Ming Tong1,2*, Xiquan Yan2,3*, Yu Jiang2*, Zhaoxia Jin4, Shengjiao Zhu5, Lianhong Zou2, Yanjuan Liu2, Qing Zheng6, Guoqiang Chen5, Ruifeng Gu5, Zhilan Zhou5, Xiaotong Han2, Jiangming He7, Siqing Yin8, Changchun Ma9, Wen Xiao2, Yong Zeng10#, Fang Chen2,3# and Yimin Zhu2,3#.
1 Department
of Infectious Diseases, Hunan Provincial People's Hospital (The
First-affiliated Hospital of Hunan Normal University), Changsha, Hunan,
China.
2 Institute of Emergency Medicine, Hunan
Provincial Key Laboratory of Emergency and Critical Care Metabonomics,
Hunan Provincial People's Hospital (The First-affiliated Hospital of
Hunan Normal University), Changsha, Hunan, China.
3 School of Life Sciences, Hunan Normal University, Changsha, Hunan, China.
4 Department of Cardiology, Huanggang Central Hospital, Huanggang, Hubei, China.
5 Department of Laboratory Medicine, Huanggang Central Hospital, Huanggang, Hubei, China.
6
Department of Geriatrics, Hunan Provincial People's Hospital (The
First-affiliated Hospital of Hunan Normal University), Changsha, Hunan,
China.
7 Department of Public Health, Huangzhou General Hospital, Huanggang, Hubei, China.
8 Huangzhou District Maternal and Child Health Hospital, Huanggang, Hubei, China.
9 Department of Neurosurgery, Huangzhou District People's Hospital, Huanggang, Hubei, China.
10 Huanggang Central Hospital, Huanggang, Hubei, China.
*Contributed equally. #Contributed equally.
Correspondence to:
Yimin Zhu, Institute of Emergency Medicine, Hunan Provincial Key
Laboratory of Emergency and Critical Care Metabonomics, Hunan
Provincial People's Hospital (The First-affiliated Hospital of Hunan
Normal University), Changsha, Hunan, China. E-mail:
cszhuyimin@163.com Fang
Chen, Institute of Emergency Medicine, Hunan Provincial Key Laboratory
of Emergency and Critical Care Metabonomics, Hunan Provincial People's
Hospital (The First-affiliated Hospital of Hunan Normal University),
Changsha, Hunan, China. E-mail:
920858110@qq.com.
Yong Zeng, Huanggang Central Hospital, Huanggang, Hubei, China. E-mail:
zengyong8000@163.com
Published: May 1, 2022
Received: December 4, 2021
Accepted: April 3, 2022
Mediterr J Hematol Infect Dis 2022, 14(1): e2022033 DOI
10.4084/MJHID.2022.033
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
COVID-19 is characterized by endothelial dysfunction and is presumed to
have long-term cardiovascular sequelae. In this cross-sectional study,
we aimed to explore the serum levels of endothelial biomarkers in
patients who recovered from COVID-19 one year after hospital discharge.
Methods: In this clinical
follow-up study, 345 COVID-19 survivors from Huanggang, Hubei, and 119
age and gender-matched medical staff as healthy controls were enrolled.
A standardized symptom questionnaire was performed, while
electrocardiogram and Doppler ultrasound of lower extremities, routine
blood tests, biochemical and immunological tests, serum soluble
vascular cell adhesion molecule-1(VCAM-1), intercellular cell adhesion
molecule-1(ICAM-1), P-selectin, and fractalkine were measured by
enzyme-linked immunosorbent assays (ELISA).
Results:
At one year after discharge, 39% of recovers possessed post-COVID
syndromes, while a few had abnormal electrocardiogram manifestations,
and no deep vein thrombosis was detected in all screened survivors.
There were no significant differences in circulatory inflammatory
markers (leukocytes, neutrophils, lymphocytes, C-reactive protein and
interleukin-6), alanine aminotransferase, estimated glomerular
filtration rate, glucose, triglycerides, total cholesterol and D-dimer
observed among healthy controls with previously mild or severe
infected. Furthermore, serum levels of VCAM-1, ICAM-1, P-selectin, and
fractalkine do not significantly differ between survivors and healthy
controls.
Conclusions: SARS-CoV-2
infection may not impose a higher risk of developing long-term
cardiovascular events, even for those recovering from severe illness.
|
Introduction
Severe
acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the pathogen of
coronavirus disease 2019 (COVID-19), is highly contagious and
pathogenic and is responsible for more than 351 million infected and
nearly 5.6 million deaths worldwide as of Jan 24, 2022.[1]
In addition, persistent and diverse post-COVID symptoms have been
described in survivors of COVID-19, including those with a mild initial
disease course.[2] Therefore, more than 340 million survivors are at high risk for post-COVID syndrome worldwide.[3]
The
available clinical evidence suggests that COVID-19, although damaging
the respiratory system initially, is a systemic disease with
extrapulmonary complications.[4] The cardiovascular system is one of the most involved systems,[5] while endotheliitis is a prominent feature of COVID-19,[6] thus is suggested to be responsible for life¬threatening thrombogenesis and coagulopathy in those with severe illness.[7]
During the acute phase of SARS-CoV-2 infection, cytokine storm and
subsequent endothelial injury and thrombosis are involved in the
pathogenesis of cardiovascular complications.[8] However, few studies have focused on the endothelial dysfunction in patients who recovered from COVID-19.
Endothelial
cells play an essential role in maintaining vascular homeostasis, such
as controlling inflammation, regulating platelet aggregation, and
preventing thrombosis.[9] Dysfunction of endothelial cells has been identified as a central feature of COVID-19.[10]
The abnormal elevation of soluble endothelial biomarkers, such as
vascular cell adhesion molecule-1 (VCAM-1), intercellular cell adhesion
molecule-1 (ICAM-1), P-selectin, and fractalkine, is closely related to
the development of arteriosclerosis,[11] which is the underlying pathology of coronary artery disease,[12] peripheral artery disease,[13] and cerebrovascular disease[14]
in most cases. Therefore, the severity of endothelial dysfunction is
associated with increased cardiovascular risks, and it is of great
significance to monitor endothelial biomarkers in patients recovering
from COVID-19.
In this study, we investigated demographics,
laboratory findings, symptoms, electrocardiogram manifestations,
screened lower extremity thrombosis and measured serum endothelial
biomarkers of participants one year after discharge, thus evaluating
long-term cardiovascular risk in patients recovered from COVID-19.
Methods
Study design and participants.
From Mar 16 to Mar 28, 2021, 473 survivors of COVID‐19, who had been
previously hospitalized from Jan 24 to Mar 18, 2020, in Huanggang,
Hubei, China, were recruited to this cross-sectional cohort study. The
inclusion criteria were adults previously diagnosed with COVID‐19
(positive in a reverse‐transcription polymerase chain reaction for
SARS‐CoV-2), and the stratification of disease severity has been
described in our published report.[15] Of these
patients, 114 cases were excluded for diabetes, suffering from chronic
systemic infection, malignant tumors or hematological and autoimmune
diseases, pregnancy, chronic smoking (defined as 20 pack-years),
long-term use of medications (angiotensin-converting enzyme inhibitors,
angiotensin II type-1 receptor antagonists, corticosteroids or
statins), and fourteen recovers did not show up for the follow-up
appointment (Figure 1). As a
result, 345 survivors were recruited into the study from Mar 1 to 30,
2021 from Mar 1 to May 30, 2021. During this time, 119 age and
sex-matched healthy controls, medical personnel at Hunan Provincial
People's Hospital, were recruited during the annual routine physical
examination. All medical staff has repeatedly undergone throat swab
screening to exclude SARS-CoV-2 infection every 1 to 2 weeks since the
pandemic. Moreover, those who were pregnant, long-term using
medications or chronic smoking, or suffering from diabetes, chronic
systemic infection, malignant tumors or hematological and autoimmune
diseases were excluded.
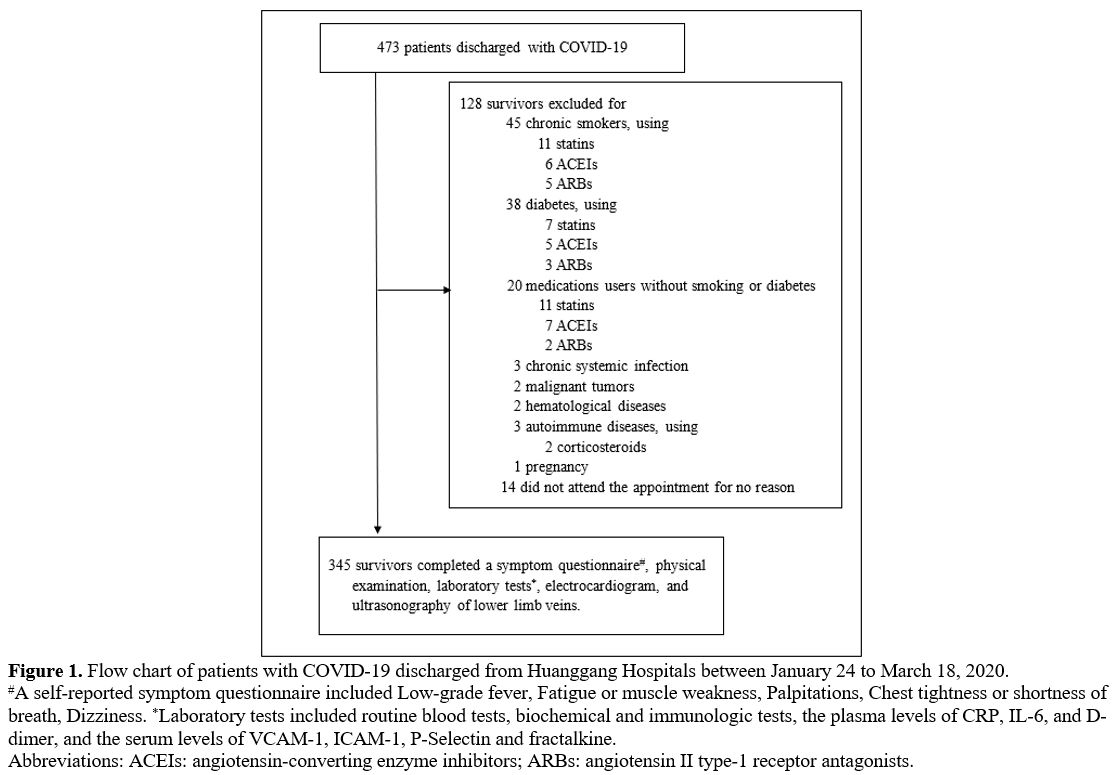 |
Figure 1. Flow chart of patients with COVID-19 discharged from Huanggang Hospitals between January 24 to March 18, 2020.
#A
self-reported symptom questionnaire included Low-grade fever, Fatigue
or muscle weakness, Palpitations, Chest tightness or shortness of
breath, Dizziness. *Laboratory tests included routine blood tests,
biochemical and immunologic tests, the plasma levels of CRP, IL-6, and
D-dimer, and the serum levels of VCAM-1, ICAM-1, P-Selectin and
fractalkine.
Abbreviations: ACEIs: angiotensin-converting enzyme inhibitors; ARBs: angiotensin II type-1 receptor antagonists.
|
Collection of clinical data.
According to the guidelines of the National Health Commission of China,
in the cohort, survivors were divided into the mild group and the
severe group according to the severity of the disease during previously
acute infection, as described in our previous report.[15]
In addition, all the survivors were subjected to a standardized symptom
questionnaire and received a physical examination, electrocardiogram,
and ultrasonography of the lower extremities for detecting deep venous
thrombosis. All data were collected and triple-checked by three
physicians.
Sample collection and processing.
Blood samples were taken from each participant by standard venipuncture
in a fasting state on the day of appointments. Routine blood tests,
biochemical and immunologic tests, and the plasma levels of C-reactive
protein (CRP), interleukin (IL)-6, and D-dimer were measured by
conventional laboratory methods. The serum for endothelial biomarker
detection was isolated by centrifugation for 15 minutes at 1500×g and
frozen at -80°C until thawed and analyzed.
This study has strictly
followed the recommendations of the Helsinki Declaration. Therefore,
the institutional review boards of the Medical Ethics Committee of the
Hunan Provincial People's Hospital approved this study (NO.2021-92),
and all participants signed informed consent.
Enzyme-linked immunosorbent assays for serum endothelial biomarkers measurement.
Quantitative measurement of serum soluble VCAM-1, ICAM-1, P-Selectin
and fractalkine was tested for survivors and healthy participants using
96-well enzyme-linked immunosorbent assay kits (Boster Biological
Technology Co. Ltd, Wuhan, China). Quality control was carried out
strictly following the manufacturer's instructions for each batch of
tests.
Statistic analysis.
Categorical variables were compared using χ2 analysis and expressed in
numbers (proportions). Continuous variables with normal distribution
were compared using independent group t-tests and expressed as mean ±
standard deviation (SD), while those not normally distributed were
compared using the Mann-Whitney U test and expressed as median and
interquartile range (IQR) values. All statistical analyses were
performed using the SPSS programme, V.19.0 (SPSS Inc., Chicago, IL,
USA), and plots were generated using GraphPad Prism, version 8
(GraphPad Software, San Diego, CA). A two-sided P-value of < 0.05 was defined as statistically significant.
Results
Clinical characteristics of the study participants.
A total of 345 COVID-19 survivors (291 recovered from the mild
situation and 54 from the severe) and 119 age and sex-matched healthy
medical volunteers participated in this study. Demographic information
and laboratory findings are shown in Table 1.
In our cohort, 46 male and 73 female medical volunteers were enrolled
as healthy controls, while 118 male and 173 female survivors and 19
adult males and 35 females who recovered from mild and severe
situations were recruited. The median ages in the control, mild, and
severe groups were 52, 53, and 54 years, respectively, and the average
visiting interval after discharge for the recovers was 375.0 days (SD,
11.0 days). No significant differences were found among the mild,
severe and control participants in sex, age, systolic or diastolic
brachial artery pressure, BMI, circulatory level of leukocytes,
neutrophils, lymphocytes, hemoglobin, D-dimer, glucose, triglycerides
(TG), total cholesterol (TC), alanine aminotransferase (ALT),
C-reactive protein (CRP) and estimated glomerular filtration rate
(eGFR). Furthermore, there were no significant differences in plasma
interleukin-6 (IL-6) levels between the mild and severe groups. For all
survivors, no venous thrombosis was observed by lower extremities
ultrasound.
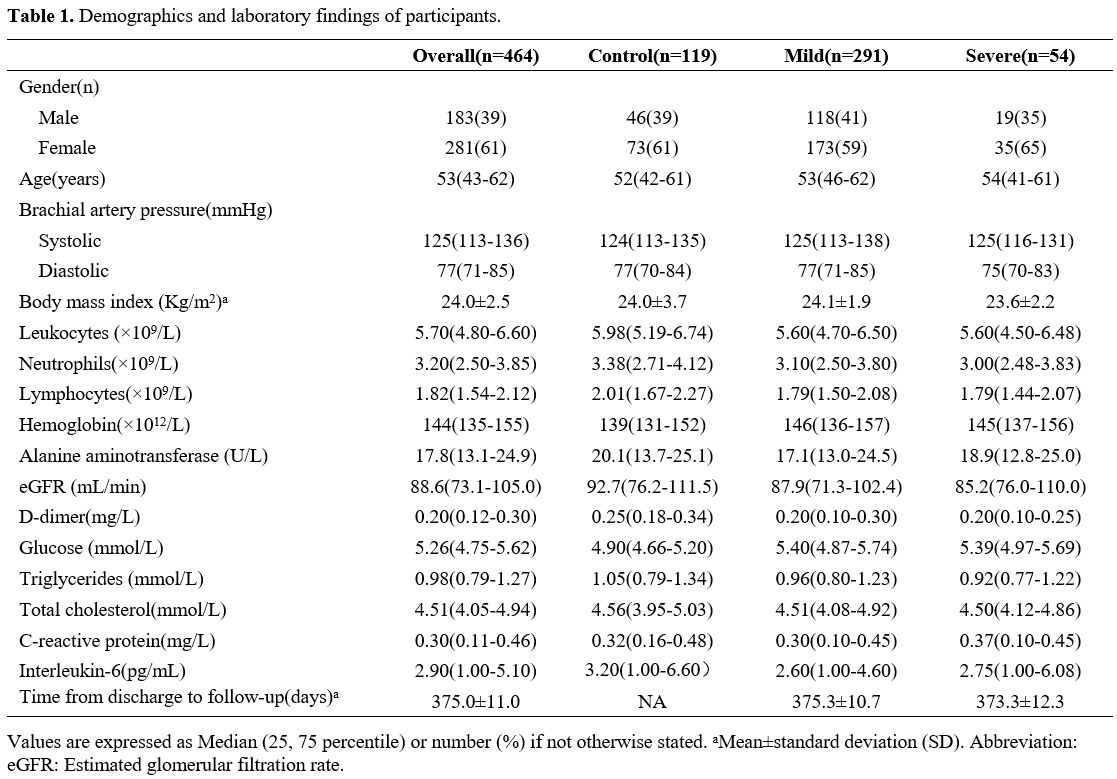 |
Table 1. Demographics and laboratory findings of participants. |
Post-COVID symptoms of participants. Regarding the post-COVID symptoms, a standardized symptom questionnaire was performed and presented in Table 2.
In general, 135 (39%) recovered patients had persistent symptoms during
the 1-year follow-up and no significant differences were found between
the severe and mild groups. Among them, 4 (1%) subjects complained of
persistent low-grade fever, and 128 (37%) recovers were troubled by
fatigue or muscle weakness. For cardiovascular disease-related
symptoms, 21 (6%) participants possessed persistent palpitations, 10
(3%) subjects had chest tightness or shortness of breath, and 8 (2%)
complained of dizziness. In addition, there was no significant
difference observed in those post-COVID symptoms between the mild and
severe groups.
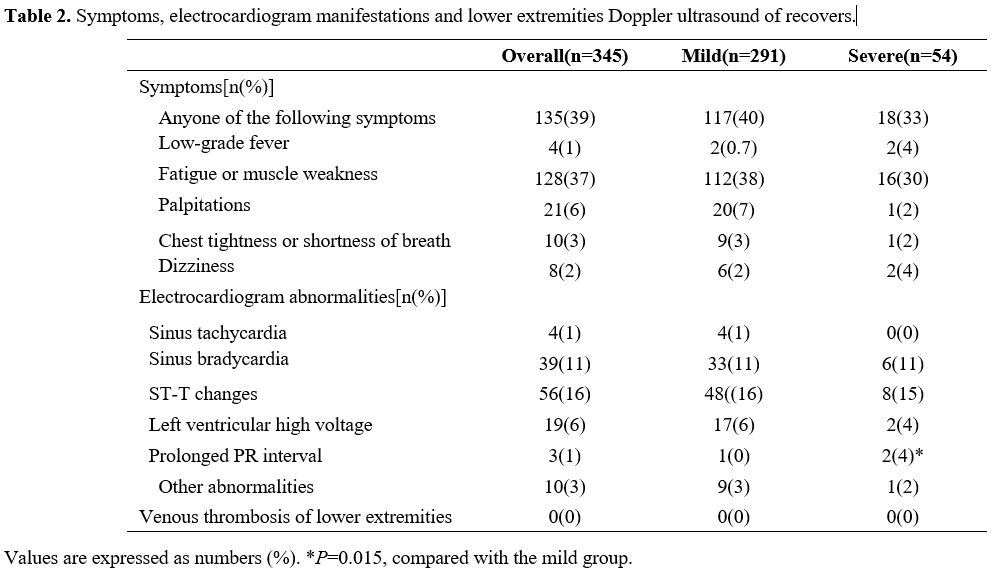 |
Table
2. Symptoms, electrocardiogram manifestations and lower extremities Doppler ultrasound of recovers. |
Electrocardiogram abnormalities in patients recovering from COVID-19.
An electrocardiogram examination was performed for each patient who
recovered from COVID-19 at a one-year follow-up. As presented in Table 2,
the most frequent abnormalities are ST-T changes (16%) and sinus
bradycardia (11%), the frequency of left ventricular high voltage (6%),
sinus tachycardia (1%), prolonged PR interval (1%) and other
abnormalities (such as ventricular premature contraction, atrial
fibrillation, prolonged Q-T interval) is relatively small. Of those
abnormalities, the frequency of prolonged PR interval seemed to be
positively related to the previously infected disease severity (P=0.015), while there was no significant difference in the frequency of other abnormalities between the mild and severe groups.
Serum levels of endothelial biomarkers.
Compared with the control group, the serum VCAM-1 levels showed no
significant differences in patients who recovered from mild (median,
1.69 vs 1.67 ng/mL, P=0.363) or severe (median, 1.69 vs 1.67 ng/mL, P=0.962) situation, in line with that between mild and severe recovers (P=0.553) (Figure 2A).
Although not significant, serum ICAM-1 levels were lower in the mild
group than in controls (median, 427.3 vs 469.7 pg / mL, P=0.139) and the severe group than in the control (median, 424.6 vs 469.7 pg/mL, P=0.789) or in the mild groups (median, 424.6 vs 427.3 pg / mL, P=0.444) (Figure 2B). Similarly, serum P-selectin levels were lower in the mild group than in controls (median, 965.6 vs 1076.6 pg / ml, P=0.296) and in the severe group than in the control (median, 960.4 vs 1076.6 pg / ml, P=0.104) or mild groups (median, 960.4 vs 965.6 pg / ml, P=0.260) (Figure 2C).
Regarding fractalkine, the mild group showed lower serum levels than
the control (mean values, 204.5±24.0 vs 206.8±25.0 pg/mL, P=0.378) and the severe groups (mean values, 204.5±24.0 vs 206.8±19.0 pg/mL, P=0.493), and there were no significant differences between the control and the severe groups (P=0.992) (Figure 2D).
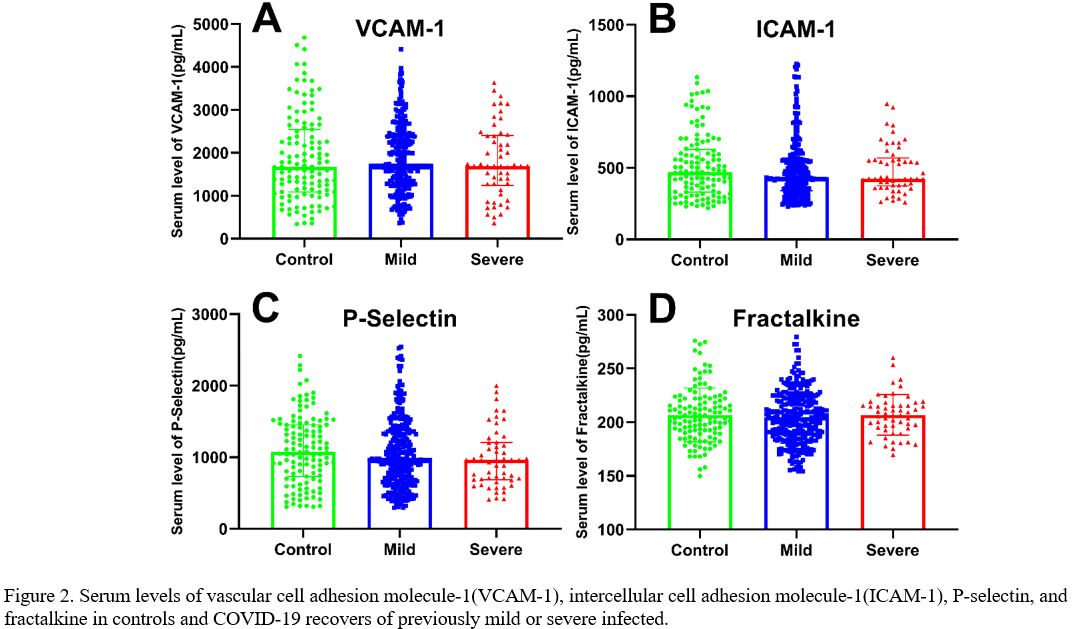 |
Figure
2. Serum levels of vascular cell adhesion molecule-1(VCAM-1),
intercellular cell adhesion molecule-1(ICAM-1), P-selectin, and
fractalkine in controls and COVID-19 recovers of previously mild or
severe infected. |
Discussion
Principal Findings of Our Study.
This study is the first to report endothelial biomarkers of patients
who recovered from COVID-19 one year after discharge. In our cohort, a
considerable number of survivors are still bothered by post-COVID
symptoms. Secondly, inflammatory markers of the normal range, including
neutrophils, CRP, and IL-6, indicate the remission of inflammatory
reactions in those survivors. In addition, significant elevated D-dimer
levels and deep vein thrombosis were absent in all screened survivors,
suggesting a relatively low risk of coagulopathy in the long term.
Furthermore, the levels of circulating endothelial biomarkers,
including VCAM-1, ICAM-1, P-selectin and fractalkine, do not show
significant differences in those survivors and healthy controls,
implying that SARS-CoV-2 infection may not impose a higher risk of the
development of long-term cardiovascular events, even for those
recovering from severe illness.
Comparison with related studies. As previously observed in the SARS epidemic,[16]
recovered patients have persistent symptoms and unexpected higher rates
of diabetes, respiratory and cardiovascular disease, named the
post-COVID syndrome after SARS-CoV-2 infection.[3,17]
Until now, few clinical studies have focused on cardiovascular sequelae
in the aftermath of COVID-19. In a 3-month follow-up study, myocardium
injury was detectable in 30% of recovered COVID-19 patients by cardiac
magnetic resonance (CMR),[18] while in a cohort of
twenty-six patients who recovered from COVID-19 who reported cardiac
symptoms and underwent CMR examinations, fifteen (58%) of them had
abnormal CMR findings, including myocardial edema, fibrosis, and
impaired right ventricle function.[19] In another
study of a cohort of 100 German patients who recently recovered from
COVID-19 infection, CMR imaging revealed cardiac involvement in 78
patients (78%) and ongoing myocardial inflammation in 60 patients
(60%).[20] The prevalence of cardiovascular
complications is alarmingly in those studies, indicating the existence
of the short to medium-term cardiovascular consequences of COVID-19.
However, a reasonable explanation is that the appearance of new or
persistent symptoms in the cohorts could increase the positive CMR
detection rate, implying that some of these patients are not genuine
'convalescent patients'. However, inconsistent with the studies above,
in a single-center longitudinal study, 13% of COVID-19 survivors
experienced significant cardiovascular symptoms three months after
discharge, including an increase in resting heart rate, occasional
palpitations, and newly diagnosed hypertension requiring blood
pressure-lowering medications.[21] At the same time,
in an observational prospective multicentre trial 60 and 100 days after
confirmed diagnosis, cardiac impairment, including reduced left
ventricular function or signs of pulmonary hypertension, was present
only in a minority of subjects.[22] Moreover, in
another preliminary 6-month follow-up study, no survivor reported any
obvious cardiopulmonary symptoms, although 29.6% (8/27) of them were
detected cardiac injury by CMR.[23] These findings
provide the contradictory prevalence of SARS-CoV-2 infection in short-
or medium-term cardiovascular sequelae, and most of the conclusions are
descriptive and imaging-based, lacking objective biomarkers and
long-term follow-up data.
A large number of studies have suggested
that endothelial function reflects the comprehensive influence of
various risk factors on the vascular system,[24] and
endothelial dysfunction is an early predictor of subclinical
atherosclerosis[25] and subsequent long-term cardiovascular events.[26]
Therefore, early detection of soluble endothelial biomarkers
contributes to early detection of disease, quantification of risk, and
early intervention to reduce the incidence of cardiovascular adverse
events in patients.[27] Both indirect induction by
hypercytokinemia (e.g., IL-1, IL -6 and tumor necrosis factor-alpha),
hyperchemokinemia and coagulopathy (named after a high-inflammatory
response),[28] and direct damage to endothelial cells by SARS-CoV-2 infection contributed to endothelial injuries in patients with COVID-19,[6,29] thus improving the expression of endothelial biomarkers, including ICAM-1, VCAM-1, P-selectin, and fractalkine.[15,30]
However, few studies have focused on the alterations of endothelial
biomarkers and cytokines in patients recovering from COVID-19. In a
prospective longitudinal multicenter cohort study, regulators of
endothelial activation such as vascular endothelial growth factor
(VEGF), brain-derived neurotrophic factor (BDNF), and macrophage
inflammatory protein-1β (MIP-1β) were persistently elevated in
convalescence patients with COVID-19, potentially promoting the
development of atherosclerosis and cardiovascular sequelae.[31]
In another 3-month follow-up study, persistent abnormal levels of
endothelial biomarkers, pro-inflammatory cytokines and chemokines
(VCAM-1, ICAM-1, TNF-α, MIP-1α, and MIP-1β) were observed in those
recovered from COVID-19, especially in severe/critical patients.[32]
Therefore, by describing the post-COVID symptoms and abnormal ECG
changes, detection of both lower extremities thrombosis, and measuring
the circulatory levels of inflammatory factors and endothelial
biomarkers, our study may provide a more comprehensive cardiovascular
perspective for COVID-19 recovers one year after discharge.
In
our cohort, the influence of various confounders, such as older age,
pregnancy, chronic smoking, preexisting conditions (malignancy,
diabetes mellitus, hyperlipidemia, obesity), and current medications
was strictly excluded, thus may not reflect the overall situation of
cardiovascular sequelae in COVID-19 recovers with a preexisting higher
risk of endothelial dysfunction. In the study, relatively low levels of
circulating endothelial biomarkers at one-year follow-up for those
survivors without preexisting endothelial dysfunction risks were
observed, although injuries to endothelium cells are believed to have
long-lasting effects.[33] Several mechanisms were
speculated to be majorly ascribed to the remission of endothelial
biomarkers. First, the clearance of SARS-CoV-2 infection in all
recruited patients, confirmed by repeated screening after discharge, is
conducive to the remission of endothelial biomarkers. Second, with the
clearance of viral infection, the levels of inflammatory markers (such
as neutrophils, CRP, and IL-6) returned to normal, and a coordinated
and dynamic immune response, characterized by reduced inflammation, was
developed.[34] The indirect mechanism of endothelial
dysfunction induced primarily by high inflammatory responses could be
interrupted. Third, the activation of endothelial cells leads to a
procoagulant phenotype, which in turn continuously activates
endothelial injuries during the acute infection phase.[35]
In addition to the normal ranged D-dimer, deep venous thrombosis of
lower extremities was excluded by ultrasonography in our cohort,
consistently with the previous study,[17] indicating
the termination of this vicious cycle that sustained activating
endothelial injuries. Therefore, our study could help to explain why
COVID-19 survivors no longer need to endure the risk of long-term
thrombosis at the level of endothelial phenotype.
Conclusions
Our
findings are encouraging, in light of the endothelial dysfunction is
involved in the pathogenesis of venous thromboembolism and vasculitis
in patients with COVID-19 during the acute phase, thus arousing
widespread concern for cardiovascular sequelae in long-term. In our
cohort of COVID-19 survivors one year after discharge, significantly
higher levels of endothelial biomarkers and higher risk of deep vein
thromboembolism in the lower extremities were absent, although the
longer-term risk of cardiovascular disease development remains to be
elucidated.
Limitations
Limitations
should be noted before interpreting the results of this study. First,
due to the inaccessibility of the samples from COVID-19 patients during
the acute phase, the lack of comparative longitudinal data makes it
impossible to dynamically observe the changes of endothelial biomarkers
and electrocardiogram, which may affect the causal inference of the
SARS-CoV-2 infection and the incidence of cardiovascular events, as
well as the accuracy of the conclusions in this cross-sectional study.
Second, parameters that more comprehensively reflect vascular function
(such as the vascular stiffness index and the intima/media thickness
ratio) may better predict future cardiovascular events. However, due to
the convenience of the equipment, the failure to combine these
parameters with endothelial biomarkers is one major limitation in our
study. Third, additional mechanistic work is required to understand
better the potential role of the adaptive immune response in the
recovery process of endothelial biomarkers and inflammatory markers in
patients with COVID-19.
Acknowledgements
On
behalf of the authors, we sincerely thank the medical staff of
Huanggang Central Hospital, Huangzhou District Maternal and Child
Health Hospital, and Huangzhou District People's Hospital, Huanggang,
Hubei, China, as well as the healthy volunteers recruited at Hunan
Provincial People's Hospital, who contributed outstandingly to this
study. More importantly, we would like to thank all our patients and
their families who volunteered to participate in this study.
Data availability
The
datasets used and/or analyzed are provided in this paper. Any other raw
data supporting the findings of this study are available from the
corresponding authors upon reasonable request.
Authors' Contributions
YMZ,
FC, and YZ conceptualized and designed the studies. ZXJ, SJZ, LHZ, GQC,
RFG, ZLZ, XTH, JMH, SQY, and CCM collected the clinical data. MT, YJ,
and YJL performed an ELISA. YMZ put forward the outline of the article
with XQY and WX. MT, QZ, and XQY performed data analysis and drew
pictures. MT and YJ drafted the manuscript, YMZ, XQY, and FC revised
the article, and all authors read and approved the final version.
Funding
This work was supported by the Key Research and Development Program of Hunan Province (grant number 2020SK3011).
References
- Johns Hopkins University. Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html Accessed Oct 30 2021.
- Song
WJ, Hui CKM, Hull JH, et al. Confronting COVID-19-associated cough and
the post-COVID syndrome: role of viral neurotropism, neuroinflammation,
and neuroimmune responses. The Lancet Respiratory Medicine 2021;
9:533-44. https://doi.org/10.1016/S2213-2600(21)00125-9
- Ayoubkhani
D, Khunti K, Nafilyan V, et al. Post-covid syndrome in individuals
admitted to hospital with covid-19: retrospective cohort study. BMJ
(Clinical research ed) 2021; 372:n693. https://doi.org/10.1136/bmj.n693
PMid:33789877 PMCid:PMC8010267
- Gupta A,
Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of
COVID-19. Nature Medicine 2020; 26:1017-32.
https://doi.org/10.1038/s41591-020-0968-3 PMid:32651579
- Zheng
YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system.
Nature Reviews Cardiology 2020; 17:259-60.
https://doi.org/10.1038/s41569-020-0360-5 PMid:32139904 PMCid:PMC7095524
- Ackermann
M, Verleden SE, Kuehnel M, et al. Pulmonary Vascular Endothelialitis,
Thrombosis, and Angiogenesis in Covid-19. The New England Journal of Medicine 2020; 383:120-8. https://doi.org/10.1056/NEJMoa2015432
PMid:32437596 PMCid:PMC7412750
- Gu SX,
Tyagi T, Jain K, et al. Thrombocytopathy and endotheliopathy: crucial
contributors to COVID-19 thromboinflammation. Nature Reviews Cardiology
2021; 18:194-209. https://doi.org/10.1038/s41569-020-00469-1
PMid:33214651 PMCid:PMC7675396
- Dou Q, Wei
X, Zhou K, Yang S, Jia P. Cardiovascular Manifestations and Mechanisms
in Patients with COVID-19. Trends in Endocrinology and Metabolism: TEM
2020; 31:893-904. https://doi.org/10.1016/j.tem.2020.10.001
PMid:33172748 PMCid:PMC7566786
- Li X, Sun
X, Carmeliet P. Hallmarks of Endothelial Cell Metabolism in Health and
Disease. Cell Metab 2019; 30:414-33.
https://doi.org/10.1016/j.cmet.2019.08.011 PMid:31484054
- Libby
P, Lüscher T. COVID-19 is, in the end, an endothelial disease. European Heart Journal 2020; 41:3038-44.
https://doi.org/10.1093/eurheartj/ehaa623 PMid:32882706 PMCid:PMC7470753
- Caligiuri
G. CD31 as a Therapeutic Target in Atherosclerosis. Circ Res 2020;
126:1178-89. https://doi.org/10.1161/CIRCRESAHA.120.315935 PMid:32324506
- Ford
TJ, Ong P, Sechtem U, et al. Assessment of Vascular Dysfunction in
Patients Without Obstructive Coronary Artery Disease: Why, How, and
When. JACC Cardiovascular Interventions 2020; 13:1847-64.
https://doi.org/10.1016/j.jcin.2020.05.052 PMid:32819476
PMCid:PMC7447977
- Polonsky TS, McDermott
MM. Lower Extremity Peripheral Artery Disease Without Chronic
Limb-Threatening Ischemia: A Review. Jama 2021; 325:2188-98.
https://doi.org/10.1001/jama.2021.2126 PMid:34061140
- Blevins
BL, Vinters HV, Love S, et al. Brain arteriolosclerosis. Acta Neuropathologica 2021; 141:1-24.
https://doi.org/10.1007/s00401-020-02235-6 PMid:33098484
PMCid:PMC8503820
- Tong M, Jiang Y, Xia D,
et al. Elevated Expression of Serum Endothelial Cell Adhesion Molecules
in COVID-19 Patients. The Journal of Infectious Diseases 2020;
222:894-8. https://doi.org/10.1093/infdis/jiaa349 PMid:32582936
PMCid:PMC7337874
- Zhang P, Li J, Liu H, et
al. Long-term bone and lung consequences associated with
hospital-acquired severe acute respiratory syndrome: a 15-year
follow-up from a prospective cohort study. Bone Research 2020; 8:8.
https://doi.org/10.1038/s41413-020-0084-5 PMid:32128276 PMCid:PMC7018717
- Huang
C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients
discharged from hospital: a cohort study. Lancet 2021; 397:220-32.
https://doi.org/10.1016/S0140-6736(20)32656-8
- Wang
H, Li R, Zhou Z, et al. Cardiac involvement in COVID-19 patients:
mid-term follow up by cardiovascular magnetic resonance. Journal of
Cardiovascular Magnetic Resonance: Official Journal of the Society for
Cardiovascular Magnetic Resonance 2021; 23:14.
https://doi.org/10.1186/s12968-021-00710-x PMid:33627143
PMCid:PMC7904320
- Huang L, Zhao P, Tang D,
et al. Cardiac Involvement in Patients Recovered From COVID-2019
Identified Using Magnetic Resonance Imaging. JACC Cardiovascular Imaging 2020; 13:2330-9. https://doi.org/10.1016/j.jcmg.2020.05.004
PMid:32763118 PMCid:PMC7214335
- Puntmann
VO, Carerj ML, Wieters I, et al. Outcomes of Cardiovascular Magnetic
Resonance Imaging in Patients Recently Recovered From Coronavirus
Disease 2019 (COVID-19). JAMA Cardiology 2020; 5:1265-73.
https://doi.org/10.1001/jamacardio.2020.3557 PMid:32730619
PMCid:PMC7385689
- Xiong Q, Xu M, Li J, et
al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a
single-centre longitudinal study. Clinical microbiology and infection :
the official publication of the European Society of Clinical
Microbiology and Infectious Diseases 2021; 27:89-95.
https://doi.org/10.1016/j.cmi.2020.09.023 PMid:32979574 PMCid:PMC7510771
- Sonnweber
T, Sahanic S, Pizzini A, et al. Cardiopulmonary recovery after
COVID-19: an observational prospective multicentre trial. The European Respiratory Journal 2021; 57.
- Wu X, Deng
KQ, Li C, et al. Cardiac Involvement in Recovered Patients From
COVID-19: A Preliminary 6-Month Follow-Up Study. Frontiers in Cardiovascular Medicine 2021; 8:654405.
https://doi.org/10.3389/fcvm.2021.654405 PMid:34055936 PMCid:PMC8155269
- Münzel
T, Sinning C, Post F, Warnholtz A, Schulz E. Pathophysiology, diagnosis
and prognostic implications of endothelial dysfunction. Annals of Medicine 2008; 40:180-96. https://doi.org/10.1080/07853890701854702
PMid:18382884
- Celermajer DS, Sorensen KE,
Gooch VM, et al. Non-invasive detection of endothelial dysfunction in
children and adults at risk of atherosclerosis. Lancet 1992;
340:1111-5. https://doi.org/10.1016/0140-6736(92)93147-F
- Kitta
Y, Obata JE, Nakamura T, et al. Persistent impairment of endothelial
vasomotor function has a negative impact on outcome in patients with
coronary artery disease. J Am Coll Cardiol 2009; 53:323-30.
https://doi.org/10.1016/j.jacc.2008.08.074 PMid:19161880
- Koga
H, Sugiyama S, Kugiyama K, et al. Elevated levels of
VE-cadherin-positive endothelial microparticles in patients with type 2
diabetes mellitus and coronary artery disease. J Am Coll Cardiol 2005;
45:1622-30. https://doi.org/10.1016/j.jacc.2005.02.047 PMid:15893178
- Perico
L, Benigni A, Casiraghi F, Ng LFP, Renia L, Remuzzi G. Immunity,
endothelial injury and complement-induced coagulopathy in COVID-19.
Nature Reviews Nephrology 2021; 17:46-64.
https://doi.org/10.1038/s41581-020-00357-4 PMid:33077917
PMCid:PMC7570423
- Varga Z, Flammer AJ,
Steiger P, et al. Endothelial cell infection and endotheliitis in
COVID-19. Lancet 2020; 395:1417-8.
https://doi.org/10.1016/S0140-6736(20)30937-5
- Goshua
G, Pine AB, Meizlish ML, et al. Endotheliopathy in COVID-19-associated
coagulopathy: evidence from a single-centre, cross-sectional study. The
Lancet Haematology 2020; 7:e575-e82.
https://doi.org/10.1016/S2352-3026(20)30216-7
- Ong
SWX, Fong SW, Young BE, et al. Persistent Symptoms and Association With
Inflammatory Cytokine Signatures in Recovered Coronavirus Disease 2019
Patients. Open Forum Infectious Diseases 2021; 8:ofab156.
https://doi.org/10.1093/ofid/ofab156 PMid:34095336 PMCid:PMC8083585
- Zhou
M, Yin Z, Xu J, et al. Inflammatory profiles and clinical features of
COVID-19 survivors three months after discharge in Wuhan, China. The
Journal of infectious diseases 2021.
- Daiber
A, Steven S, Weber A, et al. Targeting vascular (endothelial)
dysfunction. British Journal of Pharmacology 2017; 174:1591-619.
https://doi.org/10.1111/bph.13517 PMid:27187006 PMCid:PMC5446575
- Kared
H, Redd AD, Bloch EM, et al. SARS-CoV-2-specific CD8+ T cell responses
in convalescent COVID-19 individuals. The Journal of Clinical Investigation 2021; 131. https://doi.org/10.1172/JCI145476
PMid:33427749 PMCid:PMC7919723
- Jin Y, Ji
W, Yang H, Chen S, Zhang W, Duan G. Endothelial activation and
dysfunction in COVID-19: from basic mechanisms to potential therapeutic
approaches. Signal Transduction and Targeted Therapy 2020; 5:293.
https://doi.org/10.1038/s41392-020-00454-7 PMid:33361764
PMCid:PMC7758411
[TOP]

