Elisabetta Schiaroli1, Giuseppe Vittorio De Socio1, Anna Gidari2, Lisa Malincarne1,Sara Benedetti1, Alessandra Lanzi1, Sabrina Bastianelli1, Sara Pierucci1, Chiara Busti1, Benedetta Fagotti3, Ilaria Vicenti4, Maurizio Zazzi4 and Daniela Francisci1.
1 Unit of Infectious Diseases, Department of Medicine and Surgery, University of Perugia, Perugia, Italy.
2
Unit of Infectious Diseases, Department of Medicine and Surgery,
University of Perugia, Santa Maria Hospital of Terni, Terni, Italy.
3 Pharmaceutical Department of Foligno Hospital, Italy.
4 Department of Medical Biotechnologies, University of Siena, Siena, Italy.
Correspondence to:
Elisabetta Schiaroli, MD. Unit of Infectious Diseases, Department of
Medicine, University of Perugia, Perugia, Italy, Hospital "Santa Maria
della Misericordia", Piazzale Menghini, 1 – 06156, Perugia, Italy. Tel:
+39-075-5784375, Fax: +39-075-5784346. E-mail:
elisabetta.schiaroli@unipg.it
Published: July 1, 2022
Received: February 9, 2022
Accepted: June 14, 2022
Mediterr J Hematol Infect Dis 2022, 14(1): e2022050 DOI
10.4084/MJHID.2022.050
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background and Objective:
In patients with mild-to-moderate COVID-19 and at high risk of
progression, casirivimab/imdevimab and bamlanivimab/etesivimab were
utilized in Umbria from late April to November 2021. This period was
characterized by an initial prevalence of alpha (B1.1.1.7) and its
progressive substitution with the delta variant (B1.617.2). Many delta
infections occurred in patients already recently vaccinated.
Our
study aimed to observe the clinical outcome of patients treated with
mAbs associations in a subgroup in which viral isolation was obtained,
the pre and post-infusion neutralizing antibody activity against their
viral isolate.
Methods: In
this retrospective observational study, the clinical outcome before and
30 days after infusion, the baseline neutralizing activity of sera
against their viral isolate, and the titers of neutralizing antibodies
(NAbTs) one-hour post-infusion relative to the type of mAbs
associations were evaluated.
Results:
Better efficacy of the mAbs combinations relative to monotherapy
regarding global hospitalization (p = 0.021) and 30 days symptoms
(p<0.001) were seen. Infections after vaccination mostly occurred in
the absence of neutralizing antibody titers (NAbT). SARS-CoV-2 delta
variants were isolated within 2-4 months from vaccinations without
NAbTs, or in the presence of high specific neutralizing activity after
5-6 months. NAbTs were higher after casirivimab/imdevimab infusion
(p=0.001).
Conclusions:
Alpha infections occurred prevalently in unvaccinated patients or after
5-6 months, while delta infections prevailed in vaccinated ones. A poor
neutralizing activity in most of these patients was seen. A higher NAbT
after infusion of casirivimab/imdevimab was observed.
|
Introduction
The development of therapeutic monoclonal antibodies (mAbs) is currently at the forefront of fighting COVID-19 infection.
Most
mAbs with activity against SARS-CoV-2 bind to the S1 subunit with their
FAb domain and show their neutralizing activity, inhibiting virus
engagement to the cell surface receptor ACE2.[1]
In
November 2020 and in February 2021 US Food and Drug Administration
(FDA) authorized the monoclonal antibody bamlanivimab as monotherapy[2] and casirivimab/imdevimab[3]
and bamlanivimab/etesevimab[4] for emergency use in outpatients
affecting a mild to moderate coronavirus disease (COVID 19) and at high
risk of progression towards severe COVID-19 and/or hospitalization.
The emergency authorization was based on clinical trials in 2021,[5,6]
which had been performed using neutralizing mAbs developed from
convalescent COVID-19 patients infected by viral variants of SARS-CoV-2
before the emergence of the new SARS-CoV-2 Variants of Concern (VoC)
alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1) and Delta (B.1.617.2)
which have been associated with ever greater transmissibility, altered
virulence, or the ability to escape natural infection and
vaccine-mediated immunity or current diagnostic tests.[7]
Indeed,
on April 16, 2021, the FDA revoked the emergency use of bamlanivimab
alone based on the increase of SARS-CoV-2 variants that were resistant
to it.[8]
Few studies have reported the
effectiveness of combinations of mABs: Falcone M. et al. in patients
infected by alpha and gamma SARS-CoV-2 variants receiving combinations
of bamlanivimab /etesevimab or casirivimab/imdevimab[9] observed that
bamlanivimab/etesivimab should be used with caution in gamma variants
because of a high risk of disease progression.
In our recent
retrospective observational study, carried out from March to early
April 2021, we found a clinical and virological outcome largely below
the expected one of the phase 2 BLAZE 1 with bamlanivimab monotherapy,[5] both in the hospitalization rate and the improvement of 30-day symptoms.[10]
The
aim of this study was to evaluate the efficacy of early administration
of the combination of bamlanivimab /etesevimab or casirivimab/
imdevimab in outpatients infected by different SARS-CoV-2 viral
variants with mild -to moderate COVID-19 and at high risk to
progression, in reducing the global hospitalization rate, the COVID19
pneumonia hospitalization and clinical symptoms after 30 days from
therapy, in Umbria. Moreover, in Perugia-treated patients, we evaluated
the pre and post infusion neutralizing antibody titer (NAbT) towards
their own viral isolate to highlight any different efficacy of the two
combinations. The primary outcome was to evaluate any differences in
the effectiveness of the two monoclonal combinations on their
neutralizing capacity in the post-infusion sera of patients from
Perugia in relation to VOCs. Secondary outcomes were the evaluation of
the whole hospitalization rate, the COVID19 pneumonia hospitalization,
and clinical symptoms after 30 days of therapy relative to bamlanivimab
monotherapy.
Materials and Methods
This
prospective observational study was carried out from April to November
2021. We describe the characteristics, clinical outcomes, and
comorbidities of patients reported by their general practitioners
according to AIFA criteria who were admitted to the Day Hospital of
Infectious Diseases Clinic of Perugia and the COVID Hospital of Spoleto
to receive a single 2100 mg intravenous (IV) infusion of
bamlanivimab/etesivimab or 1200 mg casirivimab /etesivimab.
Demographic, medical history, main comorbidities, vaccination, and
clinical data were collected from the medical records. Moreover, we
evaluated all patients' symptoms presented at admission. And at thirty
days after the infusion.
Furthermore, at Infectious Diseases
Clinic in Perugia, before the infusion of mAbs, we took a
nasopharyngeal swab for virus isolation and routine blood samples for
laboratory analyses and neutralizing antibodies' activity.
Temperature,
blood pressure, respiratory rate, and oxygen saturation (SpO2) in
resting-state were measured before and one hour after the infusion of
mABs.
We also calculated the timeliness of the treatment (within 72 hours) from the onset of symptoms.
Thirty
days after the infusion, patients were called and interviewed about
their health state, the presence of mild adverse effects, the date and
results of subsequent nasopharyngeal swabs, and any changes in pre and
post-treatment symptoms.
Moreover, in Perugia, we cultured the
virus from the patients' nasopharyngeal swabs in VERO E6 cells, as
previously described.[11] Viral sequencing and variant identification
were performed at the Virology laboratory of the Department of Medical
Biotechnologies, University of Siena, Italy.
Neutralization assays
of sera pre and one-hour post-infusion against their own isolates were
performed at the Virology laboratory of the Infectious Disease Clinic,
University of Perugia, as previously reported.[12]
Lastly, in patients with the viral isolate, we evaluated the NAbTs related to the viral variant and type of association.
The
study was approved by Umbria regional Ethic Committee: MONO-COVID
observational study, protocol number 21647/21/OV, 30/04/2021.
Statistical Analysis
Standard
descriptive statistics were used to summarize data, such as mean,
standard deviation (SD), and percentage. The data were expressed as
mean ± SD, frequencies, or percentages with 95% confidence intervals
(95% CI). The Pearson Chi-square test was used to compare the
distribution of categorical variables. Numeric variables normally
distributed were analyzed by t-test, while variables that were not
normally distributed were analyzed by Mann–Whitney test. A p-value <
0.05 was considered for statistical significance. Patients'
demographic, epidemiological, and clinical characteristics were
compared between the two groups according to the combination of
bamlanivimab/etesevimab or casirivimab/imdevimab vs. bamlanivimab
monotherapy.
SPSS statistical package release 24.0 (SPSS Inc., Chicago, IL) was used for all statistical analyses.
Results
Seventy-nine
outpatients received treatment with monoclonal combinations, 25 with
bamlanivimab /etesivimab and 54 with casirivimab/imdevimab. Forty
(50.6%) were male, and the average age was 64. Sixty patients were
treated at the DH of Infectious Diseases Clinic of Perugia, 19 at the
Covid Hospital of Spoleto.
Neutralizing activity pre and post monoclonal infusion in Perugia subgroup patients.
Only in 42 out of the 60 patients treated with a monoclonal combination
in Perugia was the virus isolated: 17 were affected by a
SARS-Cov-2 alpha, 23 by a delta, and two by a gamma variant infection.
Twenty-two had already been vaccinated (2 with one dose), and 20 had
not. Nevertheless, the evaluation of NAbTs towards its own viral
isolate was performed in all of them before and one hour post infusion.
Table 1a shows the baseline NAbTs, related to the vaccination status and their own viral isolate.
All
alpha and gamma variant infections occurred by May 2021, all delta
between July and November. Among the 22 already vaccinated subjects,
the delta variant was by far the most prevalent one (86.36%), whereas,
among unvaccinated individuals, the alpha variant was the prevalent one
(75%).
The infection occurred within four months in 14/22
vaccinated patients (11 delta, 2 alpha, and 1 gamma). At baseline, the
NAbTs were ≤ 1:10 towards 17/22 of own viral isolates (1 alpha, 1
gamma, 15 delta), and 9 within 2 months of the vaccination. Instead, in
the two delta patients with NAbTs of 1: 160, the infection occurred
approximately 5 months after vaccination.
Table 1b shows
the post-infusion NAbTs according to the type of mAbs. After
casirivimab/ imdevimab infusion, no patients affected by alpha or
delta variants had NAbTs ≤ 1: 2560, 22 of them (75.86%) had NAbTs
≥ 10240. Instead, after bamlanivimab/etesevimab, in the two patients
affected by gamma variant, no NAbTs were obtained; in 9/11 patients
with alpha and delta variants (81.8%), NAbTs <1: 2560 and in 2 a
titer of 1:2560 was seen. Noteworthy, different NAbTs in favor of
casirivimab/imdevimab both for the alpha and delta variants were
observed, and the statistical difference for the alpha variant was very
significant (p=0.001).
Two out of the 42 patients with viral isolation
were hospitalized, both fully vaccinated, affected by delta variant,
one treated with casirivimab/imdevimab, and one with
bamlanivimab/tesevimab. Although they had been vaccinated two and three
months
earlier, both didn't have NAbTs at baseline. The patient on
casirivimab/imdevimab had a post-infusion NAbTs of 1: 10240, the
treated one with bamlanivimab / etesevimab of 1: 320.
 |
Table 1a.. Baseline neutralizing activity for already vaccinated patients and not for SARS-CoV2. |
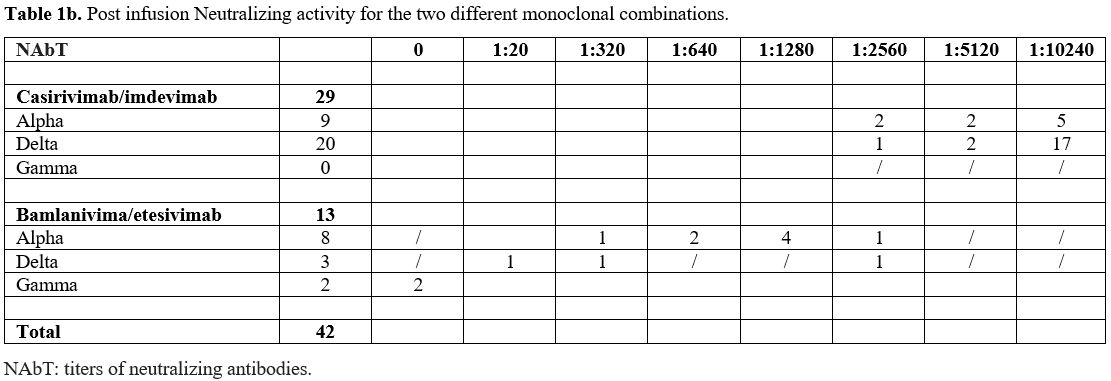 |
Table 1b. Post infusion Neutralizing activity for the two different monoclonal combinations. |
Overall clinical efficacy of two monoclonal antibodies associations in Umbria. Table 2
shows the demographic, clinical, and virological characteristics of all
the patients treated with the mAbs combinations from April to November
2021 related to those of patients previously treated with bamlanivimab
monotherapy. The most frequent comorbidities observed did not
statistically differ from the observed ones in the previous study.
However, renal insufficiency in dialysis, chronic obstructive pulmonary
disease, and congenital or acquired cardiovascular diseases were less
represented.
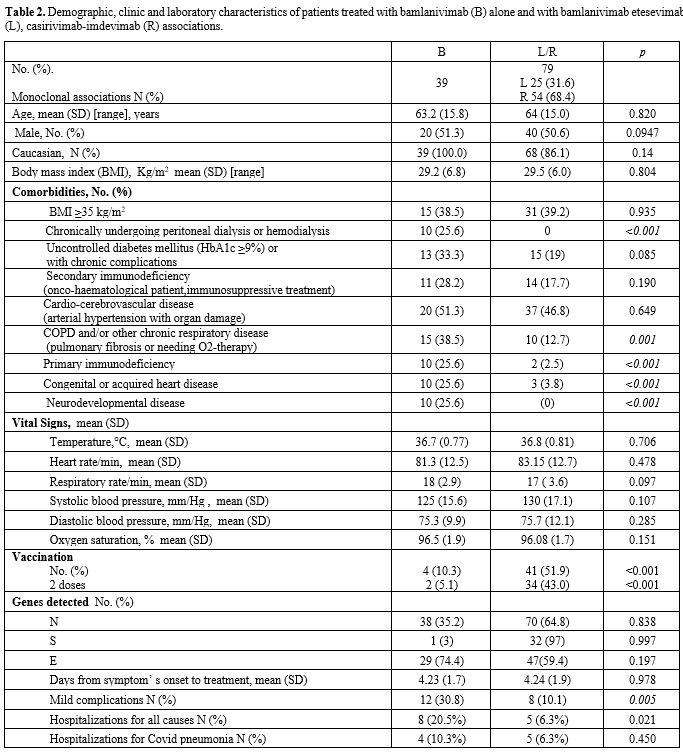 |
Table
2. Demographic,
clinic and laboratory characteristics of patients treated with
bamlanivimab (B) alone and with bamlanivimab etesevimab (L),
casirivimab-imdevimab (R) associations. |
The
diagnosis was performed with a molecular assessment. Genes N, S, and E
were detected by PCR assay in 70/79, 32/79, and 47/79, respectively. In
addition, the S deletion, suggestive of the alpha variant, was observed
in 47 patients (59.5%), unlike in the previous experience (almost all
the cases).
The vaccination rate was different (p < 0.001).
Fifty-two percent of the subjects had been vaccinated with at least one
dose, 43% with two doses compared to 10% and 5%, respectively. Most of
the unvaccinated were observed in May and June.
Baseline vital
signs were comparable to the previous experience, and some main
symptoms such as fever and asthenia but not myalgia and dyspnea.
The
mAbs infusion occurred about four days after the onset of symptoms, and
no adverse reactions were observed. Bamlanivimab/etesivimab was infused
on 12 S drop out viral isolates (48%) and casirivimab /etesivimab on 35
(64.8%).
In the following 30 days, 5/79 (6.3%) patients were hospitalized for COVID 19 pneumonia.
One
of the 5 hospitalized patients was unvaccinated (1/38, 2,63%). Overall,
2 patients showed a gene S drop out at the PCR assay and were
considered alpha variants, 2 were delta variants (viral isolation and
sequencing), 1 expressed S gene and was of uncertain attribution. The 2
delta infections appeared 2 and 3 months after vaccination, while the 2
alpha infections 6 and 7 months after. The untyped variant
hospitalization was related to the unvaccinated patient. The 2 S gene
dropout variants had been treated with casirivimab/etesivimab as well
as one delta variant and the isolate of uncertain attribution, while
one delta variant with bamlanivimab/etesivimab.
The
secondary outcome about the global hospitalization rate was observed,
6.3% vs 20.5% in the previous experience (p = 0.021), but the COVID19
pneumonia hospitalization (6.5% vs 10%, p = 0.45), and the alpha
variant pneumonia (4.25% vs 10.25%, p = 0.274) weren’t
reached. Cardiovascular disease (60%), BMI ≥ 35 (40%),
uncontrolled diabetes mellitus (20%), secondary immunodeficiency (20%),
COPD (20%) were the main comorbidities.
Thirty days after the infusion, the most common symptoms were less represented (Graphic 1).
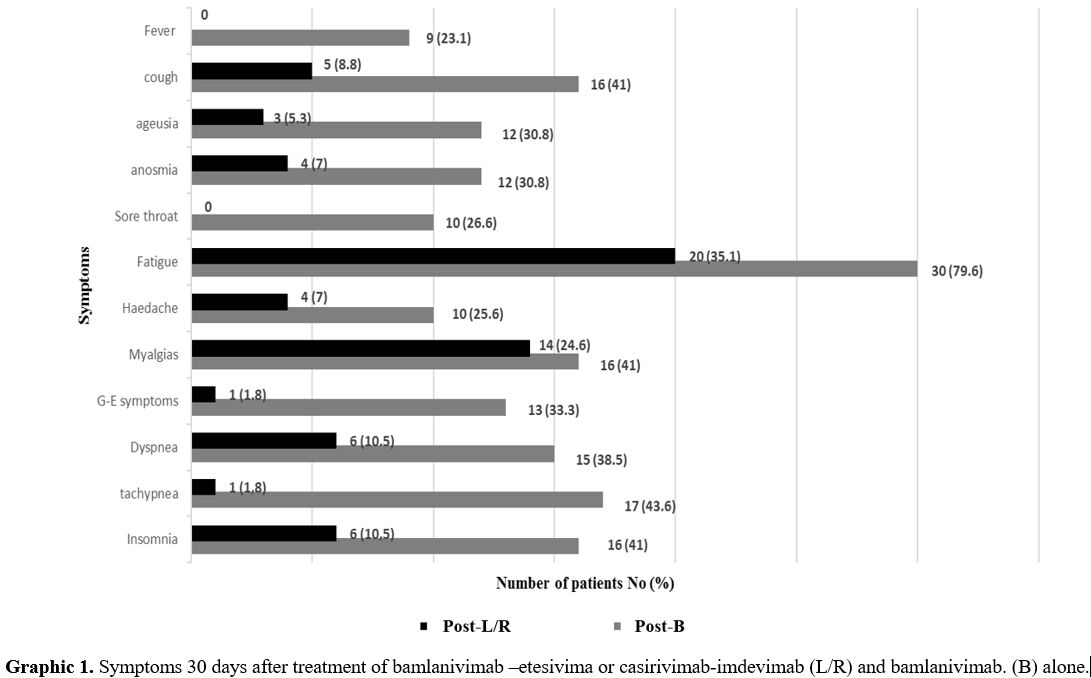 |
Graphic 1. Symptoms
30 days after treatment of bamlanivimab –etesivima or
casirivimab-imdevimab (L/R) and bamlanivimab. (B) alone. |
It
should be noted that insomnia, not present at baseline in both groups
of patients, was present thirty days after the infusion. Therefore, the
symptom numbers as shown in Figure 1 are significantly lower at 30 days in Abs combination vs. monotherapy.
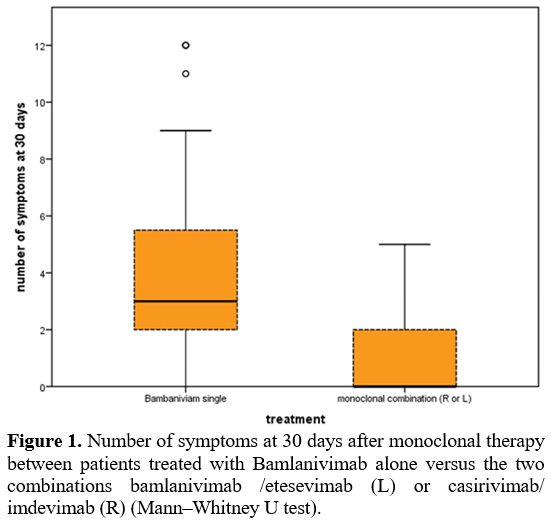 |
Figure 1. Number
of symptoms at 30 days after monoclonal therapy between patients
treated with Bamlanivimab alone versus the two combinations
bamlanivimab /etesevimab (L) or casirivimab/ imdevimab (R)
(Mann–Whitney U test). |
Discussion and Conclusions
This
study is an extension of our previous experience in treating COVID 19
with bamlanivimab monotherapy until its revocation.[10] It refers to
the subsequent use of the monoclonal combinations casirivimab/imdevimab
and bamlanivimab/ etesivimab from late April to November 2021 in
Umbria. Still, particular aspects of this study consist in having
evaluated in a subgroup of patients, before and after the infusion, the
neutralizing activity of their serum towards its own viral isolate.
The
spread of the SARS COV 2 virus in this period was characterized by an
initial prevalence of the alpha variant (B1.1.1.7) and its progressive
substitution with the delta variant (B1.617.2). Moreover, recent data
have shown the delta variant to be resistant to neutralization by
anti-RBD mAbs, including bamlanivimab.[13] In Italy, the delta variant emerged in May 2021; from July 20 its prevalence was 94.8%, while the alpha variant was 3.2%.[14]
However, we suspended this observational study in November 2021 due to
the rapid and progressive spread of the omicron variant (B.1.1.529)
because, very early, it proved to be resistant to the two mAbs
combinations in use until then.[15]
Our study is
observational, not randomized, and conditioned by mAbs combinations
available at the hospital pharmacies on that given day.
Elements
of interest consist in having isolated in 42 patients from Perugia the
viral variant and evaluated the NAbTs before and after the infusion
towards its own viral isolate, in the context of an overall population
different from the previous experience[10] by risk factors, vaccination status, involved VOCs and type of mAbs treatment.
The
comorbidities reported in this whole experience highlight a set of
patients with a lower risk of progression compared to those treated
with bamlanivimab alone. Overall, the global threat of hospitalization
was lower than previously observed; however, half of the cases were
determined by a worsening of their comorbidities.
Our data show
a higher hospitalization risk than that observed in the randomized
trials,[5,6] but in line with other experiences drawn from real-life.[9]
Both
combinations of mAbs, made from NAb convalescent COVID-19 patients
infected in the first half of 2020 and evaluated before the news VOCs,
cannot equally counteract the viral variants that subsequently spread.
Moreover, in this study, which also involves patients with the delta
variant that has been circulating in Italy since May 2020, the
hospitalization rate for the alpha variant was 4.25% (2/47), in line
with the report by Falcone et al. for the same variant.[9]
However, the risk was higher in the remaining cases, equal to 9.37% and
8.7% for delta, as a disharmonious use of combinations. In fact, in
the 20 treated patients with casirivimab/imdevimab, the risk of
hospitalization was only 5%, and in the 3 treated with
bamlanivimab/etesevimab was 33%.
Eighty percent of hospitalized
patients were vaccinated (4/5) with two doses. However, the time-lapse
from the vaccination was greater than 6 months for the alpha variant
and 2 and 3 months for delta. The hospitalization rate was 9.75% and
2.63% for unvaccinated individuals.
Thirty days after the infusion
of the mAbs, an important improvement in symptoms was observed,
independent of treatment timing, viral variant, vaccination status, and
type of monoclonal combinations (Graphic 1 and Figure 1). In addition, the rate of sequelae at 30 days appears in line with the observations made close to the infection.[16,17]
The
sub-study performed in Perugia made it possible to investigate some
pathogenic aspects of the infection and the sensitivity of viral
isolates to mAbs infused. Indeed, the viral agent's isolation,
characterization, and sequencing were possible in 42/60 patients, 22
vaccinated (two with one dose and both with alpha infection), and 20
unvaccinated. Among the 22 vaccinated subjects, the delta variant was
by far the most prevalent one (19/22), whereas, among unvaccinated
individuals, the alpha variant was the prevalent one (15/20). Moreover,
it should be emphasized that 11 delta variant infected patients had
been vaccinated within 4 months, most of the nineteen didn't have
neutralizing activity at baseline, and, worth highlighting, half of
these last had been vaccinated in the previous two months.
These
data are in line with those in the literature. In a study in which the
neutralizing activity of the serum of subjects vaccinated with BNT162b2
was studied against SARS-CoV-2 VOCs, a lower protective efficacy
against the delta and alpha variants compared to the wild type and for
the delta versus alpha was documented, as well as a significant
reduction in the NAbTs over time for all VOCs and not protective NAbTs
at 3 months for some subjects towards beta and delta isolates.[18]
To be emphasized is that the absence of the neutralizing activity
observed in most of our patients with delta infection at 2-3 months can
be explained by the different characteristics of our patients (infected
versus healthy subjects).
The inevitable consequence is that, in
the physiological decline of the antibody response after vaccination,
the protective efficacy decays earlier for the variants towards which
the baseline neutralizing activity is lower.
Our data can be
supported by a greater diffusion capacity of the delta variant and the
simultaneous progression of the vaccination campaign without a
cause/effect relation, but also by a greater sensitivity of the alpha
variant to the vaccination so that more easily delta variant took over.
Our
data about the neutralizing activity after infusion are also worthy of
interest. The greater neutralizing efficacy of casirivimab/imdevimab
may be justified by the action of the two monoclonals on different
antigenic epitopes of protein S, unlike antigenic overlapping with
bamlanivimab/etesivimab. Moreover, the poor efficacy against the delta
variant of bamlanivimab/etesivimab can be explained by the presence of
L452R, an escape mutation for bamlanivimab.[19]
Indeed,
monoclonal antibodies targeting immuno-dominant epitopes of the spike
are susceptible to a different efficacy in variants characterized by
other mutations in protein S.
Therefore, there is a need for
different therapeutic options available simultaneously with potential
activity against different viral variants, given the need to provide
treatment with mAbs in a very short time.
Conclusions
Some indications can be drawn from our report, which was done in the presence of two dominant VOCs (alpha and delta).
Although
a poor sample, this clinical study demonstrated better efficacy of the
mAbs combinations relative to monotherapy, although fewer risk factors
were present.
In
this experience, we have been unable to choose the type of mAbs, at
least based on gene S dropout, due to the conditioned availability at
the hospital pharmacies on that given day.
Alpha
variant infections occurred mainly in unvaccinated subjects, after the
first dose or after 5-6 months from the vaccination; delta infections
usually occurred within four months of full vaccination.
NAb activity at baseline against its own isolate was practically absent in most subjects.
A different NAb activity of the two associations was seen (casirivimab/imdevimab > bamlanivimab/etesevimab).
The
difficulty in proposing targeted mAbs, in a context of a rapid
succession of new viral variants and a missing context of adequate and
rapid viral sequencing leads to an even wider utilization of drugs that
inhibit viral replication.
Funding
This
research was funded by Fondazione Cassa di
Risparmio di Perugia, project "Studio prospettico sulla
durata della contagiosità e sul monitoraggio dei pazienti con
infezione da Sars -CoV-2 in isolamento: chi è infettato è anche
infettivo?". Grant number 19663(2020.05.08)
References
- Eugenia Quiros-Roldan, Silvia Amadasi, Isabella
Zanella, Melania Degli Antoni, Samuele Storti, Giorgio Tiecco,
Francesco Castelli. Monoclonal Antibodies against SARS-CoV-2: Current
Scenario and Future Perspectives. Pharmaceuticals (Basel) 2021 Dec
6;14(12):1272. https://doi.org/10.3390/ph14121272 PMid:34959672 PMCid:PMC8707981
- Food and Drug Administration. Letter to Eli Lilly and Company. November 10 2020. https://www.fda.gov/media/143602/download
- Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19 November 21, 2020
- Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19. February 09, 2021
- Peter
Chen, Ajay Nirula, Barry Heller, Robert L Gottlieb, Joseph Boscia,
Jason Morris, Gregory Huhn, Jose Cardona, Bharat Mocherla, Valentina
Stosor, Imad Shawa, Andrew C Adams, Jacob Van Naarden, Kenneth L
Custer, Lei Shen, Michael Durante, Gerard Oakley, Andrew E Schade,
Janelle Sabo, Dipak R Patel, Paul Klekotka, Daniel M Skovronsky,
BLAZE-1 Investigators. SARS-CoV-2 Neutralizing Antibody LY-CoV555 in
Outpatients with Covid-19. N Engl J Med 2021 January 21; 384(3):
229-237. https://doi.org/10.1056/NEJMoa2029849 PMid:33113295 PMCid:PMC7646625
- Robert
L Gottlieb, Ajay Nirula, Peter Chen, Joseph Boscia, Barry Heller,
Jason Morris, Gregory Huhn, Jose Cardona, Bharat Mocherla, Valentina
Stosor, Imad Shawa, Princy Kumar, Andrew C Adams, Jacob Van Naarden,
Kenneth L Custer, Michael Durante, Gerard Oakley, Andrew E Schade,
Timothy R Holzer, Philip J Ebert, Richard E Higgs, Nicole L
Kallewaard, Janelle Sabo, Dipak R Patel, Paul Klekotka, Lei Shen,
Daniel M Skovronsky. Effect of Bamlanivimab as Monotherapy or in
Combination With Etesevimab on Viral Load in Patients With Mild to
Moderate COVID-19: A Randomized Clinical Trial.JAMA. 2021 Feb
16;325(7):632-644. https://doi.org/10.1001/jama.2021.0202 PMid:33475701 PMCid:PMC7821080
- Tracking SARS-CoV-2 Variants. Available online: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants (accessed on November 15 2021
- Food and Drug Administration.April 16, 2021 https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab.
- Marco
Falcone, Giusy Tiseo, Beatrice Valoriani, Chiara Barbieri, Sara
Occhineri, Paola Mazzetti, Maria Linda Vatteroni, Lorenzo Roberto
Suardi, Niccolo' Riccardi, Mauro Pistello, Danilo Tacconi, Francesco
Menichetti. Efficacy of Bamlanivimab/Etesevimab and
Casirivimab/Imdevimab in Preventing Progression to Severe COVID-19 and
Role of Variants of Concern. Infect Dis Ther. 2021 Dec;10(4):2479-2488.
https://doi.org/10.1007/s40121-021-00525-4 PMid:34435337 PMCid:PMC8386337
- Elisabetta
Schiaroli, Giuseppe Vittorio De Socio, Laura Martinelli, Lisa
Malincarne, Martina Savoia , Anna Laura Spinelli, Daniela Francisci.
Early Treatment with Bamlanivimab Alone does not Prevent COVID 19
Hospitalization and Its Post-Acute Sequelae. A Real Experience in
Umbria, Italy. Mediterr J Hematol Infect Dis. 2021 Nov 1;13(1):
e2021061. https://doi.org/10.4084/MJHID.2021.061 PMid:34804435 PMCid:PMC8577560
- Anna
Gidari, Samuele Sabbatini, Sabrina Bastianelli, Sara Pierucci, Chiara
Busti, Desirée Bartolini, Anna Maria Stabile, Claudia Monari, Francesco
Galli, Mario Rende, Gabriele Cruciani, Daniela Francisci. SARS-CoV-2
Survival on Surfaces and the Effect of UV-C Light. Viruses 2021;13:408.
https://doi.org/10.3390/v13030408 PMid:33807521 PMCid:PMC7998261
- Anna
Gidari, Samuele Sabbatini, Sabrina Bastianelli, Sara Pierucci, Chiara
Busti, Claudia Monari , Barbara Luciani Pasqua, Filippo Dragoni,
Elisabetta Schiaroli, Maurizio Zazzi, Daniela Francisci.
Cross-neutralization of SARS CoV2 B.1.1.7 and P.1 variants in
vaccinated, convalescent and P.1 infected. J Infect. 2021
Oct;83(4):467-472. https://doi.org/10.1016/j.jinf.2021.07.019 PMid:34320390 PMCid:PMC8310664
- Delphine
Planas, David Veyer, Artem Baidaliuk, Isabelle Staropoli, Florence
Guivel-Benhassine, Maaran Michael Rajah, Cyril Planchais, Françoise
Porrot, Nicolas Robillard, Julien Puech, Matthieu Prot, Floriane
Gallais, Pierre Gantner, Aurélie Velay, Julien Le Guen, Najiby
Kassis-Chikhani, Dhiaeddine Edriss, Laurent Belec, Aymeric Seve, Laura
Courtellemont, Hélène Péré, Laurent Hocqueloux , Samira Fafi-Kremer,
Thierry Prazuck, Hugo Mouquet, Timothée Bruel, Etienne Simon-Lorière,
Felix A Rey, Olivier Schwartz. Reduced sensitivity of SARS-CoV-2
variant Delta to antibody neutralization. Nature. 2021;596:276-8 https://doi.org/10.1038/s41586-021-03777-9 PMid:34237773
- https://www.iss.it/documents/20126/0/FLASH+SURVEY+Varianti_SARS-CoV-2_30luglio.pdf/6c1c9969-e62c-cf19-6d1e-d9679e21692a?t=1627655177533.
ISS, July 30th 2021. Press
Release N°37/2021 COVID-19: in Italy with over 90% of cases, the 'Delta
variant' has replaced the 'Alpha' variant, Published 30/07/2021 -
Edited 10/01/2022.
- https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-limits-use-certain-monoclonal-antibodies-treat-covid-19-due-omicron.
FDA STATEMENT Coronavirus (COVID-19) Update: FDA Limits Use of Certain
Monoclonal Antibodies to Treat COVID-19 Due to the Omicron Variant,
Patrizia Cavazzoni, January 24, 2022
- Stephen
J Halpin, Claire McIvor, Gemma Whyatt, Anastasia Adams, Olivia
Harvey, Lyndsay McLean, Christopher Walshaw, Steven Kemp, Joanna
Corrado, Rajinder Singh, Tamsin Collins, Rory J O'Connor, Manoj
Sivan. Postdischarge symptoms and rehabilitation needs in survivors of
COVID-19 infection: A cross-sectional evaluation. J Med Virol. 2021
Feb;93(2):1013-1022. https://doi.org/10.1002/jmv.26368 PMid:32729939
- Claudia
Carvalho-Schneide, Emeline Laurent, Adrien Lemaignen, Emilie Beaufils,
Céline Bourbao-Tournois, Saïd Laribi, Thomas Flament, Nicole
Ferreira-Maldent, Franck Bruyère, Karl Stefic, Catherine
Gaudy-Graffin, Leslie Grammatico-Guillon, Louis Bernard. Follow-up of
adults with noncritical COVID-19 two months after symptom onset. Clin
Microbiol Infect. 2021 Feb;27(2):258-263. https://doi.org/10.1016/j.cmi.2020.09.052 PMid:33031948 PMCid:PMC7534895
- Emma
C Wall, Mary Wu , Ruth Harvey, Gavin Kelly, Scott Warchal, Chelsea
Sawyer, Rodney Daniels, Philip Hobson, Emine Hatipoglu, Yenting Ngai,
Saira Hussain, Jerome Nicod, Robert Goldstone, Karen Ambrose, Steve
Hindmarsh, Rupert Beale, Andrew Riddell, Steve Gamblin, Michael Howell,
George Kassiotis, Vincenzo Libri, Bryan Williams, Charles Swanton,
Sonia Gandhi, David Lv Bauer. Neutralising antibody activity against
SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet.
2021 Jun 19;397(10292):2331-2333. https://doi.org/10.1016/S0140-6736(21)01290-3
- Delphine
Planas, David Veyer, Artem Baidaliuk, Isabelle Staropoli, Florence
Guivel-Benhassine, Maaran Michael Rajah, Cyril Planchais, Françoise
Porrot, Nicolas Robillard, Julien Puech, Matthieu Prot, Floriane
Gallais, Pierre Gantner, Aurélie Velay, Julien Le Guen, Najiby
Kassis-Chikhani, Dhiaeddine Edriss, Laurent Belec, Aymeric Seve, Laura
Courtellemont, Hélène Péré, Laurent Hocqueloux, Samira Fafi-Kremer
Thierry Prazuck, Hugo Mouquet, Timothée Bruel, Etienne Simon-Lorière,
Felix A. Rey, Olivier Schwartz. Reduced sensitivity of SARS-CoV-2
variant Delta to antibody neutralization. Nature Vol. 596, pages276-280
(2021) Published: 08 July 2021. https://doi.org/10.1038/s41586-021-03777-9 PMid:34237773
[TOP]




