Stasa Krasic1, Sasa Popovic1, Ruzica Kravljanac2,3, Sergej Prijic1,2 and Vladislav Vukomanovic1,2.
1 Cardiology Department, Mother and Child Health Institute of Serbia; Belgrade, Serbia.
2 School of Medicine, University of Belgrade; Belgrade, Serbia.
3 Neurology Department, Mother and Child Health Institute of Serbia; Belgrade, Serbia.
Correspondence to:
Vladislav
A. Vukomanovic MD, PhD. Pediatric cardiologist , Mother and Child
Health Care Institute of Serbia "Dr. Vukan Cupic", R. Dakica St. 6-8,
11070 Belgrade, Serbia. Professor of Pediatrics, School of Medicine -
University of Belgrade, Serbian representative in AEPC. Tel:
+381658405885, Fax: +381112697232. E-mail:
vvukomanovicdr@gmail.com
Published: March 1, 2022
Received: December 15, 2021
Accepted: February 12, 2022
Mediterr J Hematol Infect Dis 2022, 14(1): e2022028 DOI
10.4084/MJHID.2022.028
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
To the editor
A
multisystem inflammatory syndrome in children (MIS-C) associated with
COVID-19, a newly described condition, is a hypercoagulable state
caused by hyper inflammation and cytokine storm.[1-4]
Although more than half of the patients with MIS-C (76%) have
biochemical evidence of coagulopathy, the prevalence of deep vein
thrombosis or pulmonary emboli is low (8%). On the other hand, the most
extensive published MIS-C case series have not reported thrombotic
complications.[1-4]
Intracardiac thrombus is not
a common condition in otherwise healthy children, and the most common
location is the right-sided chambers. A left-sided intracardiac
thrombus is almost always associated with left ventricular (LV)
dysfunction or arrhythmia.[5]
We presented a
left ventricle thrombus in a three-year-old boy with the normal
systolic function of the left ventricle in MIS-C associated with
COVID-19.
Clinical-Description
A three-year-old boy with a history of previous COVID-19 was admitted to the hospital due to a seven-day high fever (40,3°C),
macular erythematous rash, bilateral nonexudative conjunctivitis,
palmar-plantar edema, diarrhea, and vomiting. He was initially treated
with oral antibiotics. At the admission, he was febrile (38,6°C)
with normal vital signs (heart rate 150/min, blood pressure 90/48 mmHg,
respiratory rate 20/min). The physical examination revealed
mucocutaneous (strawberry tongue and rash) and conjunctival changes.
Laboratory findings showed mild anemia, hypoalbuminemia, hyponatremia,
elevated C-reactive protein (CRP), pro-BNP, and mildly elevated liver
enzymes D-dimers (Table 1).
Routine urine examination showed sterile pyuria. The antibodies IgM and
IgG classes against SARS-CoV-2 were detected in the blood sample by
ELISA technique. ECG and thoracic X-ray were normal. Echocardiographic
finding pointed out a normal systolic and diastolic function of the LV.
The dimension of the proximal coronary arteries was average. According
to physical examination and the results of the performed analysis,
diagnosis of MIS-C associated with COVID-19 was made. Three pulses of
intravenous methylprednisolone were administered at a 24 hours
interval. The patient became afebrile after the first IVMP. Control
laboratory parameters were in the reference range, and after 7 days of
in-hospital stay, the discharge was planned.
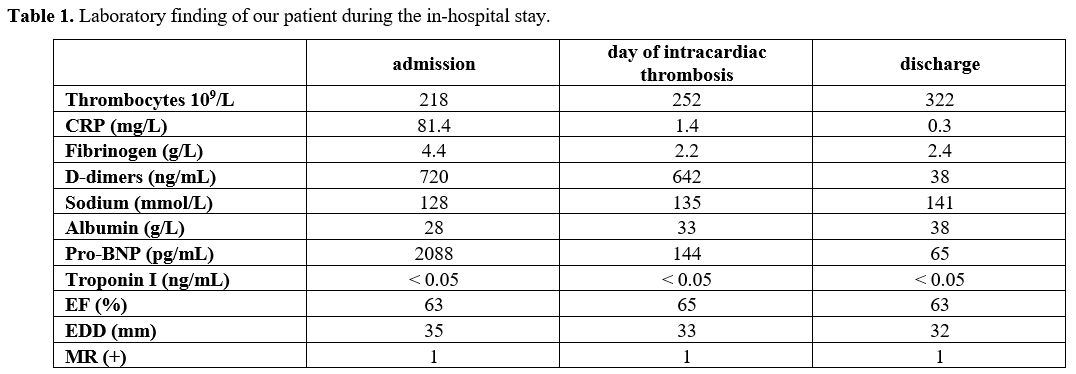 |
Table
1. Laboratory finding of our patient during the in-hospital stay. |
Before
the planned discharge, control echocardiography was performed. The
echocardiogram showed no valvular abnormalities, normal LV systolic
function with an ejection fraction of 65%, and a pedunculated bifid
mobile 14x10 mm mass at the apex of the LV (Figure 1).
He was immediately transferred to the pediatric intensive care unit.
Anticoagulation and anti-aggregating drugs were administered.
Laboratory findings pointed out the normal range of D-dimer, fibrinogen
and platelets (Table 1).
Additional hematology evaluation found elevated coagulation factor VIII
(FVIII) (280,5%, reference range 50-150%), and XII (FXII) (209,8%,
reference range 50-150%), while protein S and protein C activity levels
were in the normal range. Anti-cardiolipin IgG and IgM antibodies were
within normal limits. Factor V Leiden and FII gene mutations were not
present. After 36 hours of verified intracardiac thrombosis, he became
febrile again and developed right-sided hemiplegia and facial palsy
associated with eye deviation (Prévost's sign) toward the left side,
and aphasia. The same evening, in the control echocardiographic finding
floating, LV mass was not observed. Urgent endocranial computerized
tomography (CT) angiography was performed and, a perfusion defect of
the M2 segment of the left middle cerebral artery (MCA) was described.
Brain magnetic resonance imaging (MRI) and MRI angiography showed a
sizeable ischemic zone without signs of hemorrhage due to lack of
perfusion in the MCA territory, especially in its terminal branches (Figure 2).
Electroencephalographam (EEG) was done within 8 hours from the
appearance of neurological signs and showed asymmetric background
activity with slow theta activity above the left side without epileptic
discharges (Supplement 1). Due to the multi-day of fraxiparine
management, thrombolysis was contraindicated, and the physician's
council decided to continue anticoagulant and antiplatelet therapy,
along with other symptomatic and supportive measures. During the one
month in-hospital stay, the general condition improved, while
neurological recovery lasted for a long time, and the boy was
discharged with significant hemiplegia with improvement in speech and
facial palsy. The control brain MRI showed cortico-subcortical atrophy
of the basal ganglia limited zones of cytotoxic edema in the left
parietal cortex with secondary ventriculomegaly one month after acute
cerebral stroke. A significant reduction of ramification and flow in
the terminal branches of the left MCA was described.
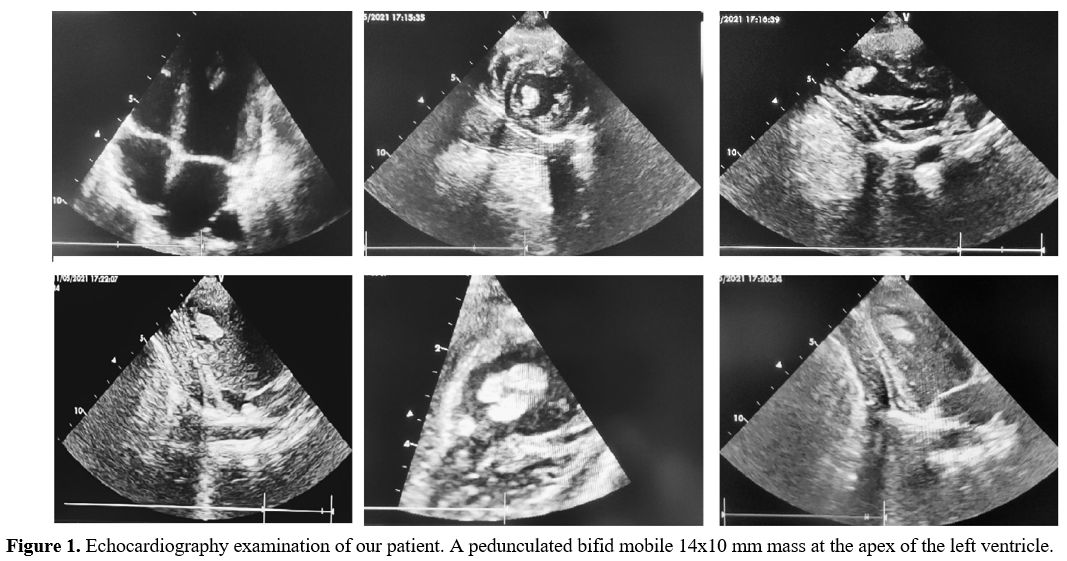 |
Figure 1. Echocardiography
examination of our patient. A pedunculated bifid mobile 14x10 mm mass
at the apex of the left ventricle. |
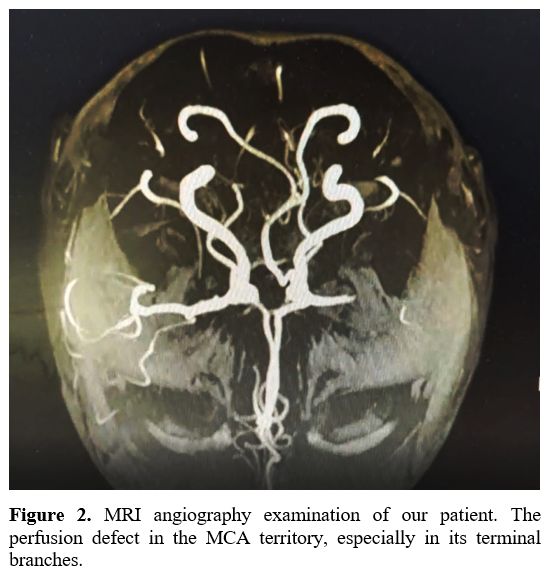 |
Figure
2. MRI angiography examination of our patient. The perfusion defect in the MCA territory, especially in its terminal branches. |
Discussion
We
presented the left ventricle thrombus in the three-year-old boy with
the normal systolic function of the LV in MIS-C associated with
COVID-19. Namely, the occurrence of one or more Virchow triad
components predisposes intravascular or intracardiac thrombosis.
Bigdelian et al. presented three patients with acute intracardiac
thrombosis during COVID-19; all of those children had preserved EF. In
compression to patients with COVID-19 (2.1%, 95% CI, 1% to 4%) and
asymptomatic SARS-CoV-2 infection (0.7%, 95% CI, 0.1% to 2.4%),
patients with MIS-C had the highest incidence at 6.5% (95% CI, 3% to
12%) of thrombotic events.[6] Among patients with
thrombotic events (20 pts) only 3 had intracardiac thrombosis and
associated comorbidities - two with acute COVID-19 and cancer, and one
with MIS-C and obesity. All of those patients had catheter-related
thrombosis.[6] Schroder J. et al. presented a healthy
17-years-old boy with systolic dysfunction of LV and a mural thrombus
near the posteromedial papillary muscle in the LV apex.[7] Our patient had normal LV systolic function, but despite this, LV thrombus was developed at the time of intended discharge.
In
SARS-CoV-2 infection, increased acute-phase reactants such as
fibrinogen and CRP may contribute to the hypercoagulable state.[1-4]
Our patient had normal coagulation status, including fibrinogen and
D-dimers, but markedly elevated FVIII and FXII. Factor VIII and von
Willebrand (VWF) have previously been described as acute phase
reactants.[8,9] Strong independent associations have been proved between elevated FVIII and an increased risk of arterial thrombosis.[8,9]
Although endothelial cells produce FVIII and VWF, SARS-CoV-2 might
induce their accelerated synthesis acting on endothelial ACE-2
receptors. Coagulation factor XII (FXII, Hageman factor) is a plasma
protease that initiates the procoagulant and proinflammatory contact
system. In addition to its role in thrombosis, FXIIa contributes to
inflammation by activating the inflammatory bradykinin-producing
kallikrein-kinin system.[10] SARS-CoV-2 inducted endothelial dysfunction leads to activation of the external coagulation pathway.[1-4]
On the other hand, increased IL-6 and other cytokines establish a prothrombotic state by disabling the natural anticoagulants.[8]
Consequently, our patient had a transient hypercoagulable state, likely
secondary to the recent SARS-CoV-2 infection. To the best of our
knowledge, that is the first case of a three-year-old boy with
intracardiac thrombosis in LV with normal systolic function during
MIS-C associated with COVID-19.
Conclusions
Floating
intracardiac thrombus in children with normal LV systolic function is
hazardous with excellent potential for thromboembolic complications.
The hypercoagulable state might be one of the most critical risk
factors for intracardiac thrombosis event systolic function of LV is
preserved. Recent SARS-CoV-2 infection leads to coagulopathy and
hypercoagulable condition with increased risk of vessels thrombosis and
embolism; pharmacological thromboprophylaxis in MIS-C should be highly
recommended.
References
- Sharathkumar AA, Faustino EVS, Takemoto CM. How we
approach thrombosis risk in children with COVID-19 infection and MIS-C.
Pediatr Blood Cancer. 2021; 68: 1-9. https://doi.org/10.1002/pbc.29049 PMid:33955167 PMCid:PMC8206673
- Whitworth
H, Sartain SE, Kumar R, et al. Rate of thrombosis in children and
adolescents hospitalized with COVID-19 or MIS-C. Blood. 2021; 138:
190-198. https://doi.org/10.1182/blood.2020010218 PMid:33895804 PMCid:PMC8079262
- Al-Ghafry
M, Vagrecha A, Malik M, et al. Multisystem inflammatory syndrome in
children (MIS-C) and the prothrombotic state: Coagulation profiles and
rotational thromboelastometry in a MIS-C cohort. J Thromb Haemost.
2021; 19: 1764-1770. https://doi.org/10.1111/jth.15340 PMid:33872443
- Aguilera-Alonso
D, Murias S, Martínez-De-Azagra Garde A, et al. Prevalence of
thrombotic complications in children with SARS-CoV-2. Arch Dis Child.
2021; 106: 1129-1132. https://doi.org/10.1136/archdischild-2020-321351 PMid:33931403
- Çetin
II, Ekici F, Ünal S, et al. Intracardiac thrombus in children: The fine
equilibrium between the risk and the benefit. J Pediatr Hematol Oncol.
2014; 31: 481-487. https://doi.org/10.3109/08880018.2014.919546 PMid:24933192
- Bigdelian
H, Sedighi M, Sabri MR, et al. Case Report: Acute intracardiac
thrombosis in Children with Coronavirus Disease 2019 (COVID-19). Front
Pediatr. 2021; 9: 1-4. https://doi.org/10.3389/fped.2021.656720 PMid:34249807 PMCid:PMC8267003
- Schroder
J, Lund MAV, Vejlstrup N, Juul K, Nygaard U. Left ventricular thrombus
in multisystem inflammatory syndrome in children associated with
COVID-19. Cardiol Young. 2021: 1-4. https://doi.org/10.1017/S1047951121002456 PMid:34082849 PMCid:PMC8220022
- Lelas
A, Greinix HT, Wolff D, Eissner G, Pavletic SZ, Pulanic D. Von
Willebrand Factor, Factor VIII, and Other Acute Phase Reactants as
Biomarkers of Inflammation and Endothelial Dysfunction in Chronic
Graft-Versus-Host Disease. Front Immunol. 2021; 12: 1-11. https://doi.org/10.3389/fimmu.2021.676756 PMid:33995421 PMCid:PMC8119744
- Zivkovic
I, Milacic P, Mihajlovic V, et al. Surgical treatment of ascending
aorta floating thrombus in a patient with recent SARS-CoV-2 infection.
Cardiovasc Diagn Ther. 2021; 11: 467-471. https://doi.org/10.21037/cdt-20-1010 PMid:33968624 PMCid:PMC8102247
- Nickel
KF, Long AT, Fuchs TA, Butler LM, Renné T. Factor XII as a therapeutic
target in thromboembolic and inflammatory diseases. Arterioscler Thromb
Vasc Biol. 2017; 37: 13-20. https://doi.org/10.1161/ATVBAHA.116.308595 PMid:27834692
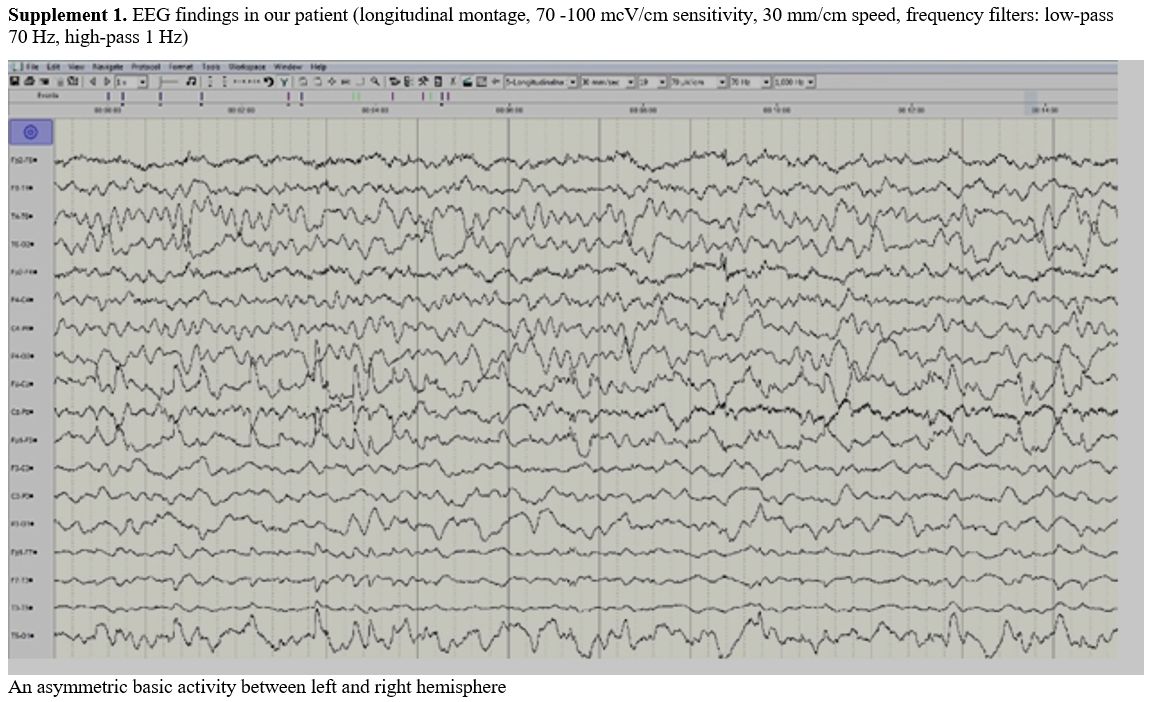 |
Supplement 1. EEG findings
in our patient (longitudinal montage, 70 -100 mcV/cm sensitivity, 30
mm/cm speed, frequency filters: low-pass 70 Hz, high-pass 1 Hz) |
[TOP]



