Araya Satdhabudha1, Chanapai Chaiyakulsil1, Paskorn Sritipsukho1,2, Phakatip Sinlapamongkolkul1, Utairat Chaumrattanakul3, Auchara Tangsathapornpong1,2, Pornumpa Bunjoungmanee1, Patcharapa Thaweekul1, Amolchaya Kwankua3, Onsuthi Pharadornuwat1, Tananya Lokanuwatsatien1, Pichaya Tantiyavarong4, Pakarat Pranudomrat1 and Chatchai Mingmalairak5.
1 Department
of Pediatrics, Faculty of Medicine, Thammasat University. 95 M 8
Phahonyothin Rd, Khlong nueng, Khlong Luang District, Pathum Thani,
12120, Thailand.
2 Center of Excellence in Applied
Epidemiology, Thammasat University, 95 M 8 Phahonyothin Rd, Khlong
nueng, Khlong Luang District, Pathum Thani,12120, Thailand.
3
Department of Radiology, Faculty of Medicine, Thammasat University. 95
M 8 Phahonyothin Rd, Khlong nueng, Khlong Luang District, Pathum Thani,
12120, Thailand.
4 Department of Clinical Epidemiology,
Faculty of Medicine, Thammasat University. 95 M 8 Phahonyothin Rd,
Khlong nueng, Khlong Luang District, Pathum Thani, 12120, Thailand.
5
Department of Surgery, Faculty of Medicine, Thammasat University. 95 M
8 Phahonyothin Rd, Khlong nueng, Khlong Luang District, Pathum Thani,
12120, Thailand.
Correspondence to:
Araya Satdhabudha, MD, Assoc. Prof. Department of Pediatrics,
Faculty of Medicine, Thammasat University. 95 M 8 Phahonyothin Rd,
Khlong nueng, Khlong Luang District, Pathum Thani, 12120, Thailand.
Tel.: +0662-9269999. E-mail:
araya221@gmail.com
Published: May 1, 2022
Received: February 17, 2022
Accepted: April 15, 2022
Mediterr J Hematol Infect Dis 2022, 14(1): e2022044 DOI
10.4084/MJHID.2022.044
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Few studies had focused on the epidemiological and clinical
characteristics of pediatric COVID-19 (SARS-CoV-2) during Delta and
pre-Delta eras in Asia, despite it being a pandemic.
Objective:
To study the epidemiological and clinical characteristics of three
waves of pediatric COVID-19 infections in a tertiary-care setting in
Thailand.
Methods: This
retrospective study reviewed all PCR-confirmed pediatric (0-18 years of
age) COVID-19 infections between January 13, 2020, and October 31,
2021, in a tertiary care system in Thailand.
Results:
1,019 patients, aged 0.02 - 18 years, 552 (54.2%) male, and 467 (45.8%)
female, with a median age of 9.2 years, were enrolled. Asymptomatic
cases accounted for 35.7%, of which 106 (18.9%) had abnormal chest
X-ray findings. Most cases were classified as having mild clinical
symptoms, with only 8 (0.8%) and 4 (0.4%) developing a severe and
critical illness, respectively. There were no deaths. The Delta variant
appeared more transmissible than previous ones, but we did not see any
difference in disease severity. Upper respiratory tract symptoms were
predominant, while few cases had lower respiratory tract involvement.
The sensitivity and specificity of dyspnea symptoms to predict
radiologically confirmed pneumonia were 14% and 95%, respectively, with
a likelihood ratio of 3.37. The overall prognosis was good, with only
13 (1.3 %) needing respiratory support. All cases showed clinical
improvement with a decent recovery.
Conclusion:
Pediatric COVID-19 during the Delta variant predominance era generally
appeared more transmissible but benign. One-fifth of cases had
pneumonia, but few cases needed respiratory support. Prevention remains
important for disease control.
|
Introduction
Severe
acute respiratory syndrome coronavirus (SARS-CoV-2) and the related
disease (COVID-19) were declared a pandemic on March 11, 2020, by the
WHO.[1] SARS-CoV-2/COVID-19 arose as an outbreak at the end of 2019 in Wuhan, China, and rapidly spread worldwide.[2,3]
SARS-CoV-2/COVID-19 can present as asymptomatic, mild symptoms, severe
to critical illness, and can cause death. Morbidity and mortality rates
were high among older adults. On the contrary, the infection rate and
symptom severity are disproportionately low in children.[4-13]
In
Thailand, the first imported case of COVID-19 infection was detected on
January 12, 2020, and the first COVID-19 outbreak was in March 2020,
with around 100 cases/day. The Department of Disease Control, Ministry
of Public Health (DDC MoPH) stated the outbreak was under control after
four months due to strict disease prevention measures. However, in
December 2020, a second outbreak occurred among foreign workers in
Samut Sakhon, a province south of Bangkok, with a maximum of about
1,700 cases/day. Again, the DDC MoPH brought in patient-screening
measures in accordance with US CDC guidelines. PUI (Persons Under
Investigation) were screened and confined at the early stages, and
again, the outbreak was slowed. However, in April 2021, a third wave
occurred, the fast-moving Alpha variant, which resulted in a
drastically higher infection rate of over 2,000 cases/day.
The
situation worsened with the June 2021 Delta variant outbreak in
Thailand, with the infection rate reaching more than 20,000 cases/day,
with around 0.83% mortality. Delta variant spread rapidly and was more
severe, resulting in insufficient medical resources; thus, the
government added external health care facilities such as field
hospitals and home isolation options. Zoning also began for patients:
green (asymptomatic or mild symptoms), yellow (mild dyspnea/high-risk
group with mild symptoms), red (clinical pneumonia/oxygen desaturation
SpO2 < 96%/severe symptoms). Green
patients went into field hospitals or home isolation, while yellow or
red patients were hospitalized. Various successful vaccination
campaigns also occurred. With strict public health measures and
vaccination coverage, the infection rate gradually declined at the end
of October 2021.
In the pediatric context, COVID-19 cases are estimated at < 0.1-15% of all confirmed cases in the first half of 2021.[4-13] In the second half of the year, however, infection grew to 28%[14]
in the US, yet the pediatric severe disease rate remains significantly
lower compared to adults. Worldwide, pediatric severe cases and deaths
are approximately 0.6-5% and 0.3%, respectively.[4-6,8-13] There was limited data on the epidemiological and clinical characteristics of pediatric COVID-19 patients in Thailand.[15,17] Our aim was to study this and compare the clinical characteristics and symptom severity of each wave.
Materials and Methods
Participants.
This retrospective study reviewed all PCR-confirmed pediatric (patients
aged 0-18 years) COVID-19 infections between January 13, 2020, and
October 31, 2021, at Thammasat University Hospital, a university
hospital in a province adjacent to Bangkok. Approval for the study was
granted by the ethics committee of Thammasat University
(MTU-EC-PE-1-293/64). Children with perinatal infection, non-acute
COVID-19 infection, and MIS-C (Multisystem inflammatory syndrome in
children) were excluded. Initially, all COVID-19 cases were admitted to
the hospital, but during the third wave, medical resources were
limited; green patients were admitted into field hospitals or home
isolation. Hospitalization was reserved for yellow or red patients. All
hospitalized patients underwent chest x-ray (CX-R) and complete blood
count (CBC); no patient had done computerized tomography. CX-R and
other laboratory exams were only performed for the green group if
clinically indicated. After discharge, all COVID-19 patients were
advised to quarantine at home for 14 days.
Demographic data and
clinical information were collected through a manual chart review. An
online standardized database was set up using REDCap (Research
Electronic Data Capture), with the main coordinating center in
Thammasat University. Data were extracted from medical records in the
Electronic Public Health Information System (E-PHIS) and entered by
experienced pediatricians. Clinical information was collected:
underlying medical conditions, nutritional status, clinical history,
initial vital signs, laboratory results (hemoglobin, white blood cell,
transaminase, C-reactive protein, CX-R), disease severity, medical
management, and clinical outcome.
Nutritional statuses were
classified into five groups: severe malnutrition, moderate
malnutrition, normal, overweight, and obese. For children < 5 years
of age, overweight or obesity was defined as weight-for-height > 2
and 3 standard deviations (SD) above the WHO Child Growth Standard
median, respectively.[18-19] Children and adolescents
aged between 5 and 18 years were defined as overweight or obese if
BMI-for-age was > 1 or 2 SD above the WHO Growth Reference median,
respectively.[18-20] Moderate malnutrition was
defined as weight-for-height or BMI-for-age between -2 and -3 SD.
According to the WHO Child Growth Standard, severe malnutrition was
defined as those < -3 SD.[21] Clinical lower
respiratory tract signs composing the dyspnea group were defined as a
history of dyspnea/shortness of breath, chest pain, tachypnea, and SpO2
< 95%. To ensure accuracy, CX-R was reviewed independently by three
investigators (two radiologists and one pediatric pulmonologist). CX-R
categorization was based on the International Expert Consensus
Statement from six countries[22] and was divided into
four categories: typical, atypical, intermediate, and negative. A final
record was made based on the consensus from two out of three
investigators.
Disease severity categorization was based on The National Institutes of Health (NIH)[23]
classification of five groups: asymptomatic, mild (mild symptoms
without pneumonia), moderate (pneumonia without hypoxemia (SpO2 ≥ 94%), or no symptoms but abnormal CX-R), severe (pneumonia with SpO2
< 94%), or critically ill (acute respiratory distress syndrome or
septic shock). Prior to the Omicron variant outbreak, which began after
this study, the MoPH recognized three COVID-19 waves in Thailand: first
(January 13, 2020 – December 15, 2020), second (December 16, 2020 –
March 31, 2021), third (April 1, 2021, to October 31, 2021). Thus, we
have classified the confirmed COVID-19 cases into three groups based on
the MoPH nationwide surveillance data of SARS-CoV2 variants: Dominant
Beta group - diagnosed before April 1, 2021 (no detection rate
recorded); Alpha dominant group from April 1, 2021, to June 30, 2021
(detection rate of 65 - 90%); Delta dominant group from July 1, 2021,
to October 31, 2021 (detection rate of 62 - 92%).
Statistical analysis.
Data were analyzed using STATA for Windows v14.0. Clinical
characteristics and laboratory results for continuous data were
reported as median with interquartile range (IQR); categorical data
were reported as the frequency with percentage. One-way ANOVA, Wilcoxon
rank-sum test, and Kruskal-Wallis test were used to compare continuous
data; nominal data analysis used a Chi-square test adjusted for
multiple comparisons: P-value ≤ 0.05 was considered statistically
significant.
Results
A
total of 1,040 confirmed pediatric SARS-CoV-2/COVID-19 cases were
enrolled. Of this, 21 cases were excluded: incomplete data (7),
referred to other hospitals (8), non-acute SARS-CoV-2 infection (1),
perinatal infection (4), and MIS-C (1). Among the remaining 1,019
patients, 552 (54.2%) were male with a median age of 9.2 years (0.02-18
years), with the highest prevalence in those aged 5 - 15 years. 555
(54.9%) patients had normal nutritional status with a mean bodyweight
of 36.6 kg, while 59 (5.8%) had severe malnutrition, and 194 (19.2%)
were obese. Only 7.8% had underlying diseases: chronic lung disease
(0.1%), allergies (1.3%), cardiovascular (0.3%), endocrine (0.3%),
neurological (0.3%), chronic renal (0.2%), hematological (1.2%),
oncological disease (0.2%), and genetic or developmental-behavioral
disorders (1.2%). Very few patients had immunocompromised conditions
(0.007%). Regarding the source of exposure, 502 (49%) patients reported
known exposure information, the most common contact route being
household (39.3%), followed by cluster or community (6.6%),
neighborhood (1.8%), school (0.7%), and travel (0.1%).
The first
pediatric COVID-19 infection at Thammasat Hospital was reported on
February 13, 2021. The distribution, peak severity, and baseline
demographic data of the 1019 confirmed pediatric COVID-19 cases are
shown in Figure 1 and Table 1.
In the Beta group, there were 17 patients, Alpha 324 and Delta 678.
Baseline demographic data of the three groups did not significantly
differ except for the source of exposure (p < 0.001) and the proportion of children in different age groups (p
= 0.019). The Alpha and the Delta period had high prevalence in younger
age group than the Beta period. The proportion of children younger than
one year was higher during the Delta period, while school-age to
adolescence was more frequently found in the Beta period. The Delta
group frequently reported unknown sources of exposure more than other
groups. However, most of them were still household contact from the
source of exposure reported. The details are shown in Table 1.
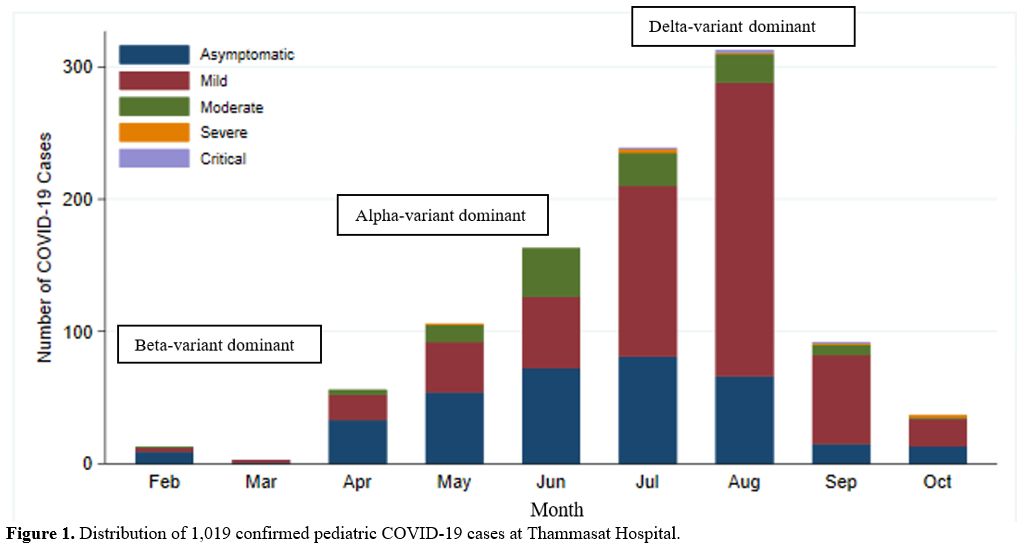 |
Figure 1. Distribution of 1,019 confirmed pediatric COVID-19 cases at Thammasat Hospital. |
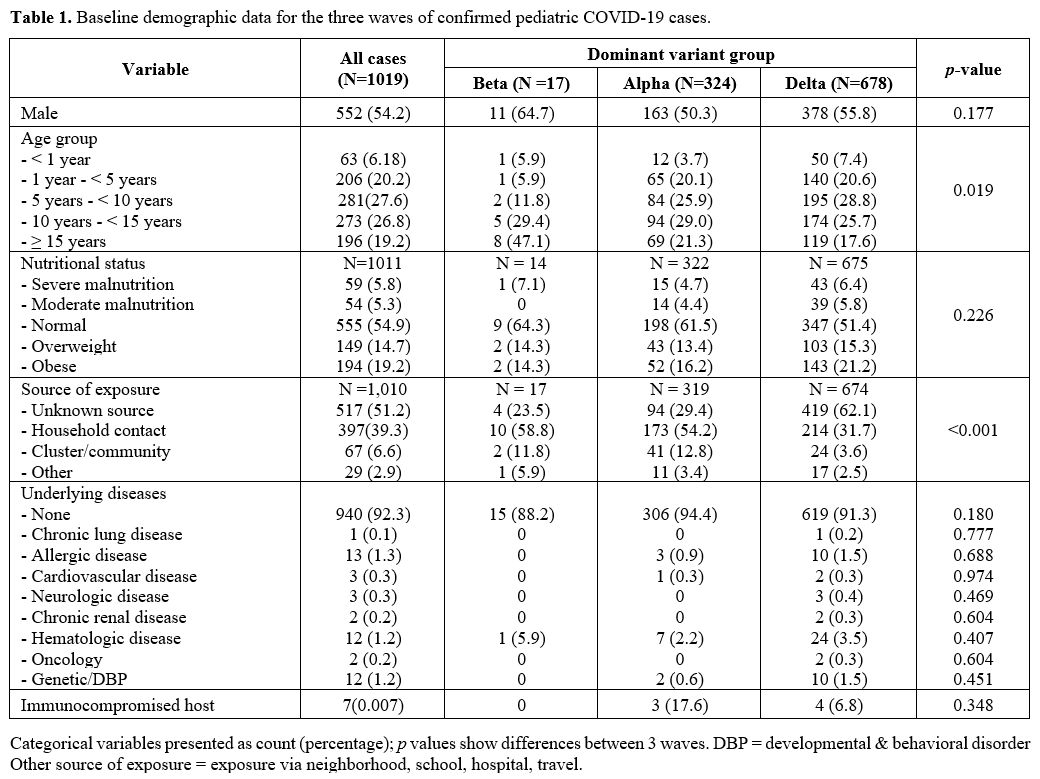 |
Table 1. Baseline demographic data for the three waves of confirmed pediatric COVID-19 cases. |
The
median day from symptom to diagnosis was one day (IQR 0-1 day); the
median day from symptom to hospitalization was three days (IQR 2-6
days). Days from symptoms to hospitalization were significantly
different among the variants (p
< 0.001). 34.3% of patients were admitted into home isolation, 46.5%
to field hospital, 18.9% to Thammasat Hospital, with 0.3% to the
pediatric intensive care unit (PICU). Most of the cases were admitted
to Thammasat Hospital in the Beta period, but the admission site
shifted to the field hospital and home isolation during the Alpha and
the Delta period. Patients reported as asymptomatic on arrival were
35.7%. For all symptomatic patients, upper respiratory signs were most
common: cough (42%), fever (31%), rhinorrhea (23%), sore throat (13%),
anosmia (11.5%), nasal congestion (5.4%), and loss of taste (4.8%).
Lower respiratory signs were few: dyspnea or shortness of breath (3%),
chest pain (0.8%), tachypnea (3%) and SpO2 < 95% (0.1%). Severe and critically ill cases had significantly more fever, cough, dyspnea, tachypnea, and SpO2 < 95% at presentation compared with mild to moderate cases (p
< 0.001). Gastrointestinal symptoms included diarrhea (7.3%),
vomiting (2.1%), and abdominal pain (0.9%). Other symptoms were
headache (7.1%), fatigue (2.1%), myalgia/arthralgia (1.5%), rash (2%),
numbness (0.2%) and palpitation (0.3%). Clinical manifestations were
also significantly different among the variants. During the Delta
variant group, there was a significantly lower number of asymptomatic
patients compared to the other two groups (p
< 0.001). Nevertheless, most of the symptomatic patients had only
mild symptoms, including fever, cough, rhinorrhea, sore throat,
anosmia, and loss of taste. In the Beta and the Alpha period, most
cases were asymptomatic. The Beta group was found to have more
prominent lower respiratory tract signs than the other groups. Only 1%
had desaturation on arrival, with no significant difference between
groups. For extra-respiratory tract symptoms, diarrhea and headache
were frequently found in the Beta and the Delta period compared to the
Alpha period with p = 0.002 and 0.031, respectively. Fatigue and myalgia were more prominent in the Beta period (p ≤ 0.001) (Table 2).
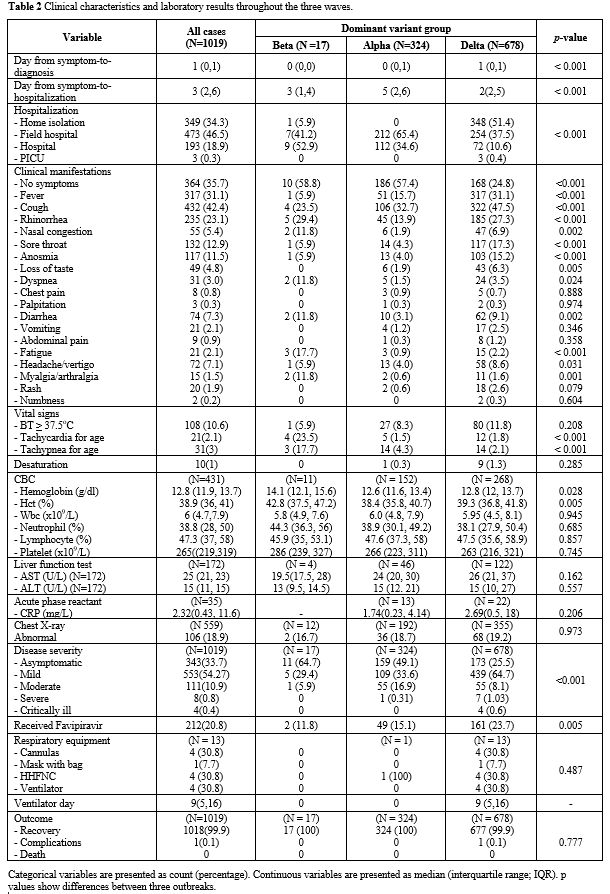 |
Table
2. Clinical characteristics and laboratory results throughout the three waves. |
Among
cases with laboratory investigations, the median hemoglobin was 12.8
(IQR 11.9 - 13.7 g/dl), the median white blood cell counts were 6 x 109/L (IQR 4.7- 7.9 x 109/L)
with the median neutrophils count of 39 (IQR 28 - 50%), median
lymphocyte counts of 47 (IQR 37 - 58%), and median platelet counts of
265 x 109/L (IQR 219-319 x 109/L
), the median AST was 25 (IQR 21 - 23 U/L), median ALT of 15 (IQR 11 -
15 U/L), and median CRP of 2.3 (IQR 0.4 - 11.6 mg/L). CX-R and
laboratory investigations data did not significantly differ among
variants, except for the highest hemoglobin level during the Beta
period. These data were demonstrated in Tables 2 and 3.
CX-R
and laboratory investigations were not routine. Only 564 patients
(55.3%) had CX-R, and among them, 106 (18.8%) had abnormal findings:
81% patchy or ground-glass opacities, 16% interstitial, and 2.9%
nodular. For abnormal distribution, 62% had peripheral infiltration,
37% central, 46% unilateral, 35% bilateral and 19% multifocal. Two
cases had atelectasis, and one had a pneumothorax. We attempted to
correlate abnormal CX-R findings with lower respiratory tract signs
(dyspnea symptoms). Abnormal CX-R results were more likely found in
patients with dyspnea than in non-dyspnea patients (44 vs. 17%; p
< 0.0001). When we evaluated dyspnea symptoms for prediction of
pneumonia (abnormal CXR), there was low sensitivity of 14% (95% CI, 8.1
- 22.3) but high specificity of 95% (95% CI, 93.5 - 97.5), with the
likelihood ratio being 3.37 (95% CI, 1.77 - 85.8). Positive predictive
value was 44% (95% CI, 27.2 - 62.1), and negative predictive value was
82.8% (95% CI, 79.15 - 85.8). Details are shown in Table 3.
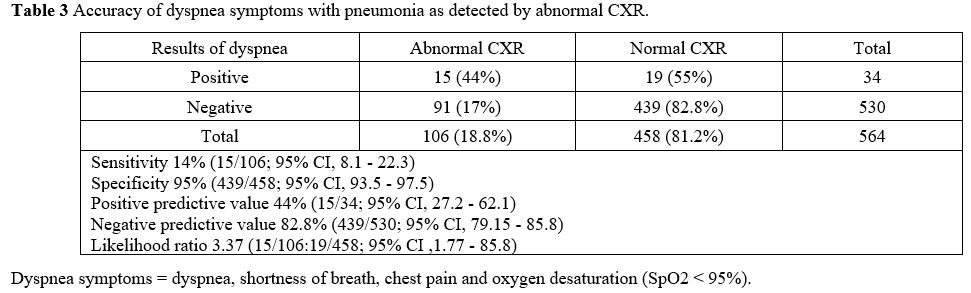 |
Table 3. Accuracy of dyspnea symptoms with pneumonia as detected by abnormal CXR. |
Within
the 1,019 patients, asymptomatic cases were 33.7%, mild symptoms 54.3%,
moderate 10.9%, severe 0.8%, and critically ill 0.4%. The proportion of
patients with underlying conditions was significantly higher in
severe/critically-ill patients when compared to the non-severe groups
(7.5% vs. 0.6%; p < 0.001). Patients beyond infancy tended to be more asymptomatic than infants (35% vs. 13%; P < 0.001), and the infant group were more likely to have critical illness than other groups (1.6% vs. 0.39%; p = 0.001). We also found that the severe group patient had more lymphopenia than the mild to moderate group (26 % vs. 46%; p =
0.001). Among severe cases, 5 (38%) patients required low-flow oxygen,
4 (31%) needed humidified high-flow nasal cannulas (HHFNC), and 4 (31%)
required invasive ventilation. All showed clinical improvement with
full recovery except for one case which experienced neurological
sequelae due to venous sinus thrombosis. We had no deaths. Compared
among groups, symptomatic patients were more likely to be found in the
Delta group (p < 0.001),
but most cases presented with mild upper respiratory tract symptoms. We
saw no significant differences in each wave for the moderate to severe
and critically ill groups. There was a slight increase in severity from
Beta to Alpha, Delta dominant groups. Critically ill patients were
found only during the Delta period. The details are shown in Table 2.
Discussion
Our
study described the epidemiological and clinical characteristics of
pediatric COVID-19 infection cases in our Thai tertiary care center. We
found that pediatric COVID-19 was not severe, with severe and critical
illness rates being only 0.8 and 0.4%, respectively. This seemed less
dire than previous studies reporting severe/critical pediatric cases at
around 0.8 - 5.3%.[4-6,8-13] We also
observed a decrease in asymptomatic infections in the Delta dominant
group compared to the Alpha one (24.8% vs. 57.4 %). However, most
symptomatic patients had mild upper respiratory tract symptoms, and no
significant changes appeared in regard to severe disease. Our data was
in line with Byung-Han R et al.,[24] their asymptomatic Delta group being 29.3% and their non-Delta group 43.4%.
Interestingly,
Delta variants did not appear to demonstrate worse clinical outcomes
than prior lineages since most patients were classified as having mild
to moderate symptoms. Delahoy et al.[25] also
reported increased ER visits and hospitalizations, especially in
unvaccinated children, during the Delta wave, but indicators of severe
disease (ICU admission, receiving invasive mechanical ventilation, or
death) did not significantly increase from the previous outbreaks.
Their study concluded that the Delta variant was more transmissible
than the previously circulating SARS-CoV-2 variants; however, it
remains uncertain whether it causes more severe disease.
There
was no significant change in the proportion of the severe disease among
groups for the disease severity. Nevertheless, we found interesting
data that no severe case was found despite a higher proportion of
patients presenting with lower respiratory tract signs during the Beta
period. More severe/critically-ill patients were seen during the Delta
versus the pre-Delta period. This might be due to the high
transmissibility combined with limited medical resources, resulting in
delayed hospitalization and treatment. However, after the Thai
government added additional health care facilities in the Alpha and the
Delta period to enhance access to medical care, more patients were
readily cared for in the field hospital and home isolation. The
government then implemented preventive measures in vaccination
campaigns and media knowledge sharing. As a result, the situation
improved, especially regarding day-of-symptoms-to-hospitalization data
and the infection rate, which gradually declined during the end of the
Delta season. Preventive measures, including vaccination of those
eligible, universal mask-wearing, social distancing, and quarantining
after exposure to persons with COVID-19, appeared to have worked and
remain important for disease control.
We found slightly more males than females afflicted, consistently with previous studies.[4,6,8,16,24]
However, no significant gender difference was observed in our study.
The median age was 9.2 years (2 months - 18 years), with the highest
prevalence in children aged 5-15, suggesting that COVID-19 occurs
throughout childhood. For source of exposure, due to the wide community
transmission during the Delta period, it was not feasible to trace the
source of exposure in most patients. Most contacts were from the
household in the cases with a known exposure, followed by
cluster/community. Especially in the Alpha dominant group, the
infection started in a boxing stadium and certain pubs in downtown
Bangkok, which implies the possibility of person-to-person transmission
in any closed environment. This was the start of strict social
distancing measures imposed by the government. Most patients had no
comorbidities in our study, but we found that significantly more
severe/critically-ill patients had underlying conditions. One with
congenital heart disease case developed venous sinus thrombosis with
acute respiratory failure after COVID-19 infection and needed special
care in the PICU. Finally, the patient was discharged with
anticoagulants, and the neurological deficit slightly improved.
However, when diseases were analyzed separately into risk groups,
including asthma, chronic lung disease, congenital heart disease,
oncology, and obesity, the findings show that none of these diseases
had any severe effects.
The prevalence of asymptomatic pediatric patients with COVID-19 was 35.7%, higher than other studies reporting 1.3% - 40%.[4-6,8-13]
This percentage varied with time and space. Upper respiratory tract
signs were common for symptomatic patients, while lower respiratory
tract signs were found less frequently. Patients who had lower
respiratory tract issues seemed to have more severe diseases. When we
evaluated dyspnea symptoms in order to predict pneumonia, we found low
sensitivity (14%), high specificity (95%), and low positive predictive
value (44%) but a high negative predictive value (82.8%), with a
likelihood ratio of 3.37. The dyspnea symptoms were not a good
screening test for predicting pneumonia, although patients with dyspnea
symptoms had significantly abnormal CX-R more often than those without.
We also found that non-dyspnea patients usually had normal CX-R.
Therefore, we suggested that CX-R should be done in dyspnea cases, but
we cautioned that it might not be necessary for non-dyspnea patients.
In our study, the low positive predictive value might be explained by
the low sensitivity of dyspnea symptoms to predict pneumonia and the
low prevalence of pneumonia in pediatric COVID-19 infections. In areas
with a high prevalence of pneumonia, the positive predictive value
might be higher.
We had seen little data on the clinical
characteristics of pediatric COVID-19 in Asia, especially during the
Delta variant wave. In our study’s favor, we had access to a large
population in our tertiary care hospital, so we were able to compare
clinical characteristics during three different outbreaks and strains
of COVID-19. According to our data, the overall disease severity of
pediatric COVID-19 was low, but with a rapid increase in the number of
cases, we received more patients with severe and critical illnesses
during the Delta versus the pre-Delta period. The mutation variants may
be linked to greater transmissibility, increased risk of reinfection,
and severe disease. Therefore, preventive measures, including
vaccination of those eligible, universal mask-wearing, and social
distancing, should be warranted for disease control. In October 2021
(At the end of this research), the vaccination campaign for children
older than 12 was initiated to reduce disease transmission and limit
the new cases of severe disease.
Despite the favors, we had some
limitations. First, as this was a retrospective study, there was the
possibility that some records might not be complete as some might be
collected via phone call and relied on respondent recall. Data such as
body weight, height, symptom time span before admission to health care
unit, source of contact, etc., might not be accurate. Secondly, most
patients were treated in an isolated room or cohort ward, and the
treatment was usually done by telemedicine; therefore, there would be
limitations in physical examination. As a result, the diagnosis of
pneumonia can only be based on the history of dyspnea, tachypnea for
age, desaturation, and abnormal CX-R, leading to more CX-R being
performed than normal. Thirdly, during all three outbreaks,
particularly in the Delta wave, there was an overload of cases in the
health care systems, resulting in delayed treatment leading to greater
severity. Finally, our study did not confirm data on the SARS-CoV-2
strains. We relied on the nationwide surveillance data of SAR-CoV2
variants from the MoPH Thailand to determine when and where the strains
were predominantly active.
Conclusions
The
epidemiological and clinical characteristics of pediatric COVID-19
cases in our tertiary care center in Thailand were similar to previous
reports. COVID-19 occurs in all ages of childhood, with no gender
difference. Most cases presented as mild upper respiratory tract
symptoms. However, infants were vulnerable to COVID-19 infection. The
Delta variant is more transmissible than previous ones, but we saw no
difference in disease severity compared to pre-Delta waves.
Acknowledgments
The
authors are grateful to the Thammasat Research Grant that partially
supported this research and the pediatric department staff and
healthcare workers responsible for the patient care at Thammasat
University Hospital.
References
- World Health Organization. Coronavirus disease (COVID-2019) situation reports. 2020. https://www.who.int/emergencies/diseases/novel-corona virus-2019/situation-reports/ Accessed May 29 2020.
- Zhu
N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Weifeng Shi,
Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Wenjie Tan W,
China Novel Coronavirus Investigating and Research Team. A Novel
Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med.
2020; 382(8):727-33. https://doi.org/10.1056/NEJMoa2001017 PMid:31978945 PMCid:PMC7092803
- Harapan
H, Itoh N, Yufika A, Winardi W, Keam S, Te H, Megawati D, Hayati Z,
Wagner AL, Mudatsir M. Coronavirus disease 2019 (COVID-19): A
literature review. J Infect Public Health. 2020 May;13(5):667-73 https://doi.org/10.1016/j.jiph.2020.03.019 PMid:32340833 PMCid:PMC7142680
- Dong
Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, Tong S. Epidemiology of COVID-19
Among Children in China. Pediatrics 2020 June;145(6):e20200702. https://doi.org/10.1542/peds.2020-0702 PMid:32179660
- COVID-19
CDC response Team, Bialek S, Gierke R, Hughes M, McNamara LA,
Pilishvili T, Skoff T. Coronavirus Disease 2019 in Children - United
States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep 2020 April
10;69(14):422-6. https://doi.org/10.15585/mmwr.mm6914e4 PMid:32271728 PMCid:PMC7147903
- Guo
CX, He L, Yin JY, Meng XG, Tan W, Yang GP, Bo T, Liu JP, Lin XJ, Chen
X. Epidemiological and clinical features of pediatric COVID-19. BMC
Med. 2020;18:250. https://doi.org/10.1186/s12916-020-01719-2 PMid:32762696 PMCid:PMC7408975
- Wang E, Brar K. COVID-19 in children: an epidemiology study from China. J Allergy Clin Immunol 2020;8:2118-20. https://doi.org/10.1016/j.jaip.2020.04.024 PMid:33427648 PMCid:PMC7172907
- Wong
JJM, Abbas Q, Chuah SL, Malisie RF, Pon KM, Katsuta T, Dang H, Lee PC,
Jayashree M, Sultana R, Maha Q, Gan CS, Shimizu N, Xu F, Tang SF, Shi
L, Lee JH, Thoon KC, Yung CF, PACCOVRA Investigators of the PACCMAN
Research Group. Comparative Analysis of Pediatric COVID-19 Infection in
Southeast Asia, South Asia, Japan, and China. Am J Trop Med Hyg. 2021
Jun;105(2):413-20. https://doi.org/10.4269/ajtmh.21-0299 PMid:34129517 PMCid:PMC8437183
- Hoang
A, Chorath K, Moreira A, Evans M, Burmeister-Morton F, Burmeister F,
Naqvi R, Petershack M, Moreira A. COVID-19 in 7780 pediatric patients:
a systematic review. EClinicalMedicine. 2020 Jul;24:100433. https://doi.org/10.1016/j.eclinm.2020.100433 PMid:32766542 PMCid:PMC7318942
- Badal
S, Bajgain KP, Badal S, Thapa R, Bajgain BB, Santana MJ. Prevalence,
clinical characteristics, and outcomes of pediatric COVID-19 : A
systematic review and meta-analysis. J Clin Virol. 2021 Feb;135:104715.
https://doi.org/10.1016/j.jcv.2020.104715 PMid:33348220 PMCid:PMC7723460
- Irfan
O, Muttalib F, Tang K, Jiang L, Lassi ZS, Bhutta Z. Clinical
characteristics, treatment and outcomes of paediatric COVID-19: a
systematic review and meta-analysis. Arch Dis Child. 2021
May;106(5):440-8. https://doi.org/10.1136/archdischild-2020-321385 PMid:33593743 PMCid:PMC8070630
- Ding Y, Yan H, Guo W. Clinical Characteristics of Children With COVID-19: A Meta-Analysis. Front Pediatr. 2020; 8:431. https://doi.org/10.3389/fped.2020.00431 PMid:32719759 PMCid:PMC7350605
- Wang
Z, Zhou Q, Wang C, Shi Q, Lu S, Ma Y, Luo X, Xun Y, Li W, Baskota M,
Yang Y, Zhai H, Fukuoka T, Ahn HS, Lee MS, Luo Z, Liu E, Chen Y,
COVID-19 Evidence and Recommendations Working Group. Clinical
characteristics of children with COVID-19: a rapid review and
meta-analysis. Ann Transl Med. 2020 May;8(10):620. https://doi.org/10.21037/atm-20-3302 PMid:32566557 PMCid:PMC7290619
- Children and COVID-19: State-Level Data Report. Available online at: https://www.aap.org/en/pages/2019-novel-coronavirus-COVID-19-infections/children-and-COVID-19-state-level-data-report/.
- Pongpirul
WA, Mott JA, Woodring JV, Uyeki TM, MacArthur JR, Vachiraphan A,
Suwanvattana P, Uttayamakul S, Chunsuttiwat S, Chotpitayasunondh T,
Pongpirul K, Prasithsirikul W. Clinical Characteristics of Patients
Hospitalized with Coronavirus Disease, Thailand. Emerg Infect Dis. 2020
July;26(7):1580-5. https://doi.org/10.3201/eid2607.200598 PMid:32267826 PMCid:PMC7323520
- Bruminhent
J, Ruangsubvilai N, Nabhindhakara J, Ingsathit A, Kiertiburanakul S, et
al. Clinical characteristics and risk factors for coronavirus disease
2019 (COVID-19) among patients under investigation in Thailand. PLoS
ONE. 2020;15(9):e0239250. https://doi.org/10.1371/journal.pone.0239250 PMid:32931517 PMCid:PMC7491739
- Anugulruengkitt
S, Teeraananchai S, Chantasrisawad N, Promsena P, Jantarabenjakul W,
Puthanakit T. Clinical outcomes of pediatric COVID-19 in a tertiary
care center in Bangkok, Thailand. IJID Regions. 2021 Dec;1:159-62. https://doi.org/10.1016/j.ijregi.2021.11.003 PMCid:PMC8600753
- World Health Organization. Obesity and overweight. 2021. Accessed December 16, 2021. Access from https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- World Health Organization. World Health Organization Child Growth Standards. 2006. Accessed December 16, 2021.
- de
Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J.
Development of a WHO growth reference for school-aged children and
adolescents. Bull World Health Organ. 2007;85:660-7
https://doi.org/10.2471/BLT.07.043497 PMid:18026621 PMCid:PMC2636412
- Mehta
NM, Corkins MR, Lyman B, Malone A, Goday PS, Carney LN, Monczka JL,
Plogsted SW, Schwenk WF, American Society for Parenteral and Enteral
Nutrition Board of Directors. Defining pediatric malnutrition: a
paradigm shift toward etiology-related definitions. JPEN J Parenter
Enteral Nutr. 2013 Jul;37(4):460-81. https://doi.org/10.1177/0148607113479972 PMid:23528324
- Foust
AM, Phillips GS, Chu WC, Daltro P, Das KM, Garcia-Peña P, Kilborn T,
Winant AJ, Lee EY. International Expert Consensus Statement on Chest
Imaging in Pediatric COVID-19 Patient Management: Imaging Findings,
Imaging Study Reporting, and Imaging Study Recommendations. Radiol
Cardiothorac Imaging. 2020 Apr;2(2):e200214. https://doi.org/10.1148/ryct.2020200214 PMid:33778577 PMCid:PMC7233446
- Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. https://www.COVID19treatmentguidelines.nih.gov/
- Byung-Han
R, Sun IH, Su JL, Younghwa C, Kyung-Wook H, In-Gyu B, Oh-Hyun C.
Features of COVID-19 Among Children and Adolescents Without Risk
Factors Before and After the Delta Variant Outbreak in South Korea.
Pediatr Infect Dis J. 2022 January 1;41(1):e34-5. https://doi.org/10.1097/INF.0000000000003394 PMid:34773397 PMCid:PMC8658054
- Delahoy
MJ, Ujamaa D, Whitaker M, o'Halloran A, Anglin O, Burns E, Cummings C,
Holstein R, Kambhampati AK, Milucky J, Patel K, Pham H, Taylor CA, Chai
SJ, Reingold A, Alden NB, Kawasaki B, Meek J, Yousey-Hindes K, Anderson
EJ, Openo KP, Teno K, Weigel A, Kim S, Leegwater L, Bye E, Como-Sabetti
K, Ropp S, Rudin D, Muse A, Spina N, Bennett NM, Popham K, Billing LM,
Shiltz E, Sutton M, Thomas A, Schaffner W, Talbot HK, Crossland MT,
McCaffrey K, Hall AJ, Fry AM, McMorrow M, Reed C, Garg S, Havers FP,
COVID-NET Surveillance Team; COVID-NET Surveillance Team.
Hospitalizations Associated with COVID-19 Among Children and
Adolescents - COVID-NET, 14 States, March 1, 2020-August 14, 2021. MMWR
2021;70(36):1255-60. https://doi.org/10.15585/mmwr.mm7036e2 PMid:34499627 PMCid:PMC8437052
[TOP]



