Anna Maria Testi, Paolo Musiu, Maria Luisa Moleti, Saveria Capria, Walter Barberi.
Hematology, Department of Translational and Precision Medicine, 'Sapienza' University, Rome, Italy.
Correspondence to:
Anna Maria Testi. Hematology, Department of Translational and Precision
Medicine, Sapienza, University of Rome, Via Benevento 6, 00161 Rome,
Italy. Tel: +39-06-49974739; Fax: +39-06-44241984. E-mail:
testi@bce.uniroma1.it
Published: May 1, 2022
Received: February 2, 2022
Accepted: April 14, 2022
Mediterr J Hematol Infect Dis 2022, 14(1): e2022038 DOI
10.4084/MJHID.2022.038
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
The
past three decades have brought major therapeutic advances in treating
acute promyelocytic leukemia (APL) both in adults and children. The
current state-of-the-art treatment with all-trans retinoic acid (ATRA)
and arsenic trioxide (ATO) in combination or not with chemotherapy
results in long-lasting remission and cure in more than 90% of newly
diagnosed patients. These treatments have made relapse a rare event.
The detection of PML-RARA transcript by polymerase chain reaction (PCR)
during treatment and follow-up can predict a hematological relapse. All
studies have suggested a survival benefit in patients with molecular
relapse given pre-emptive therapy compared with those treated at the
time of overt hematological relapse. ATO-based regimens seem to be
effective for achieving a second molecular complete remission (CR).
Patients in second molecular CR are generally considered candidates for
autologous hematopoietic stem cell transplant (HSCT), while for those
with a persistent molecular disease, allogeneic HSCT should be offered
if a suitable donor is identified.
Except for sporadic pediatric
reports, most of the evidence for using HSCT to treat
relapsed/refractory APL comes from adult literature. Therefore, we now
provide a review of published pediatric data that evaluated the role of
HSCT in children with refractory/recurrent APL disease.
|
Introduction
The
new treatment approaches for pediatric patients with newly diagnosed
acute promyelocytic leukemia (APL), combining a differentiation agent,
all-trans retinoic acid (ATRA) and anthracyclines, result in a complete
remission rate of 96-97%, and a virtual absence of primary resistance.[1-6]
These cure rates are currently obtained with risk-adapted treatments
where the intensity and duration of induction and consolidation therapy
are modulated according to clinical and biological parameters at
disease presentation and early molecular response to treatment. The
5-year overall survival (OS) and event-free survival (EFS) for children
treated with Italian AIDA 2000 and International ICC-APL-01 protocols,
both risk-adapted strategies combining ATRA and chemotherapy, are
94%-95% and 80-85%, respectively.[1,2] These protocols
enrolled a high number of children and adolescents (127 and 258,
respectively); better results are reported for patients defined at
standard risk (SR, WBC<10x109/L) with an OS rate of 98% compared to 89% achieved in patients at high-risk (HR, WBC≥10x109/L).
More
recently, the ATRA+ATO combination, chemo-free approach, associated or
not to chemotherapy for HR children, further decreased the relapse rate
in these patients. Results are still limited to a small number of young
patients, but more than 95% of these patients are alive and
leukemia-free at two years.[7-10]
Minimal
residual disease (MRD) monitoring based on detection of PML-RARA
transcript, employing first, qualitative and then quantitative
polymerase-chain-reaction (PCR; RT-and RQ-PCR) technologies after the
second or third consolidation course and during patients' follow-up,
gave the opportunity to show an early detection of molecular relapse in
the bone marrow, before the occurrence of hematologic relapse.[2,11,12]
Patients in molecular relapse, especially with ATO-based regimens, have
a higher probability of achieving a second molecular complete remission
(CR), with no risk of exacerbation of leukemic promyelocytes-related
coagulopathy compared with patients with overt haematologic relapse.[13]
Hematopoietic Stem Cell Transplantation as Part of APL Salvage Therapy (Allogeneic and Autologous)
In
this context, hematopoietic stem cell transplant (HSCT) is no longer
indicated in the first-line treatment, but it may play a crucial role
in consolidating remission in patients in second CR or beyond after
salvage therapy relapsed APL.[14-16] For patients in
second CR, consolidation with HSCT, either autologous (auto) or
allogeneic (allo) resulted in better survival outcomes than
non-transplant strategies. Although HSCT is generally accepted therapy
for APL in second CR, the choice of allo- versus auto-HSCT remains
controversial. It is uncertain whether the increased treatment-related
mortality (TRM) generally associated with allo-HSCT is compensated for
by a lower relapse rate due to the graft versus APL effect.[15,17]
It has also been suggested that auto-HSCT's outcome is improved if
molecularly negative cells are collected. The recent analysis of the
Acute Leukemia Working Party (ALWP) of the European Blood Bone Marrow
Transplantation (EBMT) included 341 and 228 APL patients in second CR
who underwent auto-HSCT and allo-HSCT, respectively.[16,17]
The EFS was significantly higher in the auto-group (75%) compared to
the allo-group (55%); auto-HSCT was also superior to allo-HSCT in terms
of OS. In keeping with these findings, other large studies reported
better OS for auto-HSCT compared to allo-HSCT.[13,15-17]
The benefit of the graft-versus-leukemia (GVL) effect in patients
undergoing allo-HSCT was widely counterbalanced by a higher TRM
systematically reported in this setting compared with patients
undergoing auto-HSCT. On the other hand, a second relapse after
auto-HSCT is probably a clinical situation with a higher chance of
subsequent salvage as compared to a second relapse after allo-HSCT.
Prognostic factors associated with transplant outcomes in APL in second
CR that adversely influenced overall mortality were mainly age (>40
years) and shorter first CR duration; in addition, the molecular
persistence after salvage treatment had an adverse impact on transplant
outcome in multivariate analysis.[16-18] Regarding
auto-HSCT, the previous salvage therapy with ATO was associated with
delayed hematopoietic recovery after transplantation.[19,20] A retrospective review of 58 APL patients undergoing auto-HSCT at [21]
Institutions in the United States and Japan reported that ATO exposure
prior to hematopoietic stem cells collection harmed hematopoietic
recovery after auto-HSCT. However, this delay does not significantly
impact TRM and transplant outcome.[20]
Peripheral
blood has been, by far, the preferred stem cell source used in most
studies where auto-HSCT represented the consolidation therapy in second
CR. The proportion of patients transplanted with peripheral blood in
the different studies varied from 86% to 100%, including the recent
EBMT study in which the proportion increased to 92%.[16] In contrast, the most common stem cell source in the allo-HSCT setting was bone marrow (range 64% to 87%).[16]
Myeloablative
conditioning regimens were almost universally used for auto- and
allo-HSCT, except for a few patients who received reduced-intensity
regimens (RIC).[21] TBI-based regimens were preferred
for auto-HSCT in the Center for International Blood and Marrow
Transplant Research (CIBMTR) registry data[21] and
single-centre studies; in contrast, non-TBI-based regimens were
preferred in most recent reports from EBMT registry and Japan Society
of Hematology and for Hematopoietic Cell Transplantation,[18] as well as other single-centre studies.[16] Busulfan and Cyclophosphamide was the non-TBI-based regimen more frequently used.
Pediatric Experiences
The
majority of reports on the use of HSCT for treatment of
relapsed/refractory APL deal primarily with adults, making the benefit
of these therapies for children unclear. Most of the published data on
HSCT as a treatment for relapsed childhood APL comes from small
retrospective studies. Among 31 children in the European APL93 trial
(ATRA+chemotherapy), seven achieved second CR; three patients received
auto-HSCT, and three underwent allo-HSCT.[22] One
allografted patient died from graft-versus-host-disease (GVHD), while
six patients remained in molecular second CR after 17 to 66 months (Table 1).
Termuhlen et al. (2008) reported the outcome of 5 relapsed APL children
who achieved a second molecular CR after reinduction therapy with ATO
(4 patients) or FLAG (1 patient) and, after that, underwent auto-HSCT.[23] All patients remained in second molecular CR after 20 months (Table 1).
Bourquin et al. (2004) reported 12 allo-HSCT performed in 11
relapsed/refractory pediatric (median age 13 years) APL patients
treated between 1986 and 2003.[24] Most of these
children were initially treated in the pre-ATRA era. All transplants
were performed with a myeloablative conditioning regimen; in 7/12, a
radiation-based regimen was employed. In five cases, the marrow graft
was obtained from an HLA-matched relative; in the other seven children,
the graft was obtained from an unrelated donor. All patients received
T-cell-depleted graft. The median time for neutrophil and platelet
recovery was 18.5 and 32 days. One patient relapsed 14 months after an
HLA-matched related donor transplant; he received a second unrelated
donor HSCT and became a long-term leukemia-free survivor. Five-year OS
was 73% (median follow-up 64 months); all deaths (3 patients) were from
TRM. Four of the five patients not in hematological CR at the time of
transplant are disease-free survivors (Table 1). The potent graft-versus-leukemia effect related to allo-HSCT was evident in this set of patients.
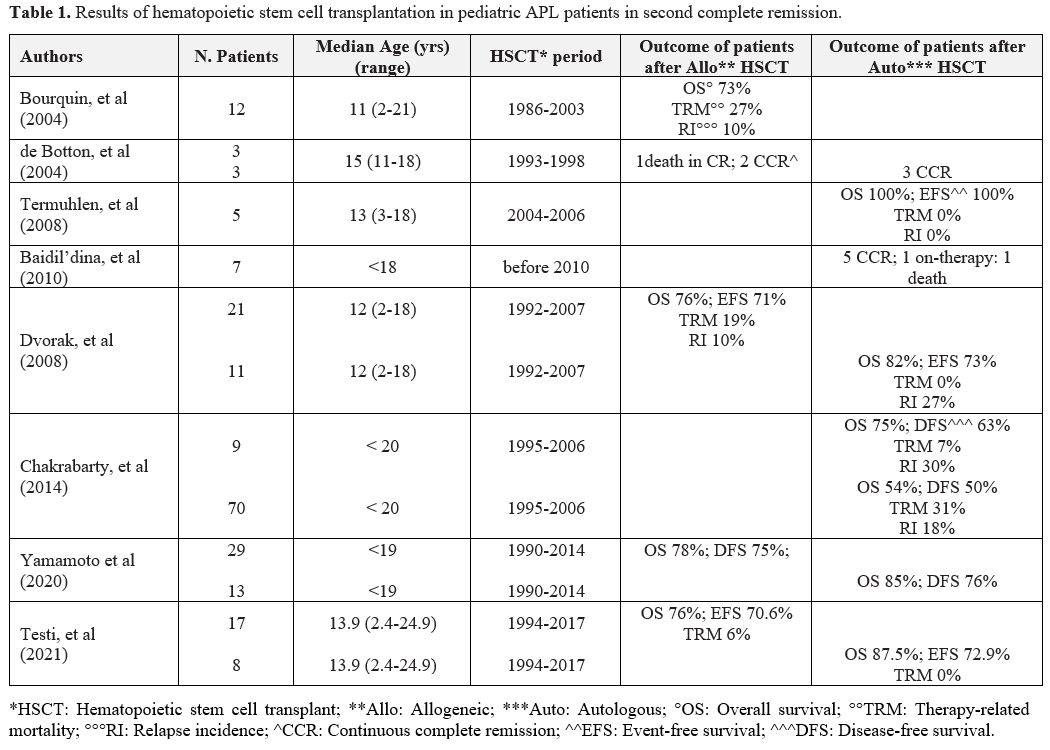 |
Table
1. Results of hematopoietic stem cell transplantation in pediatric APL patients in second complete remission. |
Another pediatric study included nine patients with recurrent APL.[25]
Salvage therapy included ATO and/or standard chemotherapy + ATRA; ATO
monotherapy was used in consolidation. CD34+ cells were mobilized at
molecular CR achievement, with high-dose cytarabine and
granulocyte-colony-stimulating factor. One patient died before therapy;
eight children achieved second molecular CR. CD34+ cell mobilization
and collection were effective in seven cases. Pre auto-HSCT
conditioning included melphalan in combination with high-dose
cytarabine (5 patients), treosulfan (1 patient) or busulfan (1
patient). Five patients became long-term survivors in molecular CR
(follow-up 30-40 months). One patient was still on treatment, and
another developed a disease recurrence and died from complications. The
authors concluded that the application of ATO and auto-HSCT in relapsed
pediatric APL is effective for achieving prolonged second molecular CR (Table 1).
Dvorak
et al. (2008) reported 32 pediatric cases with relapsed/refractory APL,
undergoing either auto- or allo-HSCT. According to the Eastern
Cooperative Group (ECOG) E2491 Trial and the Cancer and Leukemia Group
B (CALGB) C9710 trial, these children had originally received treatment
from 1992 to 2005. First-line therapy had included either ATRA alone or
a combination of cytarabine and anthracyclines (ECOG E2491) or ATRA
associated with chemotherapy randomly followed by ATRA +anthracycline
or ATO for two courses (CALGB C9710). Three children failed to achieve
CR with the initial induction protocol and underwent HSCT to treat
primary/resistant disease. The other 29 patients underwent HSCT after
relapse, which occurred at a median of 10 months from the first CR. All
these patients were in morphologic CR before transplant after various
salvage regimes; 11 patients underwent auto-HSCT and 21 allo-HSCT.
RT-PCR of the autograft product was negative in 6 and unknown in 5
patients. In most patients, conditioning regimens consisted of
cyclophosphamide combined with either total body irradiation (TBI, 13
patients) or busulfan. Other regimens added a third agent (cytarabine,
etoposide or thiotepa) to this backbone. Five patients relapsed
following HSCT (3 after auto-HSCT and 2 after allo-HSCT); the median
time to relapse was 15.3 and 13.5 months after auto and allo-HSCT,
respectively. The 5-year EFS and OS were 82% and 76% after auto-HSCT
and 73% and 71% after allo-HSCT, respectively (Figure 1). The incidence of TRM and CIR after auto- and allo-HSCT was 0% and 19% and 27% and 10%, respectively (Table 1).[26]
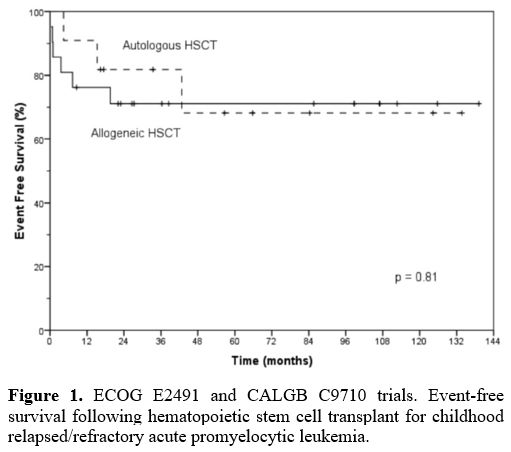 |
Figure 1. ECOG E2491
and CALGB C9710 trials. Event-free survival following hematopoietic
stem cell transplant for childhood relapsed/refractory acute
promyelocytic leukemia. |
In
the attempt to identify the favored choice of transplantation in
patients with APL in second CR, data related to 294 patients (79 of
them with age < 20 years) receiving allo-HSCT (232 patients) and
auto-HSCT (62 patients) were retrospectively analyzed to compare
toxicity, survival outcome and impact of residual molecular disease
pre-HSCT on the outcome. The use of ATO therapy prior to HSCT was also
evaluated; no impact of ATO-containing vs non-ATO pre-HSCT therapy on
the relapse risk after transplant was observed. In this large number of
patients, specific data related to the younger patients are not
available. However, the univariate analysis showed a good OS, DFS and
TRM in auto transplanted patients; 5-year OS was 75% and 54% for auto-
versus allo-recipients (Figure 2, 3). Disease-free survival (DFS) at five years also favored auto-HSCT (63% vs 50%) (Table 1).
In both auto- and allo-HSCT, molecular or cytogenetically positive
grafts were not associated with an increased risk of relapse and
overall mortality.[17]
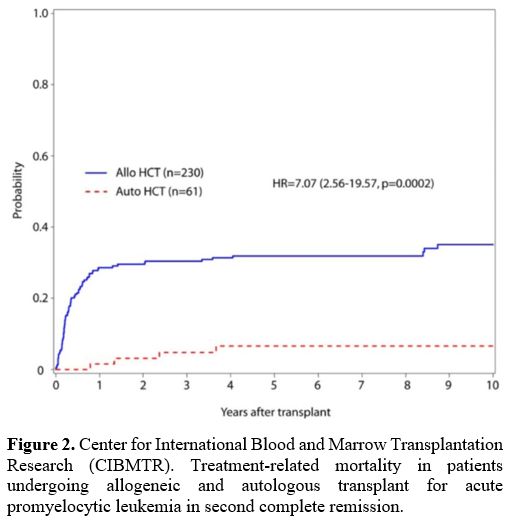 |
Figure 2. Center for
International Blood and Marrow Transplantation Research (CIBMTR).
Treatment-related mortality in patients undergoing allogeneic and
autologous transplant for acute promyelocytic leukemia in second
complete remission. |
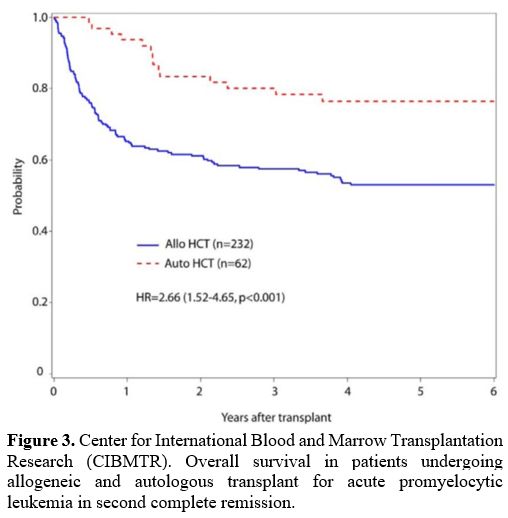 |
Figure 3. Center for
International Blood and Marrow Transplantation Research (CIBMTR).
Overall survival in patients undergoing allogeneic and autologous
transplant for acute promyelocytic leukemia in second complete
remission.
|
A
large number of pediatric APL patients (95 children; aged 0-19)
transplanted between 1990 and 2014 were identified in the Japan Society
of Hematopoietic Cell Transplantation (JSHCT) registry.[27]
Forty of them underwent auto- or allo-HSCT in first CR and 55 in other
settings (second CR: 41; third CR: 3 and no-CR: 11). HSCT was included
in the front-line treatment before ATRA-era; it was subsequently
adopted as a treatment for patients in second CR. Among the 40 patients
transplanted in first CR, 19 underwent auto- and 21 allo-HSCT. Although
there was no significant difference in 5-year OS (73% vs 86%) and DFS
(68% vs 86%) between auto- and allo-HSCT groups, the 5-year cumulative
incidence of relapse was significantly higher after auto-HSCT (32%)
than after allo-HSCT (5%). Twenty-nine of the 42 patients treated in
second CR underwent allo-HSCT and 13 auto-HSCT. There was no
significant difference in 5-year OS and DFS between auto- and allo-HSCT
groups (OS: 85% vs 78%; DFS 76% vs 75%). Both auto-and allo-HSCT were
effective in pediatric APL patients in second CR. In this study, based
on registry data, no information was available about the pre-transplant
treatments or the PML-RARA molecular status at the time of
transplantation. Even with intensive chemotherapy, achieving a CR was
the most important factor in curing relapsed APL patients who had
already received ATRA and ATO.
More recently, the 25-year
Italian experience on the outcome of relapsed/refractory APL in
children, adolescents and young adults has been reported.[28]
Fifty-one patients (age < 18 years at initial diagnosis; median age
13.9 years at the time of the study), treated between May 1994 and May
2017 in 22 AIEOP (Associazione Italiana Ematologia e Oncologia
Pediatrica) centers who experienced relapsed or refractory disease,
were included in this study. All patients had received front-line ATRA
and chemotherapy; salvage strategies were heterogeneous and, up to
January 2008, were based on ATRA and intensive chemotherapy followed by
allo- or auto-HSCT or different maintenance approaches for patients not
eligible for transplant. After January 2008, when ATO was available for
all patients, reinduction consisted of ATO + ATRA + gentuzumab
ozogamycin (GO); patients with a persistent positive molecular disease
underwent auto- or allo-HSCT. A total of 25 patients in second CR
received HSCT as consolidation therapy, and more exactly: 18 patients,
salvaged with ATRA + chemotherapy, were consolidated with either auto-
(7 patients) or allo-HSCT (11 patients); 7 out of 18 patients who had
received ATO+ATRA were consolidated with auto- (1 patient) and
allo-HSCT (6 patients) (Table 2).
The molecular status at transplant time was available in 18/25
patients, and all were negative. The univariate analysis for OS and EFS
showed no significant difference between transplanted and
non-transplanted patients (OS: HR 2.43; EFS: HR 1.10) and between those
consolidated with auto- or allo-HSCT (OS 87.5% vs 76% and EFS 72.9% vs
70.6%) (Table 1) (Figure 4, 5).
These data compared favorably with those reported in relapsed APL
adults from the European Acute Promyelocytic Leukemia Group, comparing
auto- and allo-HSCT. This study demonstrated the efficacy of auto-HSCT
performed in molecular CR (Figure 6).[29]
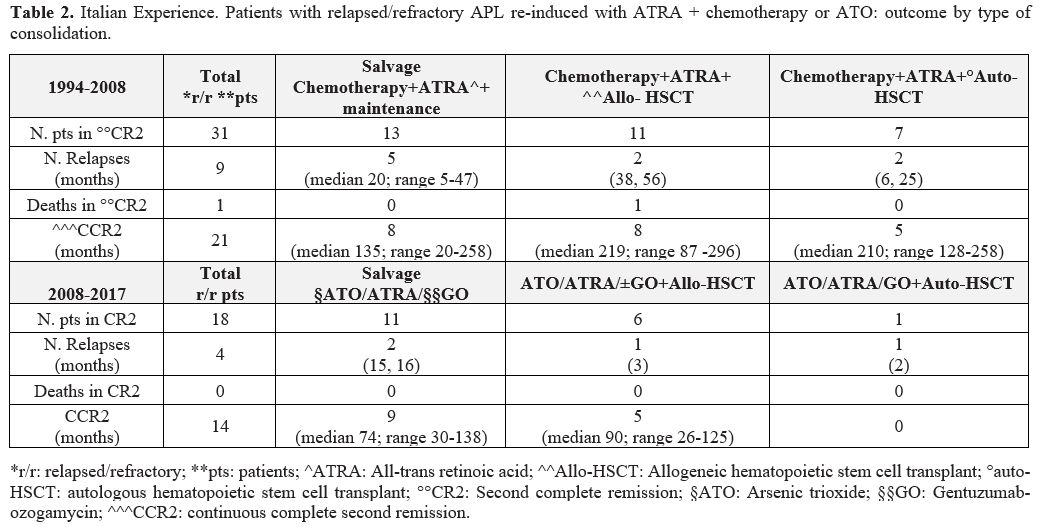 |
Table 2. Italian
Experience. Patients with relapsed/refractory APL re-induced with ATRA
+ chemotherapy or ATO: outcome by type of consolidation. |
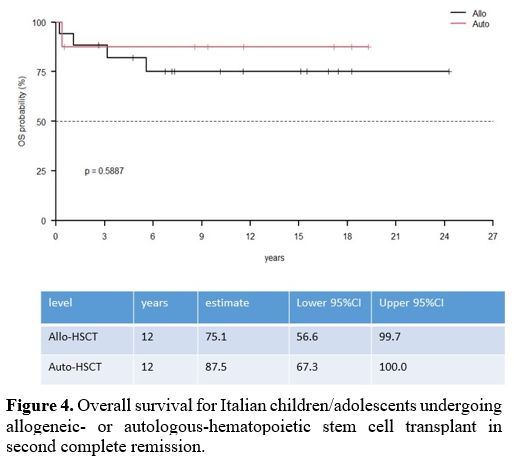 |
Figure 4. Overall survival
for Italian children/adolescents undergoing allogeneic- or
autologous-hematopoietic stem cell transplant in second complete
remission. |
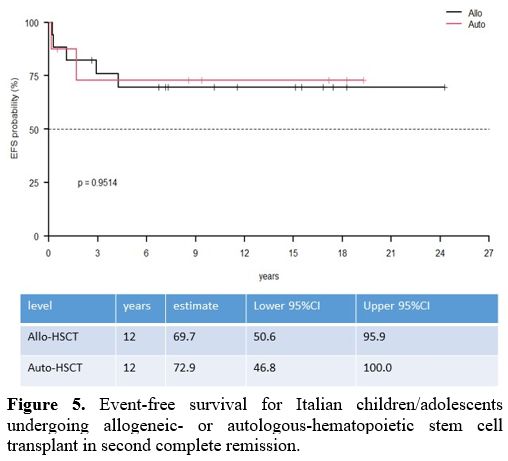 |
Figure 5. Event-free
survival for Italian children/adolescents undergoing allogeneic- or
autologous-hematopoietic stem cell transplant in second complete
remission. |
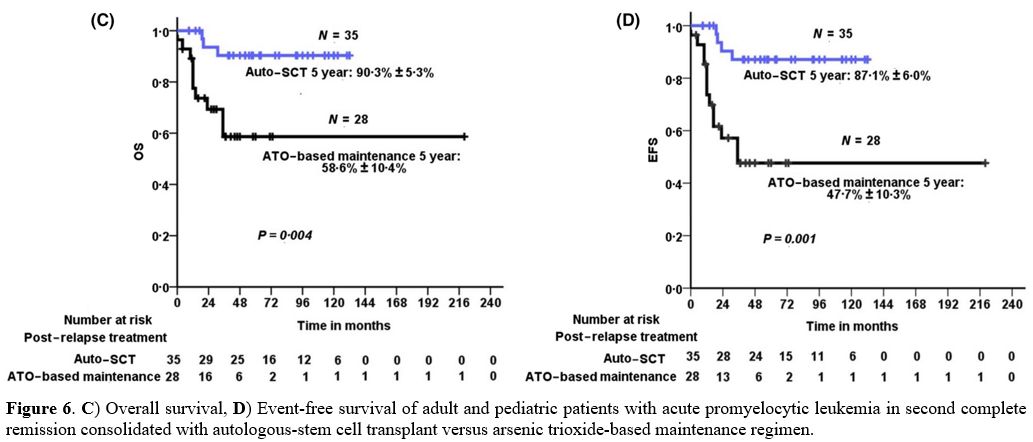 |
Figure 6. C) Overall
survival, D) Event-free survival of adult and pediatric patients with
acute promyelocytic leukemia in second complete remission consolidated
with autologous-stem cell transplant versus arsenic trioxide-based
maintenance regimen.
|
In
the pediatric age, based on literature data on relapsed APL,
recommendations on APL salvage therapy were established by members with
APL expertise from the North American Children's Oncology Group (COG)
and the International Berlin-Frankfurt–Münster Study Group (I-BFM SG).[13]
The quality of evidence for these recommendations was mainly derived
from expert opinion, while a final agreement was by consensus. Several
studies, mostly involving adults, had identified prognostic factors in
patients with relapsed APL who underwent salvage therapy with or
without HSCT.[13,17,30,31]
Time to relapse (< 18 months from diagnosis), prior ATO therapy, and
failure to clean PML-RARA transcript had an unfavourable impact on
patients' outcomes. These factors can be useful to predict the risk of
further relapse and consequently to guide which children can be treated
with further differentiating agents and/or chemotherapy and who would
benefit from either auto- or allo-HSCT. Therefore, treatment algorithms
have been created, dividing patients according to time to relapse,
previous ATO exposure and relapse site (Figure 7).
According to these recommendations, for children with relapse occurring
less than 18 months from initial diagnosis and with previous or no ATO
exposure, auto-HSCT is planned only for those who achieve a second
molecular CR after induction and consolidation strategy. Stem cell
mobilization and collection are obtained in these patients after a
further high-dose cytarabine consolidation course. For the patients
still PML-RARA positive at the end of consolidation, allo-HSCT is
recommended after a further cycle of intensive therapy. ATO-naïve
children with relapse occurring 18-36 months from initial diagnosis can
be reinduced with ATO-ATRA and GO and proceed to allo-HSCT only if
persistently PML-RARA positive at the end of consolidation. Children
with prior ATO exposure and late relapse who clear PML-RARA transcript
after four consolidation courses can be considered for auto-HSCT; for
those still positive, allo-HSCT is planned after other intensive
consolidation chemotherapy.
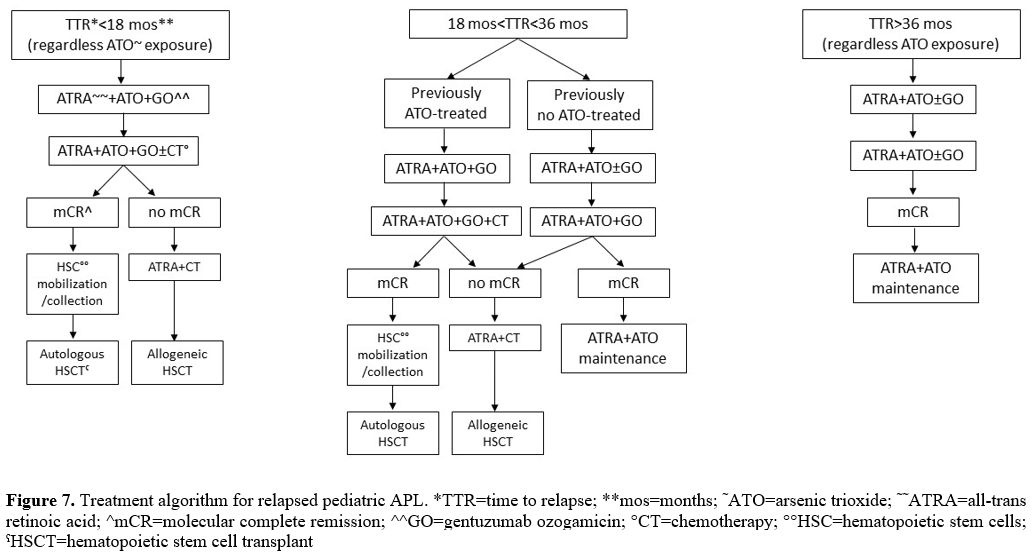 |
Figure 7. Treatment
algorithm for relapsed pediatric APL. *TTR=time to relapse;
**mos=months; ˜ATO=arsenic trioxide; ˜˜ATRA=all-trans retinoic acid;
^mCR=molecular complete remission; ^^GO=gentuzumab ozogamicin;
°CT=chemotherapy; °°HSC=hematopoietic stem cells; ˤHSCT=hematopoietic
stem cell transplant.
|
Regarding
the conditioning regimen, no advantage has been shown for TBI in acute
myeloid leukemia (AML), and in pediatric age, chemotherapy-only
regimens should be used. There is no proven best chemotherapy
conditioning regimen, though myeloablative regimens with busulfan and
cyclophosphamide and therapeutic drug monitoring of busulfan levels are
currently the standard of care. In pediatric AML, busulfan,
cyclophosphamide and melphalan have been successfully employed; the
choice is usually balanced between efficacy and toxicity with a careful
evaluation of previous drugs that may contribute to the toxicity.
All
these recommendations were made to assist in designing therapy for
children with APL relapse; since APL patients are a heterogeneous
population, these schemas may require modifications based on individual
patients' characteristics and the resources available to the treating
physicians.
Very Late Relapse
Only
sporadic reports of adult and pediatric patients with very late relapse
have been published. Very late relapse is defined as any relapse at
hematological or molecular level,
occurring > 36 months from diagnosis. The bone marrow and/or
extra-hematological sites may be involved.
Although no standard of
care for treating these relapses exists, drug resistance is unlikely in
these patients, and, irrespective of front-line ATO therapy, salvage
with ATO-based regimens remains effective; GO has also been
demonstrated to be effective.[31-35] While the role
of ATO in remission induction in these patients is well established,
the benefit of consolidation HSCT is questionable for patients
relapsing after a very prolonged first CR. The registry study of the
European LeukemiaNet collecting and analyzing the results of 155
relapsed APL patients has clearly demonstrated the role of allo-HSCT as
consolidation therapy for patients with either early or late relapse
not achieving a molecular CR and suggested auto-HSCT as a suitable
option for patients in second molecular CR.[31]
However, the transplant options could be questioned for patients
relapsing after a very prolonged first CR in whom continuing ATRA-ATO
might be curative. Though limited to a small number of patients, a
prolonged second molecular CR with ATO-based salvage therapy has been
described in the literature.[31-35] ATO+ATRA
combination in repeated consolidation courses, associated or not with
GO, and PML-RARA quantitative monitoring, will probably contribute to
avoiding HSCT in patients with very late relapse.
Extramedullary Relapse
Extramedullary
relapse in APL can involve many sites but more frequently affects the
central nervous system (CNS), skin or external auditory channel.[35,36]
The best management of children with CNS relapse of APL remains to be
determined, and the role of HSCT in isolated CNS relapse is still
controversial, although it was recommended by the European LeukemiaNet
in 2009.[14] An extensive pediatric literature reports a very low incidence of isolated CNS relapse (1.39%).[37]
Most CNS relapses are accompanied by evidence of molecular bone marrow
disease and are significantly associated with high WBC counts and/or
intracranial hemorrhage at diagnosis. There is a higher incidence of
bcr3 PML-RARA isoform, expression of antigen CD56 on the leukemic cell
surface, and microgranular M3 variant, presence of FLT3-ITD mutation
and young age are more frequently associated with extramedullary APL
relapse.[37,38] ATO
is currently the first-line therapy for refractory/relapsed APL and may
also benefit patients with CNS relapse. ATO accumulates in epidermal
tissues and penetrates the blood-brain barrier, reaching cerebral
spinal fluid (CSF) levels up to 50% of serum levels; thereafter,
therapeutic response at these sites is expected.[39]
For CNS relapse, intrathecal chemotherapy in combination with/without
ATO-ATRA has been described as a successful treatment of CNS relapse.
Drugs with high CNS penetrance, such as high-dose cytarabine, have been
used in CNS relapse, followed by auto-HSCT or cranial radiotherapy.[40]
The need for radiotherapy is also questionable, although some centers
still follow this approach. Allo-HSCT is also recommended for patients
with an available HLA-identical donor by some experts, although
auto-HSCT is preferred by others, owing to its lower TRM. For those
patients with concomitant bone marrow molecular disease, achieving a
molecular CR should be important for a successful outcome of auto-HSCT.ATO alone or in combination with ATRA to treat non-CNS isolated extramedullary relapse is a reasonable strategy.[41] HSCT is not recommended in these cases.
Comments and Conclusions
- The
widespread clinical employment of ATO and ATRA combination in pediatric
and adult APL has made relapse a rare event, especially in children
- HSCT
has ceased to play any role in the first-line treatment, and it is
relegated to consolidating patients in second complete remission or
beyond after salvage treatment
- ATO
is currently the first-line therapy for refractory/relapsed APL, but
the optimal post-remission therapy in pediatric patients with second
complete remission after either ATO or ATRA+chemotherapy remains
undefined
- No
significant differences in EFS and OS have been observed between auto-
and allo-HSCT in the two largest pediatric series, including relapsed
pediatric APL
- The molecular disease status at the time of transplant has emerged as an important prognostic variable
- In
the attempt to assist in designing therapy for children with
relapsed/refractory APL, recommendations from an international expert
panel have been published
- ATO-naive
children with late relapse (> 18 months from diagnosis) and those
with very late relapse (> 36 months from diagnosis), regardless of
ATO exposure, can be reinduced with ATO/ATRA associated or not with GO
followed by ATO consolidation without HSCT
- ATO-naive
children with early relapse (< 18 months from diagnosis) and those
with prior ATO exposure and early or late relapse (18-36 months from
diagnosis) who clear PML-RARA after four salvage courses could be
candidates to auto-HSCT
- Children
with early relapse or primary refractory APL or ≥ second relapse or
with the persistence of PML-RARA positivity after salvage treatment,
ATO treated or not, should be considered for allo-HSCT consolidation
Acknowledgments
In
every paper in which acute promyelocytic leukemia is mentioned, we
always have to remember Prof. F. Lo Coco and Prof. E.H. Estey, to whom
all our gratitude goes for having strongly contributed to the successes
in this pathology.
References
- Testi AM, Biondi A, Lo Coco F, et al. GIMEMA-AIEOP
AIDA protocol for the treatment of newly diagnosed acute promyelocytic
leukemia (APL) in children. Blood. 2005; 106: 447-453. https://doi:
10.1182/blood-2004-05-1971. https://doi.org/10.1182/blood-2004-05-1971 PMid:15677559
- Testi
AM, Pession A, Diverio D, et al. Risk-adapted treatment of acute
promyelocytic leukemia: results from the International Consortium for
Childhood APL. Blood. 2018; 132(4): 405-412. https://doi.org/10.1182/blood-2018-03-836528 PMid:29789356
- Ortega
JJ, Madero L, Martin G, et al. Treatment with all-trans retinoic acid
and anthracycline monochemotherapy for children with acute
promyelocytic leukemia: a multicenter study by the PETHEMA group. J
Clin Oncol. 2005; 23(30): 7632-7640. https://doi.org/10.1200/JCO.2005.01.3359 PMid:16234524
- Kutny
MA, Geyer S, Laumann KM, et al. Outcome of pediatric acute
promyelocytic leukemia patients at Children's Oncology Group sites on
the Leukemia Intergroup Study CALGB 9710 (alliance). Pediatr Blood
Cancer. 2019; 66(3): e27542.
https://doi.org/10.1002/pbc.27542 PMid:30393935 PMCid:PMC6392047
- Creutzig
U, Zimmemann M, Dvorzak M, et al. Favourable outcome of patients with
childhood acute promyelocytic leukemia after treatment with reduced
cumulative anthracycline doses. Br J Haematol. 2010; 143(3): 399-409. https://doi.org/10.1111/j.1365-2141.2010.08107.x PMid:20230404
- George
B, Mathews V, Poonkuzhali B, Shaji RV, Srivastava A, Chandy M.
Treatment of children with newly diagnosed acute promyelocytic leukemia
with arsenic trioxide: a single center experience. Leukemia. 2004;
18(10): 1587-1590. https://doi.org/10.1038/sj.leu.2403480 PMid:15356649
- Zhang
L, Zhu X, Zou Y, Chen Y, Chen X. Effect of arsenic trioxide on the
treatment of children with newly diagnosed acute promyelocytic leukemia
in China. In J Hematol. 2011; 93(2): 199-205. https://doi.org/10.1007/s12185-011-0768-0 PMid:21287409
- Creutzig
U, Dworzak MN, Bochennek K, et al. First experience of the
AML-berlin-Frankfurt-Munster group in pediatric patients with
standard-risk acute promyelocytic leukemia treated with arsenic
trioxide and all-trans retinoic acid. Pediatr Blood Cancer. 2017;
64(8). https://doi.org/10.1002/pbc.26461 PMid:28111878
- Strocchio
L, Gurnari C, Santoro N, et al. Arsenic trioxide and all-trans retinoic
acid treatment for childhood acute promyelocytic leukemia. Br J
Haematol. 2019; 185(2): 360-363. https://doi.org/10.1111/bjh.15507 PMid:30028005
- Kutny
MA, Alonzo TA, Gebing RB, et al. Arsenic trioxide consolidation allows
anthracycline dose reduction for pediatric patients with acute
promyelocytic leukemia: report from the Children's Oncology Group Phase
III historically controlled trial AAML0631. J Clin Oncol. 2017; 35:
3021-3029. https://doi.org/10.1200/JCO.2016.71.6183 PMid:28767288 PMCid:PMC5590801
- Diverio
D, Rossi V, Avvisati G, et al. Early detection of relapse by
prospective reverse transcriptase-polymerase chain reaction analysis of
PML/RARalpha fusion gene in patients with acute promyelocytic leukemia
enrolled in the GIMEMA-AIEOP multicenter "AIDA" trial. GIMEMA-AIEOP
Multicent. Blood. 1998; 92: 784-789. https://doi.org/10.1182/blood.V92.3.784 PMid:9680345
- Grimwade
D, Jovanovic JV, Hills RK, et al. Prospective minimal residual disease
monitoring to predict relapse of acute promyelocytic leukemia and to
direct pre-emptive arsenic trioxide therapy. J Clin Oncol. 2009; 27:
3650-3658. https://doi.org/10.1200/JCO.2008.20.1533 PMid:19506161
- Abla
O, Kutney MA, Testi AM, et al. Management of relapsed and refractory
childhood acute promyelocytic leukemia: recommendations from an
international expert panel. Br J Haematol. 2016; 175: 588-601. https://doi.org/10.1111/bjh.14313 PMid:27651168
- Sanz
MA, Grimwade D, Tallman MS, et al. Management of acute promyelocytic
leukemia: recommendation fron an expert panel on behalf of the European
Leukemia.Net. Blood. 2009; 113(9): 1875-91. https://doi.org/10.1182/blood-2008-04-150250 PMid:18812465
- Sanz
J, Montesinos P, Sanz AM. Role of hematopoietic stem cell transplant in
acute promyelocytic leukemia. Front Oncol. 2021; 11: 614215.
eCollection 2021. https://doi.org/10.3389/fonc.2021.614215 PMid:33816245 PMCid:PMC8012800
- Sanz
J, Labopin M, Sanz MA, et al. Hematopoietic stem cell transplantation
for adults with relapsed acute promyelocytic leukemia in second
complete remission. Bone Marrow Transplant. 2021; 56(6): 1272-1280. https://doi.org/10.1038/s41409-020-01162-0 PMid:33323947
- Chakrabarty
JLH, Rubinger M, Le Rademacher J, et al. Autologous is superior to
allogeneic hematopoietic cell transplantation for acute promyelocytic
leukemia in second complete remission. Biol Blood Marrow Transplant.
2014; 20(7): 1021-1025. https://doi.org/10.1016/j.bbmt.2014.03.025 PMid:24691221 PMCid:PMC4097890
- Yanada
M, Takami A, Mizuno S, et al. Autologous hematopoietic cell
transplantation for acute myeloid leukemia in adults: 25 years of
experience in Japan. Int J Hematol. 2020; 11(1): 93-102. https://doi.org/10.1007/s12185-019-02759-y PMid:31612307
- Ueki
T, Ohashi K, Jinta M, et al. Delayed hematological recovery following
autologous transplantation utilizing peripheral blood stem cells
harvested after treatment with arsenic trioxide. Pathol Oncol Res.
2008; 14(4): 387-390. https://doi.org/10.1007/s12253-008-9049-5 PMid:18553162
- Mannis
GN, Logan AC, Leavitt AD, et al. delayed hematopoietic recovery after
auto-HSCT in patients receiving arsenic-trioxide-based therapy for
acute promyelocytic leukemia: a multi-center analysis. Bone Marrow
Transplant. 2015; 50(1): 40-44. https://doi.org/10.1038/bmt.2014.201 PMid:25243620
- Holter
Chakrabarty JL, Rubinger M, Le-Rademacher J, et al. Autologous is
superior to allogeneic hematopoietic stem cell transplantation for
acute promyelocytic leukemia in second complete remission. Biol Blood
Marrow Transpl. 2014: 20(7): 1021-1025. https://doi.org/10.1016/j.bbmt.2014.03.025 PMid:24691221 PMCid:PMC4097890
- de
Botton S, Coiteux V, Chevret S, et al. Outcome of childhood acute
promyelocytic leukemia with all-trans retinoic acid and chemotherapy. J
Clin Oncol. 2004; 22: 1404-1412. https://doi.org/10.1200/JCO.2004.09.008 PMid:15084614
- Termuhlen
AM, Klopfenstein K, Olshefski R, et al. Mobilization of PML-RARA
negative blood stem cells and salvage with autologous peripheral blood
stem cell transplantation in children with relapsed acute promyelocytic
leukemia. Pediatric Blood Cancer. 2008; 51: 521-524. doi:
10.1002/pbc.21614. https://doi.org/10.1002/pbc.21614 PMid:18493994
- Bourquin
JP, Thornley I, Neuberg D, et al. Favorable outcome of allogeneic
hematopoietic stem cell transplantation for relapsed or refractory
acute promyelocytic leukemia in childhood. Bone Marrow Transplant.
2004; 34: 795-788. https://doi.org/10.1038/sj.bmt.1704676 PMid:15354207
- Baidil'dina
DD, Maschan MA, Skorobogatova EV, et al. Recurrences of acute
promyelocytic leukemia in children: experience with arsenic trioxide
therapy and autologous hematopoietic cell transplantation. Ter Arkh.
2010; 82(7): 20-25.
- Dvorak CC, Agarwal R, Dahl GV, Gregory JJ,
Feusner JH. Hematopoietic stem cell transplant for pediatric acute
promyelocytic leukemia. Biol Blood Marrow Transplant. 2008; 14:
824-830. https://doi.org/10.1016/j.bbmt.2008.04.015 PMid:18541203 PMCid:PMC2796449
- Yamamoto
S, Tomizawa D, Kudo K, et al. Hematopoietic stem cell transplantation
for pediatric acute promyelocytic leukemia in Japan. Pediatr Blood
Cancer. 2020; 67(5): e28181. https://doi.org/10.1002/pbc.28181 PMid:31965692
- Testi
AM, Mohamed S, Diverio D, et al. Outcome of relapsed/refractory acute
promyelocytic leukaemia in children, adolescents and young adult
patients - a 25-year Italian Experience. Br J Haematol. 2021; 195(2):
278-283. https://doi.org/10.1111/bjh.17637 PMid:34145572
- Fouzia
NA, Sharma V, Ganesan S, et al. Management of relapse in acute
promyelocytic leukemia treated with upfront arsenic trioxide based
regimens. Br J Haematol. 2021: 192(2): 292-299. https://doi.org/10.1111/bjh.17221 PMid:33216980 PMCid:PMC7894296
- Marjerrison
S, Antillon F, Bonilla M, et al. Outcome of children treated for
relapsed acute myeloid leukemia in Central America. Pediatr Blood
Cancer. 2014; 61(7): 1222-1226. https://doi.org/10.1002/pbc.24942 PMid:24443303
- Lengfelder
E, lo Coco F, Ades I, et al. Arsenic trioxide based therapy at relapsed
acute promyelocytic leukemia: registry results from the European
LeukemiaNet. Leukemia. 2015; 29(5): 1084-1091. https://doi.org/10.1038/leu.2015.12 PMid:25627637
- Lou
Y, Suo S, Tong V, et al. Outcomes and prognostic factors of first
relapse acute promyelocytic leukemia patients undergoing salvage
therapy with intravenous arsenic trioxide and chemotherapy. Annals of
Hematology. 2014; 93(6): 941-948. https://doi.org/10.1007/s00277-013-2000-1 PMid:24408159
- Breccia
M, Cicconi L, Minotti C, et al. Efficacy of prolonged therapy with
combined arsenic trioxide and ATRA for relapse of acute promyelocytic
leukemia. Haematologica. 2011; 96(9); 1390-1391. https://doi.org/10.3324/haematol.2011.045500 PMid:21659361 PMCid:PMC3166113
- Douer
D, Zickl LN, Schiffer CA, et al. All-trans retinoic acid and late
relapsesin acute promyelocyic leukemia: very long-term follow-up of the
North American Intergroup Study Io129. Leuk Res. 2013; 37(7): 795-801. https://doi.org/10.1016/j.leukres.2013.03.001 PMid:23528262 PMCid:PMC4174301
- Testi
AM, Moleti Ml, Canichella M, et al. Very late relapse in a patient with
acute promyelocytic leukemia (APL) rescued with a chemotherapy-free
protocol. Leuk Lymphoma. 2017; 58(4): 999-1001 https://doi.org/10.1080/10428194.2016.1222377 PMid:27658340
- Latagliata
R, Carmosino I, Breccia M, et al. Late relapses in acute promyelocytic
leukemia. Acta Haematol. 2007; 117(2): 106-108. https://doi.org/10.1159/000097385 PMid:17135723
- Chow
I, Feusner J, Isolated central nervous system recurrence of acute
promyelocytic leukemia in children. Pediatr Blood Cancer. 2009; 52(1):
11-13. https://doi.org/10.1002/pbc.21608 PMid:18816805
- de
Botton S, Sanz MA, Chevret S, et al. Extramedullary relapse in acute
promyelocytic leukemia treated with all-trans retinoic acid and
chemotherapy. Leukemia. 2006; 20(1): 35-41. https://doi.org/10.1038/sj.leu.2404006 PMid:16307026
- Helwig
A, Klemm M, Schuttig R, et al. Arsenic-induced APL differentiation in
cerebrospinal fluid. Leukemia Res. 2007; 31(5): 703-705. doi:
10.1016/j.leukres.2006.06.011. https://doi.org/10.1016/j.leukres.2006.06.011 PMid:16876245
- Scheinemann
K, Weitzman S, Hitzler J, et al. Isolated central nervous system
relapse in childhood acute promyelocytic leukemia. J Pediatr Hematol
Oncol. 2008; 30(2): 160-162. https://doi.org/10.1097/MPH.0b013e318159a582 PMid:18376270
- Au
WY, Tam S, Kwong YL. Entry of elemental arsenic into the central
nervous system in patients with acute promyelocytic leukemia during
arsenic trioxide treatment. Leuk Res. 2008; 32(2): 357-358. https://doi.org/10.1016/j.leukres.2007.06.005 PMid:17662385
[TOP]








