Wen Yao1, Xinchen Fang2, Peng Jiang3, Juan Tong1, Liangquan Geng1,Xiaoyu Zhu1, Baolin Tang1, Xiang Wan1, Kaidi Song1, Lei Zhang1, Ping Qiang1, Guangyu Sun1, Yongsheng Han1, Huilan Liu1 and Zimin Sun1.
1Department
of Hematology, The First Affiliated Hospital of USTC, Division of Life
Science and Medicine, University of Sciences and Technology of China,
Hefei, Anhui 230001, P. R. China.
2 Central Laboratory
of Medical Research Centre, The First Affiliated Hospital of USTC,
Division of Life Sciences and Medicine, University of Science and
Technology of China, Hefei, Anhui 230001, P. R. China.
3
Department of Pharmacy, The First Affiliated Hospital of USTC, Division
of Life Sciences and Medicine, University of Science and Technology of
China, Hefei, Anhui 230031, P. R. China.
Correspondence to: Sun
Z, Department of Hematology, The First Affiliated Hospital of USTC,
Division of Life Science and Medicine, University of Sciences and
Technology of China, Hefei, Anhui 230001, P. R. China. E-mail:
yaowen0511@163.com
Published: March 1, 2022
Received: November 24, 2021
Accepted: February 11, 2022
Mediterr J Hematol Infect Dis 2022, 14(1): e2022025 DOI
10.4084/MJHID.2022.025
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
To the editor
Despite
the significant progress made in treating acute myeloid leukemia (AML)
in the last decade, 10%–40% of the patients with standard induction
chemotherapy still did not achieve complete remission (CR),[1] and 50%-70% of the patients in the first CR were at risk for relapse.[2] Although few of these patients can be cured with conventional salvage therapy,[2,3]
they need to be evaluated regarding eligibility for allogeneic
hematopoietic stem cell transplantation (HSCT), the most potent
therapeutic strategy for patients who achieve CR after relapse.[2] Before transplantation, salvage chemotherapy regimens need to be employed to reduce the leukemia burden.
In
relapsed/refractory AML, fludarabine, cytarabine, combination with
granulocyte colony-stimulating factor (G-CSF) (FLAG) were used as a
reinduction therapy and resulted in only 38.2% CR.[4]
Wrzesień-Kuś A et al. utilized the combination of cytarabine,
cladribine, and G-CSF as the induction therapy in patients with
refractory or early relapsed AML, obtaining a 50% CR rate with 17%
early death.[5] Subsequent studies evaluated FLAG plus
idarubicin (FLAG-Ida) or FLAG-Ida plus gemtuzumab ozogamicin
(FLAGO-Ida) in adult patients with refractory/relapsed AML, showing
that the CR/CR with an incomplete blood count recovery (CRi) rate was
51%, with 9% of induction deaths.[6] These data
demonstrated the therapy limitation of FLAG in relapsed/refractory AML
and the prospects of FLAG combination with other specific drugs.
Chidamide
is a new histone deacetylase (HDAC) inhibitor of the benzamide class,
and it has been approved by China Food and Drug Administration (CFDA)
in treating peripheral T-cell lymphoma in China. Additionally, evidence
demonstrated that chidamide combined with cytarabine synergistically
enhanced apoptosis in AML cell lines.[7] Therefore, we
speculated that the addition of chidamide in the FLAG combination might
improve its efficacy in relapsed/refractory AML. Herein, we evaluate
the efficacy and toxicity of chidamide-FLAG (Chi-FLAG) reinduction
treatment in patients with relapsed/refractory AML and the potential
for subsequent HSCT.
Material and Methods
Patients. AML was defined by the criteria of the World Health Organization.[8] Furthermore, genetic risk grouping and remission criteria were defined according to the European Leukemia Network (ELN).[9]
Additionally, the selected patients were required to have an adequate
hepatic and renal function and no uncontrolled infections with ECOG
scores of 0-2 and could not receive investigational agents within 30
days of enrollment or myelosuppressive therapy within 14 days. Based on
these, a total of 14 consecutive patients, median age 28 years (range
from 14 to 52 years, four female and ten male), with
refractory/relapsed AML were enrolled in our current prospective study
(ChiECRCT-20180058), which had passed Ethical Committee in our
hospital.
Treatment plan. Chidamide 30 mg was given orally on days 1, 4, 8, and 11. Fludarabine 30 mg/m2 and cytarabine 2 g/m2
were given for 5 days, from day 4 to 8. G-CSF was given at a 5-10 (g/kg
body weight) dose, which started 24 h prior to fludarabine until
neutrophil recovery (Chi-FLAG regimen). Patients who were found to have
CR/CRi, PR, or even NR, as tolerated and disposed of, received HSCT.
Adverse events were graded according to the National Cancer Institute
Common Terminology Criteria for Adverse Events Version 4.0.
All
patients were treated according to standardized institutional treatment
and supportive care algorithms. Patients were maintained on infectious
disease prophylaxis, including a broad-spectrum fluoroquinolone,
anti-fungal agent, and acyclovir. Transfusions were given according to
institutional guidelines.
Statistical analysis.
Continuous variables were summarized using descriptive statistics such
as median and interquartile range (IQR). Impact on response rate was
assessed using the Mann–Whitney U test. Categorical variables were
presented as percentages and compared using Fisher’s exact test.
Finally, survival curves were compared using the Log-rank test.
Statistical analysis was conducted using Stata# data analysis and
statistical software (version 14.0; Stata-Corp LP, College Station, TX).
Results
Patient characteristics. The demographic characteristics of the 14 patients are shown in Table 1.
The median age was 28 years (range, 14-55). Six patients had a primary
refractory disease at the time of enrollment, seven were experiencing a
first relapse, and one was experiencing a second relapse. The relapse
always occurred before 12 months. Among the eight patients in the ELN
favorable-risk group, six patients had t(8;21)(q22;q22) translocation;
one had a normal karyotype with NPM1 mutation and one with CEBPA double
mutations. The intermediate group consisted of one patient with
t(8;21)(q22;q22) translocation with C-kit mutation and three patients
with normal karyotypes, including one with NPM1 FLT3-ITD mutations.
Finally, the poor-risk group contained two patients with abnormal
karyotypes with FLT3-ITD mutations (Table 1).
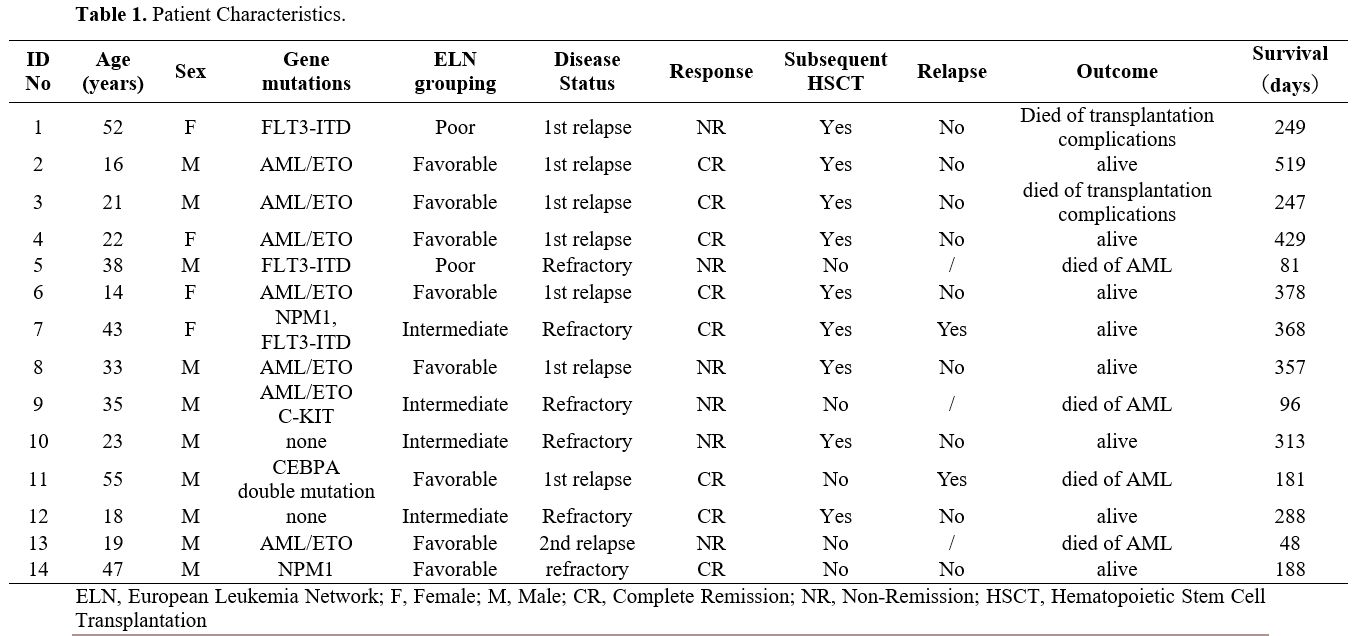 |
Table
1. Patient Characteristics. |
Response. Response rates of the 14 patients are also shown in Table 1.
Eight patients (8/14, 57.1%) achieved CR and no patients had CRi or PR
as their best response. ORR was also 57.1%, Responses occurred in 71.4%
(five of seven) of patients with first relapse, 0.0% with second
relapse, and 50.0% (three of six) with refractory AML (P = 0.417).
Patients with relapsed AML had a similar CR compared to those with
refractory AML (62.5% vs. 50%; P = 1.000). Favorable, intermediate and
poor risk patients had CR of 75.0%, 50.0% and 0.0%, respectively (P =
0.152).
Survival.
The Kaplan–Meier survival curve results showed that the 1-year overall
survival (OS) rate of patients uncensored for transplant was remarkably
higher than that of patients censored for transplant (77.8% vs. 20%, p
= 0.001, Figure 1). The 1-year
OS rate of all patients was 55.6%. (95% CI 26.4%-77.2%); In
multivariable analysis examining outcomes, neither age, sex, ELN
grouping, nor disease status impacted survival significantly.
Ultimately, four patients (28.6%) died of progressive disease.
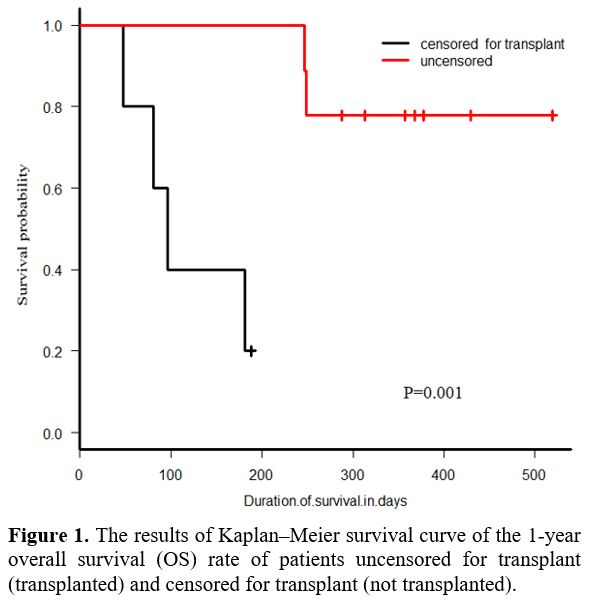 |
Figure
1. The results of Kaplan–Meier survival curve of the 1-year overall
survival (OS) rate of patients uncensored for transplant (transplanted)
and censored for transplant (not transplanted). |
Allogeneic transplant. Allogeneic transplant is the favored long-term strategy for disease control and prolonged survival,[9]
seen in this population. Nine patients (9/14, 64.3%) in this cohort
received an allogeneic transplant. The median age was 22 years (range,
14-52 years) (Table 1). Among
these nine patients, four had ELN intermediate/poor-risk disease, and
five had ELN favorable-risk disease. Six patients were in CR, and three
were in NR prior to transplant. One patient had a matched related
donor, another had a haploidentical donor, and seven were transplanted
with umbilical cord blood stem cells. The 1-year OS after allogeneic
transplant was 77.8% (95% CI 36.5%-93.9%), and 1-year disease-free
survival was 66.7% (95% CI 28.2%-87.8%). Two patients were disease-free
at about one year, of the three patients in NR at the time of
transplant.
Toxicity. Four of 14 patients (28.6%) had a grade 3-4 nonhematologic toxicity within 30 days (based on NCI CTACE v4.0).[13]
The most common toxicity was fatigue and nausea. Other toxicities
included sepsis secondary to pneumonia, neutropenic fever without
source, and thrombocytopenia. The readmission rate was 100%. Causes of
hospitalization were febrile neutropenia (50.0%), proven infection
(42.9%), cytopenia (7.1%). No patients died within 30 days of treatment
due to toxicity. No significant differences in toxicity were observed
in patients who achieved CR compared with those who did not (NR).
Discussion
In
this prospective analysis, we report the results of a phase I study to
assess the safety and activity of chidamide combined with the FLAG
regimen in all subsets of patients with relapsed/refractory AML.
Interestingly, the overall CR rate (57.1%) was comparable to prior
analysis of relapsed/refractory patients with FLAG-Ida (51%) but showed
lower treatment-related mortality (0% vs. 9%).[6] In
addition, the overall CR rate in the present study was improved
compared with previous studies of intensive reinduction regimens with
high dose cytarabine with (44%) or without (32%) mitoxantrone[11] or FLAG (33.3%).[12]
Collectively, the present work demonstrated that the combination of
chidamide and FLAG shows a promising application prospect in
relapsed/refractory AML.
Numerous HDAC inhibitors are in
clinical trials, and the reported response rates are unsatisfactory for
relapsed or refractory AML. Vey et al. reported a dose-escalation study
of oral abexinostat to treat patients with relapsed/refractory AML. It
is frustrating that the best response was stable disease in one
patient.[13] Kirschbaum et al. demonstrated that no
CR or PR had been seen in a phase 2 study of relapsed/refractory AML
patients administrated with belinostat.[14] Gojo et
al. found that only seven out of 21 attained a CR/CRi in
relapsed/refractory AML patients when treated with the combination of
vorinostat, cytarabine, and etoposide.[15] Moreover,
Walter et al. suggested that among 43 older patients with
relapsed/refractory AML treated with vorinostat combined with
gemtuzumab ozogamicin and azacitidine, 10 achieved CR, 8 achieved CRi,
and the overall response rate was only 41.9%.[16]
Chidamide is a new HDAC inhibitor of the benzamide class that specifically inhibits HDAC 1, 2, 3[7,17]
and has been approved by the CFDA in treating peripheral T-cell
lymphoma in China. A previous study demonstrated that Chidamide
significantly increased the expression of suppressors of cytokine
signaling 3, reduced the expression of Janus activated kinases 2 and
signal transducer and activator of transcription 3 (STAT3), and
inhibited STAT3 downstream genes, including c-Myc, Bcl-xL, and Mcl-1,
which are involved in cell cycle progression and anti-apoptosis,
thereby inducing G0/G1 phase arrest and apoptosis in AML cells.[18] Notably, chidamide synergistically enhances apoptosis combined with cytarabine,[19] decitabine,[20] or MLL-menin interaction targets[21] in leukemia cell lines.
At
present, there are only two reports on the application of chidamide in
relapsed/refractory AML patients. Lun et al. reported that one patient
with MLL-AF9 attained complete molecular remission after treatment with
chidamide combined with CAG regimen chemotherapy.[22]
The other report was that chidamide and decitabine combined with the
CHAG priming regimen for eight patients, five achieved CR, one achieved
PR, one’s disease progressed, and one died from complications of
chemotherapy.[23] Our regimen, the first to be used in relapsed/refractory AML, was generally well tolerated with relatively high CR.
The
Chi-FLAG regimen may be particularly useful for patients with
intermediate- or high-risk disease characteristics, especially as a
bridge to HSCT. In our series, the OS of patients with an
intermediate/poor ELN risk was at least as high as those with favorable
risk (50.0% vs. 60.0%, p = 0.750). No significant differences in OS
were observed in NR patients after reinduction chemotherapy (33.3%)
compared with those who achieved CR (72.9%, P = 0.108). However, 64.3%
of all patients underwent transplantation, and the OS was significantly
higher in patients uncensored for subsequent transplant (77.8%) than in
censored patients (20.0%). Additionally, the OS rate of all patients in
our study (55.6%) was slightly higher than that of other previous
series of relapsed/refractory patients (22.0%, uncensored for the
subsequent transplant).[24] These differences may be
related to the higher response rates to the Chi-FLAG regimen in
patients with intermediate/poor-risk disease and their ability to
undergo subsequent transplantation.
Our series adds to the
growing literature supporting the use of the Chi-FLAG regimen in
patients with relapsed/refractory AML and as a bridge to potentially
curative allogeneic transplant. However, the limitation of this paper
is the insufficient sample size. Further delineation of molecular and
cytogenetic subsets associated with higher response rates to chidamide
will be of value as future prospective trials of chidamide in
combination with new molecularly targeted agents are designed.
Acknowledgments
The
authors would like to thank all the patients and their caregivers for
participating in this study. This work was financially supported by the
Fundamental Research Funds for the Central Universities (WK9110000017).
Compliance with Ethical Standards
The
protocol was approved by our Ethics Committee and registered at
Chictr.org (ChiCTR1800015871). Informed consent, and assent when
appropriate, was obtained from all patients.
References
- Cheson BD, Bennett JM, Kopecky KJ, et al.
International Working Group for Diagnosis, Standardization of Response
Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic
Trials in Acute Myeloid Leukemia. Revised recommendations of the
International Working Group for Diagnosis, Standardization of Response
Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic
Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642-4649. https://doi.org/10.1200/JCO.2003.04.036 PMid:14673054
- Rashidi
A, Weisdorf DJ, Bejanyan N. Treatment of relapsed/refractory acute
myeloid leukaemia in adults. Br J Haematol. 2018;181(1):27-37. https://doi.org/10.1111/bjh.15077 PMid:29318584
- Xu
J, Lv TT, Zhou XF, et al. Efficacy of common salvage chemotherapy
regimens in patients with refractory or relapsed acute myeloid
leukemia: a retrospective cohort study. Medicine (Baltimore). 2018;97
(39):e12102. https://doi.org/10.1097/MD.0000000000012102 PMid:30278488 PMCid:PMC6181529
- Fiegl
M, Unterhalt M, Kern W, et al. Chemomodulation of sequential high-dose
cytarabine by fludarabine in relapsed or refractory acute myeloid
leukemia: a randomized trial of the AMLCG. Leukemia.
2014;28(5):1001-1007. https://doi.org/10.1038/leu.2013.297 PMid:24150216
- Wrzesień-Kuś
A, Robak T, Lech-Marańda E, et al. A multicenter, open,
non-comparative, phase II study of the combination of cladribine
(2-chlorodeoxyadenosine), cytarabine, and G-CSF as induction therapy in
refractory acute myeloid leukemia - a report of the Polish Adult
Leukemia Group (PALG). Eur J Haematol. 2003;71(3):155-162. https://doi.org/10.1034/j.1600-0609.2003.00122.x PMid:12930315
- Bergua
JM, Montesinos P, Martinez-Cuadrón, et al. A prognostic model for
survival after salvage treatment with FLAG-Ida +/-
Gemtuzumab-Ozogamicine in adult patients with refractory/relapsed acute
myeloid leukaemia. Br J Haematol. 2016;174(5):700-710. https://doi.org/10.1111/bjh.14107 PMid:27118319
- Huang
H, Yang WB, Dong AS, et al. Chidamide enhances the cytotoxicity of
cytarabine and sorafenib in acute myeloid leukemia cells by modulating
H3K9me3 and autophagy levels. Front Oncol. 2019;9:1276. https://doi.org/10.3389/fonc.2019.01276 PMid:31850196 PMCid:PMC6901797
- Vardiman
JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health
Organization (WHO) classification of myeloid neoplasms and acute
leukemia: rationale and important changes. Blood. 2009;114(5):937-951. https://doi.org/10.1182/blood-2009-03-209262 PMid:19357394
- Dohner
H, Estey EH, Amadori S, et al. Diagnosis and management of acute
myeloid leukemia in adults: recommendations from an international
expert panel, on behalf of the European LeukemiaNet. Blood.
2010;115(3):453-474. https://doi.org/10.1182/blood-2009-07-235358 PMid:19880497
- Common
Terminology Criteria for Adverse Events (CTCAE): National Cancer
Institute; 2010 [cited 2016 Oct 2]. Available from:
https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.Pdf
- C, Kopecky KJ, Head DR, et al. A
phase III comparison of high dose ARA-C (HIDAC) versus HIDAC plus
mitoxantrone in the treatment of first relapsed or refractory acute
myeloid leukemia Southwest Oncology Group Study. Leuk Res.
1999;23(9):787-794. https://doi.org/10.1016/S0145-2126(99)00087-9
- Li
LM, Zhang XQ, Yu HJ, et al. Low-dose hypomethylating agent decitabine
in combination with aclacinomycin and cytarabine achieves a better
outcome than standard FLAG chemotherapy in refractory/relapsed acute
myeloid leukemia patients with poor-risk cytogenetics and mutations.
Onco Targets Ther. 2018;11:6863-6870. https://doi.org/10.2147/OTT.S161919 PMid:30349319 PMCid:PMC6190628
- Vey
N, Prebet T, Thalamas C, et al. Phase 1 dose-escalation study of oral
abexinostat for the treatment of patients with relapsed/refractory
higher-risk myelodysplastic syndromes, acute myeloid leukemia, or acute
lymphoblastic leukemia. Leuk Lymphoma. 2017;58(8):1880-1886. https://doi.org/10.1080/10428194.2016.1263843 PMid:27911138
- Kirschbaum
MH, Foon KA, Frankel P, et al. A phase 2 study of belinostat (PXD101)
in patients with relapsed or refractory acute myeloid leukemia or
patients over the age of 60 with newly diagnosed acute myeloid
leukemia: a California Cancer Consortium Study. Leuk Lymphoma.
2014;55(10):2301-2304. https://doi.org/10.3109/10428194.2013.877134 PMid:24369094 PMCid:PMC4143479
- Gojo
I, Tan M, Fang HB, et al. Translational phase I trial of vorinostat
(suberoylanilide hydroxamic acid) combined with cytarabine and
etoposide in patients with relapsed, refractory, or high-risk acute
myeloid leukemia. Clin Cancer Res. 2013;19(7):1838-1851 https://doi.org/10.1158/1078-0432.CCR-12-3165 PMid:23403629 PMCid:PMC4332848
- Walter
RB, Medeiros BC, Gardner KM, et al. Gemtuzumab ozogamicin in
combination with vorinostat and azacitidine in older patients with
relapsed or refractory acute myeloid leukemia: a phase I/II study.
Haematologica. 2014;99(1):54-59. https://doi.org/10.3324/haematol.2013.096545 PMid:24142996 PMCid:PMC4007917
- Gong
K, Xie J, Yi H, Li W. CS055 (Chidamide/HBI-8000), a novel histone
deacetylase inhibitor, induces G1 arrest, ROS-dependent apoptosis and
differentiation in human leukaemia cells. Biochem J.
2012;443(3):735-746. https://doi.org/10.1042/BJ20111685 PMid:22339555
- Zhao
S, Guo J, Zhao Y, et al. Chidamide, a novel histone deacetylase
inhibitor, inhibits the viability of MDS and AML cells by suppressing
JAK2/STAT3 signaling. Am J Transl Res. 2016;8(7):3169-3178.
- Li
X, Yan X, Guo WJ, et al. Chidamide in FLT3-ITD positive acute myeloid
leukemia and the synergistic effect in combination with cytarabine.
Biomed Pharmacother. 2017;90:699-704. https://doi.org/10.1016/j.biopha.2017.04.037 PMid:28419965
- Mao
JP, Li S, Zhao HH, et al. Effects of chidamide and its combination with
decitabine on proliferation and apoptosis of leukemia cell lines. Am J
Transl Res. 2018;10(8):2567-2578.
- Ye J,
Zha J, Shi YF, et al. Co-inhibition of HDAC and MLL-menin interaction
targets MLL-rearranged acute myeloid leukemia cells via disruption of
DNA damage checkpoint and DNA repair. Clin Epigenetics. 2019;11(1):137.
https://doi.org/10.1186/s13148-019-0723-0 PMid:31590682 PMCid:PMC6781368
- Lun
Y, Yang JJ, Wu Y. Complete molecular remission in relapsed and
rrefractory acute myeloid leukaemia with MLL-AF9 treated with
chidamide-based chemotherapy. J Clin Pharm Ther. 2017;42 (6):786-789. https://doi.org/10.1111/jcpt.12577 PMid:28646565
- Chen
L, Mi RH, Zhu ST, et al. Therapeutic effect of chidamide and decitabine
in combination with CHAG priming regimen for 8 Patients with
relapsed/refractory acute myeloid leukemia. Zhonghua Xue Ye Xue Za Zhi.
2018;39 (7):602-604.
- Ye PP, Pei RZ, Jin
J, et al. Modified cladribine, cytarabine, and G-CSF as a salvage
regimen in patients with relapsed/refractory acute myeloid leukemia: a
bridge to myeloablative allogeneic hematopoietic stem cell
transplantation. Ann Hematol, 2019 Sep;98(9):2073-2080. Epub 2019 Jun
14. https://doi.org/10.1007/s00277-019-03723-w PMid:31201514
[TOP]

