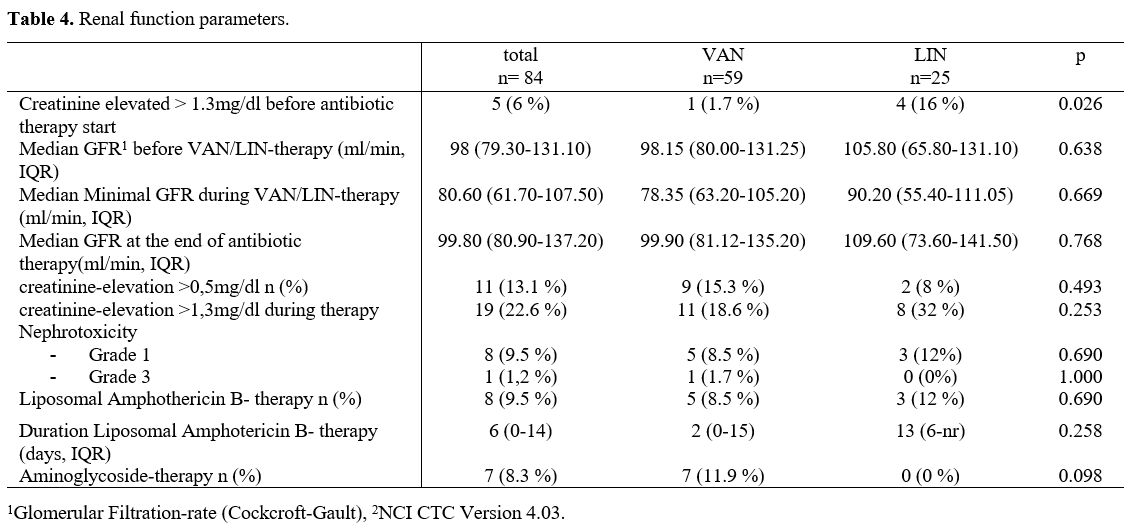 |
Table 4. Renal function parameters. |
Liver toxicity. In
one case of the VAN group, increased liver enzymes were detected (CTCAE
grade 2), which decreased when the VAN application was stopped.
Haematologic toxicity. The
patients treated with VAN showed a median duration of neutropenia of 18
days versus in the LIN treated patients, the median duration of
neutropenia was 15 days, which was not significantly different
(p=0.900).
Diarrhoea. 21 (42%) cases developed diarrhoea under treatment in the VAN group, vs 10 (43,5%) cases in the LIN group.
Only
in the VAN cohorts drug levels were measured. The minimal VAN level was
in the median of 1.95mg/dl (IQR1,0-3,5), and the maximum VAN level was
in the median of 8 mg/dl (IQR 6,8-12,1). 50% of all measured VAN drug
levels were in the therapeutic window (5-15mg/dl).
Cost analyses.
The median duration of VAN therapy was 6 days when 7.5gr. VAN were
applicated (IQR 4-12g); on the other side, LIN therapy was done in a
median for 8 days, in which 8,4g LIN were given (IQR 6,3-12).
Therefore, antibiotic therapy costs were significantly less in the VAN
group (255,20 Euro) than in the LIN treated patients (1019,17 Euro).
Regarding
all costs from the start of treatment with VAN or LIN till the
demission of the ward in the LIN group, there was a trend to lower
costs than in the VAN group (LIN 13,349,76 Euro, VAN 15697,41 Euro,
p=0.311).
Discussions
In
current guidelines, VAN or LIN are recommended as an escalation regimen
for fever in neutropenia when a gram-positive pathogen is suspected.
In
our retrospective analysis, we tested the efficacy of these two
different regimens (VAN vs LIN) and found no significant difference in
the rate of defervescence with LIN or with VAN.
The finding that
there is no significant difference in the efficacy in VAN or LIN was
also reported by Jaksic et al. In their prospective multicentric
randomised, double-blinded study, patients with haemato-oncologic
diseases and proven gram-positive infections in neutropenia were
randomised to treatment with LIN or VAN.[10] Treatment was done as 1st or 2nd
line therapy. They could not find a significant difference in the
efficacy (rate of defervescence) between the two treatment regimens.
Also, no difference in the efficacy of VAN vs LIN could be shown by Kohno et al.[11]
They tested in a multicentre study VAN vs LIN in MRSA
(methicillin-resistant Staphylococcus aureus) driven skin, mucosal
infections, pneumonia and sepsis. However, these studies were not
undertaken in patients with haematological malignancies or neutropenia
in contrast to our cohort, and we had no MRSA infection in our group.
Interestingly, the eradication rate at the end of antibiotic treatment
was significantly higher in the LIN group.
In our analysis,
bacteriaemia, pneumonia, and central venous catheter infections were
the most detected foci for infection. Also, in the work of Jaksic et
al.,[10] catheter infections and bacteriaemia were
the most found infection sites in their neutropenic cohort.
Interestingly, in a meta-analysis done by Falagas et al., empiric
therapy with Lin vs glycopeptides or beta-lactam antibiotics LIN was
significantly more effective in central venous catheters and
bacteriemia.[11] In contrast to the study of Jaksic
et al. with a low rate of pneumonia, pneumonia was more present in our
cohort, with a significant accumulation in the LIN group (8-9% vs
27-60% in our cohort). This difference in the pneumonia rate in our
data between the VAN and LIN treatment remains unclear.
In the data of Falagas et al. in pneumonia, there was no significant difference in the efficacy between LIN and glycopeptides.[11] However, in contrast, Kohno et al. could show that LIN had a significantly better efficacy on pneumonia.[12]
Because of the relatively small patient group in our analysis, this
could not be verified in our study, but eventually can help explain the
trend to a better efficacy in the LIN group in our data.
In about
half of the cases in our analysis, at least one bacterial pathogen
could be detected, mainly gram-positive bacteria, and most of them were
Staphylococcus spp. These findings are in line with Jaksic et al.[10]
In their neutropenic patients' study, Staph were the most found
bacteria, but in contrast to our patients, there was also relevant Staph aureus
detected. In our cohort, only in the VAN group two gram-negative
bacteria could be detected, but this was not significant, but could
also be an explanation for the trend of lesser efficacy in the VAN
group.
Mortality was not different between the two treatment
groups in our analysis; this result was also found in the study of
Jaksic et al.[10] Also, in a Cochrane analysis, no difference in mortality was described for VAN vs LIN.[13]
Another
issue of both antibiotic regimens was the occurrence of toxicity. The
already quoted study from Jaksic et al. reported significantly more
side effects (like nausea, vomiting, flush and erythema) in the VAN
group. However, the more frequent side effects did not lead to a more
often discontinuation in the VAN group.[10] In our
analysis, we did not find a difference in the occurrence of nausea or
vomiting in both groups, but it has to be kept in mind that our
analysis was retrospective.
In the study of Jaksic,[10]
there was no difference in the rate of diarrhoea in both groups, and
this was also the case in our analysis. Nevertheless, interestingly in
our study, the rate of diarrhoea was quite higher than in the study of
Jaksic et al.. One reason could be the retrospective manner, which
could make the evaluation of diarrhoea as a side effect of VAN or LIN
difficult because there could be other reasons for diarrhoea in
neutropenic patients after myelosuppressive chemotherapy. So this
result has to be interpreted with caution.
Another known side
effect of LIN is pancytopenia. However, in most cases, there has been a
long-term treatment (more than 30 days) with LIN as a reason.[14]
In line with this in our analysis (where the treatment with LIN had a
median of seven days), we did not find a difference in haematological
recovery between LIN and VAN. Also, Jaksic and coworkers and Nedved et
al. could not find a difference.[10,15]
In contrast, Kohno et al. could show that Lin was given for 10-28 days
in case of pneumonia and skin infections, a higher incidence of anaemia
and thrombocytopenia than the VAN group.[11]
Another
issue is the potential nephrotoxicity of VAN. Our analysis did not find
a significant difference in creatinine accelerations during VAN or LIN
treatment. In contrast to our findings, Jaksic et al. could show
significant more renal failures during VAN than LIN treatment.[10] These results were in line with Kohno et al., who showed significant renal impairment during VAN vs LIN therapy.[12]
An explanation for the divergent results of our study could be the low
vancomycin levels during VAN treatment. Only 50% of the VAN levels were
in the therapeutic window. These sub-optimal levels could explain that
we could not see a difference in renal toxicity between VAN and LIN,
and it might also explain why the efficacy of treatment in the VAN
group was lower than in the LIN group.
Also, Pritchard et al. established risk factors for VAN nephrotoxicity.[16]
Beneath the risk of high VAN blood levels (10-15mg/dl), the VAN
treatment was an important factor beyond seven days. In our analysis,
the median VAN treatment time was 6 days, and the blood levels were
mostly lower than 10mg/dl.
In our analysis, almost every second
VAN treatment was stopped and switched to another antibiotic treatment
because of fever persistence. In comparison to LIN, this was slightly
not significant. There was no treatment discontinuation of VAN or LIN
because of side effects, in contrast to the study of Jaksic et al. and
Kohno et al., where both groups (VAN and LIN) had some treatment
discontinuations because of side effects.[10,12] There was no significant difference between the VAN and LIN treatments in both studies.
Regarding
the costs of the antibiotic therapy in our analysis, the LIN therapy
was more expensive than the VAN therapy. However, we could detect a
trend to a shorter hospitalisation time in the LIN group since
treatment starts with LIN or VAN. A reason for the shorter
hospitalisation time could be that the treatment was more often
discontinued in the VAN group because of treatment failure and a new
treatment had to be started, which needed again time for response.
Therefore, due to the shorter hospital stay in the LIN group, all
treatment with LIN was cheaper regarding the total hospital costs. In
line with our findings are Patel et al.[17] Patel et
al. compared the costs of VAN and LIN for treating MRSA nosocomial
pneumonia, and they also found that the LIN treatment all in all was
cheaper than treatment with VAN. As an explanation, they mentioned more
complicated side effects during VAN treatment and a shorter
hospitalisation time in the LIN group.
Conclusions
In
our retrospective analysis of VAN or LIN treatment as escalation
therapy in patients with hematologic malignancies, neutropenic fever
and suggested gram-positive infection, the treatment with LIN showed a
trend to a better defervescence. In addition, the time of
hospitalisation was significantly shorter in the LIN group, which
reduced the LIN group's costs even if the LIN medication was more
expensive than the VAN medication. Nevertheless, it has to be kept in
mind that our study was a retrospective analysis and that the case
numbers were small. Because of these limitations, VAN may still be
considered a reasonable option in patients with neutropenic fever, and
randomised studies are needed in this field.
References
- Aapro MS, Bohlius J, Cameron DA, Dal Lago L,
Donnelly JP, Kearney N, Lyman GH, Pettengell R, Tjan-Heijnen VC,
Walewski J, Weber DC, Zielinski C (2011) 2010 update of EORTC
guidelines for the use of granulocyte-colony stimulating factor to
reduce the incidence of chemotherapy-induced febrile neutropenia in
adult patients with lymphoproliferative disorders and solid tumours,
vol 1, England https://doi.org/10.1016/j.ejca.2010.10.013 PMid:21095116
- Freifeld
AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II,
Rolston KV, Young J-AH, Wingard JR (2011) Clinical practice guideline
for the use of antimicrobial agents in neutropenic patients with
cancer: 2010 Update by the Infectious Diseases Society of America. Clin
Infect Dis 52(4): 427-431. https://doi.org/10.1093/cid/ciq147 PMid:21205990
- Maschmeyer
G, Carratala J, Buchheidt D, Hamprecht A, Heussel CP, Kahl C, Lorenz J,
Neumann S, Rieger C, Ruhnke M, Salwender H, Schmidt-Hieber M, Azoulay E
(2015) Diagnosis and antimicrobial therapy of lung infiltrates in
febrile neutropenic patients (allogeneic SCT excluded): updated
guidelines of the Infectious Diseases Working Party (AGIHO) of the
German Society of Hematology and Medical Oncology (DGHO). Ann Oncol
26(1): 21-33. https://doi.org/10.1093/annonc/mdu192 PMid:24833776 PMCid:PMC4269340
- Bow EJ (2011) Fluoroquinolones, antimicrobial resistance and neutropenic cancer patients. Curr Opin Infect Dis 24(6): 545-553. https://doi.org/10.1097/QCO.0b013e32834cf054 PMid:22001945
- Klastersky J, Aoun M (2004) Opportunistic infections in patients with cancer. Ann Oncol 15 Suppl 4: iv329-35. https://doi.org/10.1093/annonc/mdh947 PMid:15477331
- Gustinetti
G, Mikulska M (2016) Bloodstream infections in neutropenic cancer
patients: A practical update. Virulence 7(3): 280-297. https://doi.org/10.1080/21505594.2016.1156821 PMid:27002635 PMCid:PMC4871679
- Klastersky
J (2004) Management of fever in neutropenic patients with different
risks of complications. Clin Infect Dis 39 Suppl 1: S32-7. https://doi.org/10.1086/383050 PMid:15250018
- Averbuch
D, Orasch C, Cordonnier C, Livermore DM, Mikulska M, Viscoli C, Gyssens
IC, Kern WV, Klyasova G, Marchetti O, Engelhard D, Akova M (2013)
European guidelines for empirical antibacterial therapy for febrile
neutropenic patients in the era of growing resistance: summary of the
2011 4th European Conference on Infections in Leukemia. Haematologica
98(12): 1826-1835. https://doi.org/10.3324/haematol.2013.091025 PMid:24323983 PMCid:PMC3856957
- Schwab
KS, Hahn-Ast C, Heinz WJ, Germing U, Egerer G, Glasmacher A,
Leyendecker C, Marklein G, Nellessen CM, Brossart P, Lilienfeld-Toal M
von (2014) Tigecycline in febrile neutropenic patients with
haematological malignancies: a retrospective case documentation in four
university hospitals. Infection 42(1): 97-104. https://doi.org/10.1007/s15010-013-0524-x PMid:23979853
- Jaksic
B, Martinelli G, Perez-Oteyza J, Hartman CS, Leonard LB, Tack KJ (2006)
Efficacy and safety of linezolid compared with vancomycin in a
randomised, double-blind study of febrile neutropenic patients with
cancer. Clin Infect Dis 42(5): 597-607. https://doi.org/10.1086/500139 PMid:16447103
- Falagas
ME, Siempos II, Vardakas KZ (2008) Linezolid versus glycopeptide or
beta-lactam for treatment of Gram-positive bacterial infections:
meta-analysis of randomised controlled trials. Lancet Infect Dis 8(1):
53-66. https://doi.org/10.1016/S1473-3099(07)70312-2
- Kohno
S, Yamaguchi K, Aikawa N, Sumiyama Y, Odagiri S, Aoki N, Niki Y,
Watanabe S, Furue M, Ito T, Croos-Dabrera R, Tack KJ (2007) Linezolid
versus vancomycin for the treatment of infections caused by
methicillin-resistant Staphylococcus aureus in Japan. J Antimicrob
Chemother 60(6): 1361-1369. https://doi.org/10.1093/jac/dkm369 PMid:17913720
- Yue
J, Dong BR, Yang M, Chen X, Wu T, Liu GJ (2016) Linezolid versus
vancomycin for skin and soft tissue infections, vol 1, England https://doi.org/10.1002/14651858.CD008056.pub3
- Lakhani
N, Thompson W, Bombassaro AM (2005) Probable linezolid-induced
pancytopenia. Can J Infect Dis Med Microbiol 16(5): 286-288 https://doi.org/10.1155/2005/961613 PMid:18159560 PMCid:PMC2095045
- Nedved
AN, DeFrates SR, Hladnik LM, Stockerl-Goldstein KE (2016) Effect of
Linezolid on Hematologic Recovery in Newly Diagnosed Acute Myeloid
Leukemia Patients Following Induction Chemotherapy. Pharmacotherapy
36(10): 1087-1094. https://doi.org/10.1002/phar.1824 PMid:27521990
- Pritchard
L, Baker C, Leggett J, Sehdev P, Brown A, Bayley KB (2010) Increasing
vancomycin serum trough concentrations and incidence of nephrotoxicity.
Am J Med 123(12): 1143-1149. https://doi.org/10.1016/j.amjmed.2010.07.025 PMid:21183005
- Patel
DA, Michel A, Stephens J, Weber B, Petrik C, Charbonneau C (2014) An
economic model to compare linezolid and vancomycin for the treatment of
confirmed methicillin-resistant Staphylococcus aureus nosocomial
pneumonia in Germany. Infect Drug Resist 7: 273-280. https://doi.org/10.2147/IDR.S68658 PMid:25368526 PMCid:PMC4216023
[TOP]



