Claire Coutureau1,2, Philippe Nguyen3, Maxime Hentzien2,4, Peter Joe Noujaim1, Sarah Zerbib1, Damien Jolly1,2 and Lukshe Kanagaratnam1,2.
1 Department of Research and Public Health, Reims University Hospital, 51092 Reims, France.
2 UR 3797 Vieillissement, Fragilité (VieFra), faculty of medicine, University of Reims Champagne-Ardenne, 51092 Reims, France.
3 Department of Hematology Laboratory, Reims University Hospital, 51092 Reims, France.
4 Department of Internal Medicine, Clinical Immunology and Infectious Diseases, Reims University Hospital, 51092 Reims, France.
Correspondence to: Dr
Claire Coutureau. Reims University Hospitals, Robert Debré Hospital,
Department of Research and Public Health, Rue du Général Koenig -
F51092 Reims, France. Telephone Number : +33 3 26 78 45 21. E-mail:
ccoutureau@chu-reims.fr
Published: May 1, 2022
Received: March 1, 2022
Accepted: April 10, 2022
Mediterr J Hematol Infect Dis 2022, 14(1): e2022036 DOI
10.4084/MJHID.2022.036
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Severe forms of SARS-CoV-2 infections are associated with high rates of
thromboembolic complications. Professional societies and expert
consensus reports have recommended anticoagulants for COVID-19
hospitalized patients. Our study aimed to compare the effect of
therapeutic, intermediate and prophylactic doses of heparin on 6-week
survival in patients hospitalized for COVID-19.
Methods.
The study sample is a French cohort of COVID-19 patients hospitalized
between Feb 25th and Apr 30th 2020. Patients were assigned to one of 3
anticoagulation dose groups based on the maximum dose they received for
at least three days (prophylactic, intermediate or therapeutic). The
main outcome was survival up to 42 days after hospital admission.
Multivariate Cox regression models were performed to adjust analyses
for confounding factors.
Results.
A total of 323 patients were included. The mean age of the study sample
was 71.6 ± 15 years, and 56.3% were men. Treatment with the
intermediate versus prophylactic dose of anticoagulation (HR = 0.50,
95%CI = [0.26; 0.99], p = 0.047) and with therapeutic versus
prophylactic dose (HR = 0.58 95%CI = [0.34; 0.98], p = 0.044) was
associated with a significant reduction in 6-week mortality, after
adjustment for potential confounding factors. Comparison of therapeutic
versus intermediate doses showed no significant difference in survival.
Conclusions. Our results
reported a significant positive effect of intermediate and therapeutic
doses of heparin on 6-week survival for hospitalized COVID-19 patients
compared with a prophylactic dose.
|
Introduction
The
coronavirus disease 2019 (COVID-9), caused by severe acute respiratory
syndrome coronavirus-2 (SARS-CoV-2) has been responsible for the deaths
of several million persons worldwide.[1] The range of
severity of COVID-19 is broad, and most patients who require
hospitalization suffer from respiratory failure and/or sepsis. In the
most severe cases, acute respiratory distress syndrome (ARDS),
multi-organ failure and death can ensue.[2]
The cytokine storm, an excessive systemic inflammatory response,[3] is considered one of the major causes of ARDS in COVID-19 patients.[4] It is now well established that interaction between inflammation and coagulation exists.[5]
It has been observed that severe forms of SARS-CoV-2 infection are
associated with elevated levels of D-dimers and fibrinogene with high
rates of thromboembolic complications, such as pulmonary embolism.[6]
In
a meta-analysis, Malas et al. estimated that the overall rate of venous
thromboembolism was 21%, ranging from 5% among patients hospitalized in
conventional wards to 31% in patients admitted to the intensive care
unit (ICU).[7] In addition, autopsy studies from
deceased COVID-19 patients have also shown the presence of fibrinous
thrombi in small pulmonary arterioles, confirming the important role of
coagulation abnormalities.[8]
Anticoagulant treatments are used to prevent and treat thromboembolic events.[9] Moreover, heparin also has anti-inflammatory effects[10]
that may benefit patients with severe forms of SARS-CoV-2 infection.
Professional societies and expert consensus reports have recommended
anticoagulants as part of the treatment of hospitalized COVID-19
patients.[11–13] However, vascular complications,
occurring even in patients receiving prophylactic doses of
anticoagulants, led to intensified prophylactic doses (called
intermediate doses) and therapeutic doses,[14,15]
with changing indications over time. Since April 2020, the French
Society of Thrombosis and Haemostasis recommends intensified doses
depending on the patient's state (e.g. requirement for supplemental
oxygen therapy, D-dimer levels) and characteristics.[16]
Observational
studies have reported encouraging results regarding the mortality
reduction in patients treated with anticoagulants.[17–20]
However, to the best of our knowledge, no cohort study has investigated
the effect of 3 different anticoagulant doses on 6-week mortality. In
this context, we aimed to compare the effect of therapeutic,
intermediate and prophylactic doses of anticoagulants on 6-week
survival among a cohort of patients hospitalized for COVID-19 during
the first wave of the pandemic in France.
Methods
Population.
The study sample is a prospective cohort of adult patients diagnosed
with COVID-19 and admitted to Reims University Hospital, France,
between Feb 25th and Apr 30th
2020. This study received approval from the Ethics Committee (number
3838-RM), and informed consent was obtained for each patient. The study
was registered on Clinicaltrials.gov under the number NCT04553575.
Patients
were included if they were hospitalized with a diagnosis of COVID-19,
defined as a positive reverse transcriptase-polymerase chain reaction
(RT-PCR) test or the presence of characteristic findings on a computed
tomography scan associated with a typical clinical history.
Patients
were excluded if they did not receive anticoagulant treatment or were
already hospitalized for another condition before their COVID-19
diagnosis. Patients with a length of stay shorter than five days were
also excluded to prevent immortality bias[21] since they were unlikely to receive the studied doses of treatment in such a short stay.
Anticoagulation
treatments were unfractionated heparin (UFH) or low molecular weight
heparin (LMWH). Examples of prophylactic, intermediate and therapeutic
doses of different anticoagulants are presented in Table S1 (Supplementary material).
We
assigned each patient to one of the three following anticoagulant dose
groups: (1) prophylactic, (2) intermediate or (3) therapeutic. The
patient had to receive the corresponding dose for at least three days
to be assigned to a group.
Patients who received anticoagulation
at different doses during their hospital stay were assigned to the
group corresponding to the maximum dose received. If a patient received
more than three days of anticoagulation but less than three days of
intermediate or therapeutic dose in total, they were assigned to the
prophylactic dose group since there is evidence that therapeutic levels
are not reached for most patients in this short timeframe.[22]
Similarly, patients with less than three days of anticoagulation at any
dose were excluded from the study since the treatment duration was too
short of ensuring a real anticoagulation effect.
Variables and outcome.
For each patient, we recorded socio-demographic characteristics,
co-morbidities, clinical and biological data regarding the initial
severity of COVID-19, as well as treatments and outcomes. We defined
cardiovascular disease as the presence of high blood pressure, a
history of cerebral stroke, coronary heart disease, cardiac surgery or
heart failure of New York Heart Association (NYHA) class III or IV. The
updated version of the Charlson co-morbidity index by Quan et al. was
used to measure the co-morbidity status.[23] To
evaluate the severity of infection at admission, we measured and
recorded the early warning score (EWS), a modified version of the
National Early Warning Score 2 with age ≥ 65 years old as an additional
parameter.[24] Each patient was classified as low
(EWS ≤ 4), medium (EWS > 4 and ≤ 6) or high risk (EWS > 6) of
acute deterioration. In addition, we noted whether the patient received
systemic corticosteroid therapy, as it is a recommended treatment for
severe forms of COVID-19.[25] Since Mar 27th
2020, the treatment protocol in our hospital has included
corticosteroids for all patients with COVID-19 pneumonia, at a dose of
1 mg/kg equivalent per day of prednisone or methylprednisolone for 3 to
4 weeks, depending on the severity of the disease.
Information on
potential complications of anticoagulation was extracted from the
program for the medicalization of information systems (PMSI) of our
hospital. This database contains information on diagnoses,
co-morbidities, and complications for each hospital stay.
The main outcome of our analysis was survival up to 6 weeks (42 days) after hospital admission.
Statistical analysis.
Quantitative variables are described as mean ± standard deviation (SD)
or median and interquartile range (IQR), and qualitative variables as
numbers (percentage). Comparisons by anticoagulant dose group were
performed using the ANOVA test for quantitative variables and the Chi2 test for qualitative variables. In addition, validity conditions for these tests were verified.
We
constructed survival curves using the Kaplan Meier method to compare
the effect of the 3 anticoagulant doses on 6-week survival. Curves were
compared using the log-rank test.
Multivariate Cox regression
analysis was performed to adjust for potential confounders, which were
identified in the bivariate analyses or recognized confounding or
prognostic factors from the literature. The choice of variables also
took into account the risk of multicollinearity. Thus, the following
adjustment variables were chosen for our analyses: socio-demographic
characteristics (age and sex), co-morbidities evaluated with the
Charlson co-morbidity index, anticoagulant as part of regular
treatment, the severity of COVID-19 (O2 therapy needed at hospital
arrival and hospitalization in ICU) and treatment of SARS-CoV-2
infection with systemic corticosteroids. Results are expressed as
hazard ratios (HR) with a 95% confidence interval (95% CI).
All
analyses were performed using R software, version 4.0.5 (R Core Team
(2019). R Foundation for Statistical Computing, Vienna, Austria). A
p-value <0.05 was considered statistically significant.
Results
A total of 479 patients were included in the cohort between Feb 25th and Apr 30th. Thirty-seven
patients were not treated with anticoagulants, 111 patients were
hospitalized for other reasons before their COVID-19 diagnosis or were
hospitalized for less than five days, and eight patients did not
receive at least three days of any anticoagulant dose. Therefore, 323
patients were included in our analysis: 34.1% in the prophylactic
group, 25.4% in the intermediate group and 40.6% in the therapeutic
group (Figure 1).
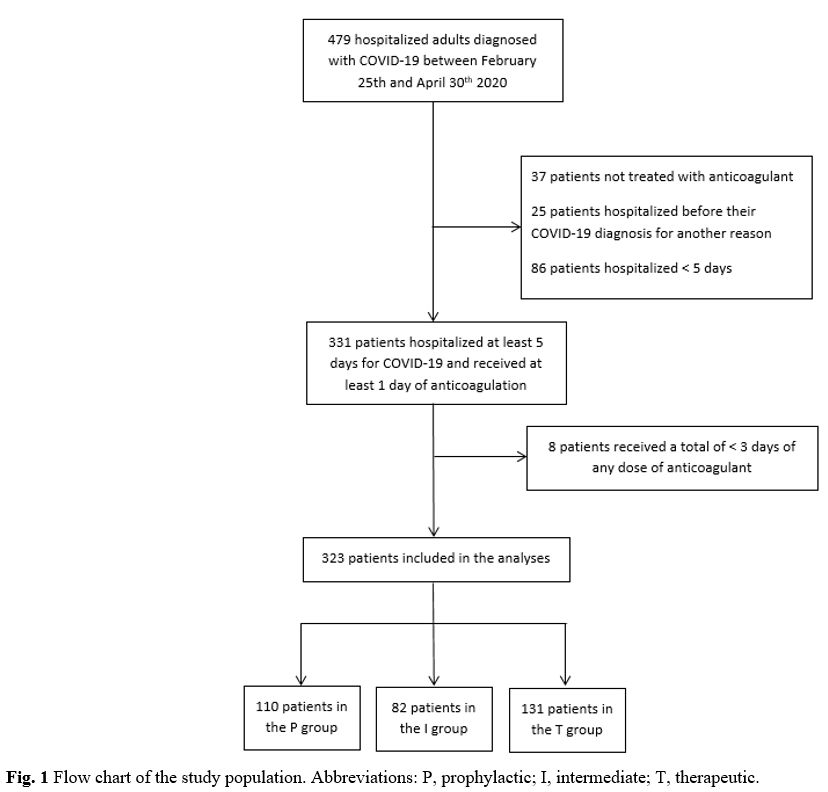 |
Figure
1. Flow chart of the study population. Abbreviations: P, prophylactic; I, intermediate; T, therapeutic. |
Overall,
the mean age of the population was 71.6 ± 15 years, and 56.3% were men.
Fifty-nine (18.3%) patients received anticoagulants, and 114 (35.3%)
had an antiplatelet agent in their regular treatment before admission.
Information
on baseline D-dimer levels was missing for 91 patients. Among those
with available data, the median D-dimer level was 0.9 mg/L (IQR = [0.7;
2.0]) in the prophylactic group, 0.8 mg/L (IQR = [0.4; 1.1]) in the
intermediate group and 1.6 mg/L (IQR = [0.6; 5.7]) in the therapeutic
group. Nineteen patients (5.9%) suffered pulmonary embolism during
their hospital stay. The characteristics of the three groups are
presented in Table 1.
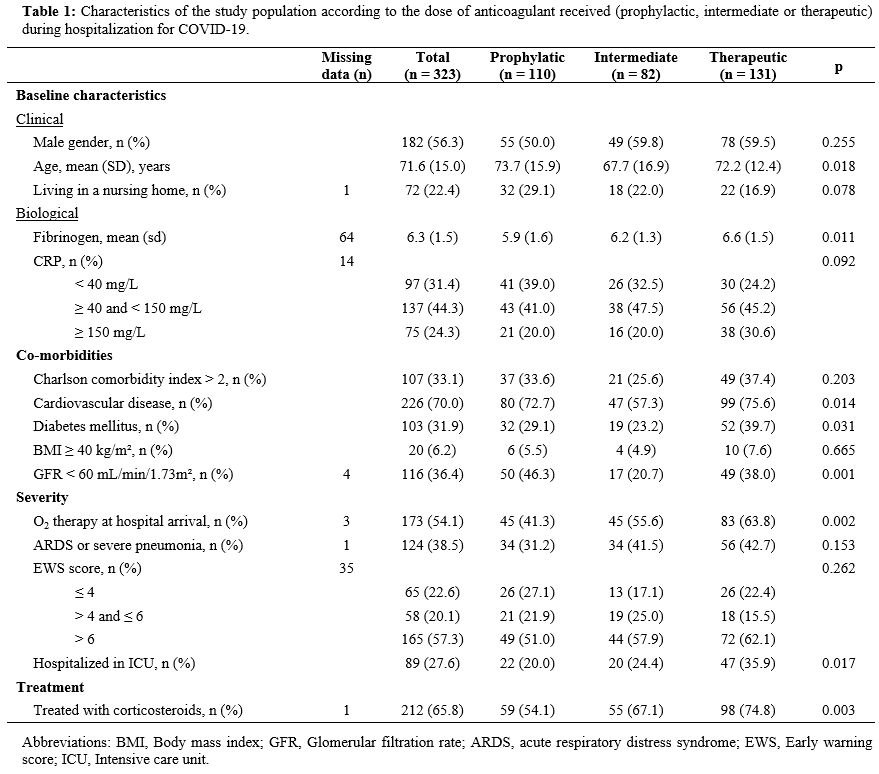 |
Table
1. Characteristics of the study population according to the dose of
anticoagulant received (prophylactic, intermediate or therapeutic)
during hospitalization for COVID-19. |
Independently
of the treatment duration, and thus of the assigned group, we also
explored the different anticoagulant doses received by each patient in
chronological order. Nearly two thirds (209) of included patients
received only one type anticoagulant regimen, 79 (24.5%) received two
regimens, 15 (4.6%) received three regimens, and 20 (6.2%) had more
complicated treatment regiments comprising several dose changes that
did not fit with any other regimen (Table 2).
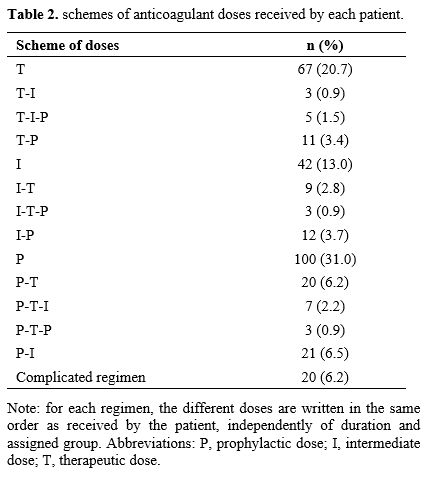 |
Table 2. Schemes of anticoagulant doses received by each patient. |
At
6 weeks post-admission, 31 (28.2%) patients in the prophylactic group,
12 (14.6%) in the intermediate group and 31 (23.7%) in the therapeutic
group had died. The Kaplan-Meier curves depicting survival across the
three groups are shown in Figure 2. There was no significant difference between groups (p = 0.066) (Figure 2).
The results of the bivariate analysis investigating the impact of
patient characteristics on 6-week survival are presented in Table S2 (Supplementary material).
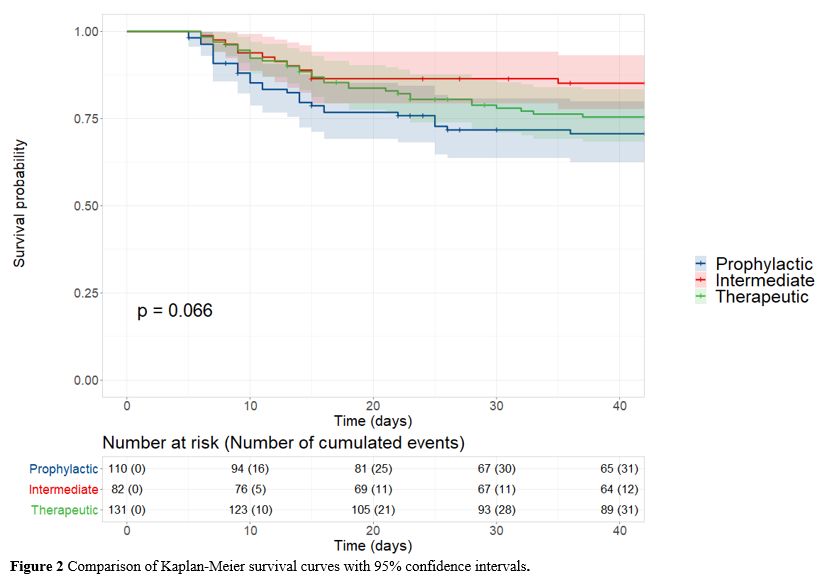 |
Figure 2. Comparison of Kaplan-Meier survival curves with 95% confidence intervals. |
A
total of 319 patients were included in the multivariate analyses; 4
were excluded because of missing data on one of the adjustment
variables.
Treatment with intermediate dose of anticoagulation
was associated with a significant reduction in the risk of 6-week
mortality, compared to prophylactic dose (HR = 0.50, 95%CI = [0.26;
0.99], p = 0.047). Similarly, therapeutic dose of heparin, compared to
prophylactic dose, was associated with a significant reduction in the
risk of mortality (HR = 0.58, 95%CI = [0.34; 0.98], p = 0.044). When
comparing the therapeutic versus the intermediate dose, no significant
difference was found (HR = 1.14, 95%CI = [0.57; 2.29], p = 0.704).
Four
patients, 1 in the prophylactic group, 1 in the intermediate group and
2 in the therapeutic group, presented a minor complication of
anticoagulant treatment (i.e. asymptomatic overdose, oral or nasal
bleeding) and 3 patients of the therapeutic group presented an
intramuscular hematoma.
Discussion
The
results of our study highlight the positive impact of intermediate and
therapeutic doses of heparin on 6-week survival among hospitalized
COVID-19 patients, as compared with prophylactic dose of anticoagulant
therapy.
It has been well established that severe SARS-CoV-2
infection is associated with a high prevalence of pulmonary thrombosis
and macro-vascular thromboembolic events. Besides its anticoagulant
activity, heparin also has anti-inflammatory and immunomodulatory
properties that may explain its beneficial effects on the outcomes of
COVID-19 patients.[26]
Our results are consistent with other observational studies[22,27]
reporting that patients receiving therapeutic doses had a higher
survival probability than those receiving only prophylactic doses. A
further study by Meizlish et al.[28] reported a
significantly lower cumulative incidence of in-hospital death for
patients receiving intermediate doses of anticoagulant compared to
prophylactic doses.
Due to the observational design of our
study, our results remain subject to hidden confounding factors. The
association between anticoagulation and survival in SARS-CoV-2 infected
patients warrants evaluation in interventional studies. Randomized
controlled trials (RCTs) in hospitalized COVID-19 patients have found
contrasting results. Two RCTs testing the effects of full dose
anticoagulant therapy showed no beneficial effect on clinical outcomes
compared to prophylactic doses.[29,30] One trial had to stop enrolment of critically ill patients prematurely due to futility and safety concerns.[31]
Nevertheless, results in non-critically ill patients showed that
therapeutic doses of heparin increased the probability of survival to
hospital discharge, with reduced use of cardiovascular and respiratory
organ support compared to prophylactic doses.[32]
Another recent study concluded that therapeutic doses of heparin
reduced major thromboembolism and death among in-patients with elevated
D-dimers, but no treatment effect was seen in ICU patients.[33]
Regarding the comparison between intermediate and prophylactic doses of
anticoagulants, two RCTs, including only patients with severe COVID-19,
found no significant difference.[34,35] There are still many on-going RCTs that will provide further evidence to determine the optimal anticoagulation doses.[36]
To
the best of our knowledge, this study is the first prospective,
observational study to compare the effect of 3 types of anticoagulant
doses on 6-week mortality. Our cohort included adult patients
hospitalized with COVID-19 independently of the severity of the
disease, the need for mechanical ventilation and the presence of
coagulation disorders. Moreover, patients were followed for 6 weeks,
whereas most published observational studies and trials focused only on
in-hospital or short-term mortality.
The precision of the data
collected enabled us to study the different dose regimens received by
the patients. Accordingly, we noted that more than a third of the study
population received at least two types of regimen (from among
prophylactic, intermediate and therapeutic doses). This could be
explained by the lack of consensus on anticoagulant treatment before
April 2020 in France, but above all, by the constant adaptation of the
treatment doses by the clinicians in response to the patient's state
and any potential complications of the disease.
Our results also
provide interesting findings as regards the comparison of therapeutic
versus intermediate doses. Indeed, we found no significant advantage of
therapeutic anticoagulation in terms of survival. While therapeutic and
intermediate doses were both found to be superior to prophylactic
doses, the use of intermediate anticoagulation may have the advantage
of reducing the risk of bleeding complications. Indeed, it has
previously been shown that therapeutic doses of anticoagulant increase
the risk of bleeding in COVID-19 patients.[37]
Our
study presents several limitations. Firstly, we did not have precise
data on the start and end dates of each dose for each patient. As a
result, we were not able to perform a time-dependent analysis, which
would have been a more appropriate approach to correctly classify the
immortal time in pharmacoepidemiology.[38] We
partially took this risk of bias into account by excluding patients
with a length of stay less than 5 days, because there was a high risk
that these patients were not hospitalized long enough to receive
intermediate or therapeutic doses of anticoagulant. Secondly, the fact
that patients with less than 3 days of intermediate or therapeutic
doses were assigned to the prophylactic group could induce
classification bias. Since the reason for the short duration of
treatment was unknown, we may have overlooked potential complications
due to higher doses of anticoagulation. However, as highlighted by
Ionescu et al., assigning patients with less than 3 days of treatment
in the intermediate or therapeutic group could induce other
biases, as therapeutic levels are often not reached in such a short
timeframe.[27] Thirdly, our study may suffer from a
lack of statistical power due to the small sample size. Finally, the
external validity of our results might be affected by the fact that
this was a single-centre study.
Conclusions
Our
study reports a significant positive effect of intermediate and
therapeutic doses of heparin, compared to prophylactic doses, on 6-week
survival in hospitalized COVID-19 patients. Results of on-going RCTs
will be helpful to determine the optimal anticoagulation doses.
Acknowledgements
Reims
COVID Study Group : Ailsa ROBBINS, Kévin DIDIER, Pauline ORQUEVAUX,
Violaine NOEL, Paola MARIANETTI, Juliette ROMARU, Dorothée LAMBERT,
Jean Luc BERGER, Sandra DURY, Maxime DEWOLF, Jean Hugues SALMON, Jérôme
COSTA, Julia SIMON, Natacha NOEL, Sara BARRAUD, Marion BARROIS, Hédia
BRIXI, Quentin LAURENT-BADR, Manuelle VIGUIER, Clélia VANHAECKE,
Laurence GUSDORF, Isabelle QUATRESOUS, Aline CARSIN-VU, Véronique
BRODARD, Antoine HUGUENIN, Morgane BONNET, Aurore THIERRY.
We extend our sincere thanks to Fiona CAULFIELD for her assistance in editing this manuscript.
References
- WHO Coronavirus (COVID-19) Dashboard [Internet]. [cited 2021 Dec 1]. Available from: https://covid19.who.int
- Zhou
F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan
L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical
course and risk factors for mortality of adult inpatients with COVID-19
in Wuhan, China: a retrospective cohort study. The Lancet. 2020
Mar;395(10229):1054-62. https://doi.org/10.1016/S0140-6736(20)30566-3
- Fajgenbaum DC, June CH. Cytokine Storm. N Engl J Med. 2020 Dec 3;383(23):2255-73. https://doi.org/10.1056/NEJMra2026131 PMid:33264547 PMCid:PMC7727315
- Ye Q, Wang B, Mao J. The pathogenesis and treatment of the 'Cytokine Storm' in COVID-19. J Infect. 2020 Jun;80(6):607-13. https://doi.org/10.1016/j.jinf.2020.03.037 PMid:32283152 PMCid:PMC7194613
- Connors JM, Levy JH. Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost. 2020;18(7):1559-61. https://doi.org/10.1111/jth.14849 PMid:32302453
- Semeraro
N., Colucci M.The prothrombotic state associated with SARS-CoV-2
infection: pathophysiological aspects. Mediterr J Hematol Infect Dis
2021, 13(1): e2021045 https://doi.org/10.4084/MJHID.2021.045 PMid:34276914 PMCid:PMC8265369
- Malas
MB, Naazie IN, Elsayed N, Mathlouthi A, Marmor R, Clary B.
Thromboembolism risk of COVID-19 is high and associated with a higher
risk of mortality: A systematic review and meta-analysis.
EClinicalMedicine. 2020 Dec;29:100639. https://doi.org/10.1016/j.eclinm.2020.100639 PMid:33251499 PMCid:PMC7679115
- Dolhnikoff
M, Duarte-Neto AN, de Almeida Monteiro RA, da Silva LFF, de Oliveira
EP, Saldiva PHN, Mauad T, Negri EM. Pathological evidence of pulmonary
thrombotic phenomena in severe COVID-19. J Thromb Haemost JTH. 2020
Jun;18(6):1517-9. https://doi.org/10.1111/jth.14844 PMid:32294295 PMCid:PMC7262093
- Amaral
F, Baptista-Silva J, Nakano L, Flumignan R. Pharmacological
interventions for preventing venous thromboembolism in patients
undergoing bariatric surgery. Cochrane Database Syst Rev [Internet].
2020;(7). https://doi.org/10.1002/14651858.CD013683 PMCid:PMC7386840
- Young E. The anti-inflammatory effects of heparin and related compounds. Thromb Res. 2008;122(6):743-52. https://doi.org/10.1016/j.thromres.2006.10.026 PMid:17727922
- Rico-Mesa
JS, Rosas D, Ahmadian-Tehrani A, White A, Anderson AS, Chilton R. The
Role of Anticoagulation in COVID-19-Induced Hypercoagulability. Curr
Cardiol Rep. 2020 Jun 17;22(7):53-53. https://doi.org/10.1007/s11886-020-01328-8 PMid:32556892 PMCid:PMC7298694
- Task
Force for the management of COVID-19 of the European Society of
Cardiology. ESC guidance for the diagnosis and management of
cardiovascular disease during the COVID-19 pandemic: part 2-care
pathways, treatment, and follow-up. Eur Heart J. 2021 Nov 16;ehab697.
- Castelli
R, Gidaro A. Abnormal Hemostatic Parameters and Risk of Thromboembolism
Among Patients With COVID-19 Infection. J Hematol. 2020 Apr;9(1-2):1-4.
https://doi.org/10.14740/jh636 PMid:32362977 PMCid:PMC7188381
- Pesavento
R, Ceccato D, Pasquetto G, Monticelli J, Leone L, Frigo A, Gorgi D,
Postal A, Marchese GM, Cipriani A, Saller A, Sarais C, Criveller P,
Gemelli M, Capone F, Fioretto P, Pagano C, Rossato M, Avogaro A,
Simioni P, Prandoni P, Vettor R. The hazard of (sub)therapeutic doses
of anticoagulants in non-critically ill patients with Covid-19: The
Padua province experience. J Thromb Haemost JTH. 2020
Oct;18(10):2629-35. https://doi.org/10.1111/jth.15022 PMid:32692874 PMCid:PMC7404507
- Dobesh
PP, Trujillo TC. Coagulopathy, Venous Thromboembolism, and
Anticoagulation in Patients with COVID-19. Pharmacotherapy. 2020/11/03
ed. 2020 Nov;40(11):1130-51. https://doi.org/10.1002/phar.2465 PMid:33006163 PMCid:PMC7537066
- Susen
S, Tacquard CA, Godon A, Mansour A, Garrigue D, Nguyen P, Godier A,
Testa S, Levy JH, Albaladejo P, Gruel Y, GIHP and GFHT. Prevention of
thrombotic risk in hospitalized patients with COVID-19 and hemostasis
monitoring. Crit Care Lond Engl. 2020 Jun 19;24(1):364. https://doi.org/10.1186/s13054-020-03000-7 PMid:32560658 PMCid:PMC7303590
- Ayerbe
L, Risco C, Ayis S. The association between treatment with heparin and
survival in patients with Covid-19. J Thromb Thrombolysis.
2020;50(2):298-301. https://doi.org/10.1007/s11239-020-02162-z PMid:32476080 PMCid:PMC7261349
- Nadkarni
GN, Lala A, Bagiella E, Chang HL, Moreno PR, Pujadas E, Arvind V, Bose
S, Charney AW, Chen MD, Cordon-Cardo C, Dunn AS, Farkouh ME, Glicksberg
BS, Kia A, Kohli-Seth R, Levin MA, Timsina P, Zhao S, Fayad ZA, Fuster
V. Anticoagulation, Bleeding, Mortality, and Pathology in Hospitalized
Patients With COVID-19. J Am Coll Cardiol. 2020 Oct 20;76(16):1815-26. https://doi.org/10.1016/j.jacc.2020.08.041 PMid:32860872 PMCid:PMC7449655
- Paranjpe
I, Fuster V, Lala A, Russak AJ, Glicksberg BS, Levin MA, Charney AW,
Narula J, Fayad ZA, Bagiella E, Zhao S, Nadkarni GN. Association of
Treatment Dose Anticoagulation With In-Hospital Survival Among
Hospitalized Patients With COVID-19. J Am Coll Cardiol. 2020 Jul
7;76(1):122-4. https://doi.org/10.1016/j.jacc.2020.05.001 PMid:32387623 PMCid:PMC7202841
- Rentsch
CT, Beckman JA, Tomlinson L, Gellad WF, Alcorn C, Kidwai-Khan F,
Skanderson M, Brittain E, King JT, Ho Y-L, Eden S, Kundu S, Lann MF,
Greevy RA, Ho PM, Heidenreich PA, Jacobson DA, Douglas IJ, Tate JP,
Evans SJW, Atkins D, Justice AC, Freiberg MS. Early initiation of
prophylactic anticoagulation for prevention of coronavirus disease 2019
mortality in patients admitted to hospital in the United States: cohort
study. BMJ. 2021 Feb 11;372:n311. https://doi.org/10.1136/bmj.n311 PMid:33574135 PMCid:PMC7876672
- Lévesque
LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in
cohort studies: example using statins for preventing progression of
diabetes. BMJ. 2010 Mar 12;340:b5087. https://doi.org/10.1136/bmj.b5087
PMid:20228141
- Trinh MA, Chang DR,
Govindarajulu US, Kane E, Fuster V, Kohli-Seth R, Ahmed S, Levin MA,
Chen MD. Therapeutic Anticoagulation Is Associated with Decreased
Mortality in Mechanically Ventilated COVID-19 Patients. medRxiv. 2020
Jun 3;2020.05.30.20117929. https://doi.org/10.1101/2020.05.30.20117929
- Holler
JG, Eriksson R, Jensen TØ, van Wijhe M, Fischer TK, Søgaard OS,
Israelsen SB, Mohey R, Fabricius T, Jøhnk F, Wiese L, Johnsen S, Søborg
C, Nielsen H, Kirk O, Madsen BL, Harboe ZB. First wave of COVID-19
hospital admissions in Denmark: a Nationwide population-based cohort
study. BMC Infect Dis. 2021 Jan 9;21(1):39. https://doi.org/10.1186/s12879-020-05717-w PMid:33421989 PMCid:PMC7794638
- Liao
X, Wang B, Kang Y. Novel coronavirus infection during the 2019-2020
epidemic: preparing intensive care units-the experience in Sichuan
Province, China. Intensive Care Med. 2020;46(2):357-60. https://doi.org/10.1007/s00134-020-05954-2 PMid:32025779 PMCid:PMC7042184
- World
Health Organization. Corticosteroids for COVID-19: living guidance, 2
September 2020 [Internet]. Geneva: World Health Organization; 2020.
Available from: https://apps.who.int/iris/handle/10665/334125
- Gozzo
L, Viale P, Longo L, Vitale DC, Drago F. The Potential Role of Heparin
in Patients With COVID-19: Beyond the Anticoagulant Effect. A Review.
Front Pharmacol. 2020 Aug 21;11:1307.
https://doi.org/10.3389/fphar.2020.01307 PMid:32973526 PMCid:PMC7472559
- Ionescu
F, Jaiyesimi I, Petrescu I, Lawler PR, Castillo E, Munoz-Maldonado Y,
Imam Z, Narasimhan M, Abbas AE, Konde A, Nair GB. Association of
anticoagulation dose and survival in hospitalized COVID-19 patients: A
retrospective propensity score-weighted analysis. Eur J Haematol. 2021
Feb;106(2):165-74. https://doi.org/10.1111/ejh.13533 PMid:33043484 PMCid:PMC7675265
- Meizlish
ML, Goshua G, Liu Y, Fine R, Amin K, Chang E, DeFilippo N, Keating C,
Liu Y, Mankbadi M, McManus D, Wang SY, Price C, Bona RD, Ochoa Chaar
CI, Chun HJ, Pine AB, Rinder HM, Siner JM, Neuberg DS, Owusu KA, Lee
AI. Intermediate-dose anticoagulation, aspirin, and in-hospital
mortality in COVID-19: A propensity score-matched analysis. Am J
Hematol. 2021 Apr 1;96(4):471-9. https://doi.org/10.1002/ajh.26102 PMid:33476420 PMCid:PMC8013588
- Lopes
RD, Silva PGM de B e, Furtado RHM, Macedo AVS, Bronhara B, Damiani LP,
Barbosa LM, Morata J de A, Ramacciotti E, Martins P de A, Oliveira AL
de, Nunes VS, Ritt LEF, Rocha AT, Tramujas L, Santos SV, Diaz DRA,
Viana LS, Melro LMG, Chaud MS de A, Figueiredo EL, Neuenschwander FC,
Dracoulakis MDA, Lima RGSD, Dantas VC de S, Fernandes ACS, Gebara OCE,
Hernandes ME, Queiroz DAR, Veiga VC, Canesin MF, Faria LM de,
Feitosa-Filho GS, Gazzana MB, Liporace IL, Twardowsky A de O, Maia LN,
Machado FR, Soeiro A de M, Conceição-Souza GE, Armaganijan L, Guimarães
PO, Rosa RG, Azevedo LCP, Alexander JH, Avezum A, Cavalcanti AB,
Berwanger O. Therapeutic versus prophylactic anticoagulation for
patients admitted to hospital with COVID-19 and elevated D-dimer
concentration (ACTION): an open-label, multicentre, randomised,
controlled trial. The Lancet. 2021 Jun 12;397(10291):2253-63. https://doi.org/10.1016/S0140-6736(21)01203-4
- Sholzberg
M, Tang GH, Rahhal H, AlHamzah M, Kreuziger LB, Áinle FN, Alomran F,
Alayed K, Alsheef M, AlSumait F, Pompilio CE, Sperlich C, Tangri S,
Tang T, Jaksa P, Suryanarayan D, Almarshoodi M, Castellucci LA, James
PD, Lillicrap D, Carrier M, Beckett A, Colovos C, Jayakar J, Arsenault
M-P, Wu C, Doyon K, Andreou ER, Dounaevskaia V, Tseng EK, Lim G,
Fralick M, Middeldorp S, Lee AYY, Zuo F, da Costa BR, Thorpe KE, Negri
EM, Cushman M, Jüni P, RAPID trial investigators. Effectiveness of
therapeutic heparin versus prophylactic
heparin on death, mechanical ventilation, or intensive care unit
admission in moderately ill patients with covid-19 admitted to
hospital: RAPID randomized clinical trial. BMJ. 2021 Oct
14;375:n2400. https://doi.org/10.1136/bmj.n2400 PMid:34649864 PMCid:PMC8515466
- REMAP-CAP
Investigators, ACTIV-4a Investigators, ATTACC Investigators, Goligher
EC, Bradbury CA, McVerry BJ, Lawler PR, Berger JS, Gong MN, Carrier M,
Reynolds HR, Kumar A, Turgeon AF, Kornblith LZ, Kahn SR, Marshall JC,
Kim KS, Houston BL, Derde LPG, Cushman M, Tritschler T, Angus DC, Godoy
LC, McQuilten Z, Kirwan B-A, Farkouh ME, Brooks MM, Lewis RJ, Berry LR,
Lorenzi E, Gordon AC, Ahuja T, Al-Beidh F, Annane D, Arabi YM, Aryal D,
Baumann Kreuziger L, Beane A, Bhimani Z, Bihari S, Billett HH, Bond L,
Bonten M, Brunkhorst F, Buxton M, Buzgau A, Castellucci LA, Chekuri S,
Chen J-T, Cheng AC, Chkhikvadze T, Coiffard B, Contreras A, Costantini
TW, de Brouwer S, Detry MA, Duggal A, Džavík V, Effron MB, Eng HF,
Escobedo J, Estcourt LJ, Everett BM, Fergusson DA, Fitzgerald M, Fowler
RA, Froess JD, Fu Z, Galanaud JP, Galen BT, Gandotra S, Girard TD,
Goodman AL, Goossens H, Green C, Greenstein YY, Gross PL, Haniffa R,
Hegde SM, Hendrickson CM, Higgins AM, Hindenburg AA, Hope AA, Horowitz
JM, Horvat CM, Huang DT, Hudock K, Hunt BJ, Husain M, Hyzy RC, Jacobson
JR, Jayakumar D, Keller NM, Khan A, Kim Y, Kindzelski A, King AJ,
Knudson MM, Kornblith AE, Kutcher ME, Laffan MA, Lamontagne F, Le Gal
G, Leeper CM, Leifer ES, Lim G, Gallego Lima F, Linstrum K, Litton E,
Lopez-Sendon J, Lother SA, Marten N, Saud Marinez A, Martinez M, Mateos
Garcia E, Mavromichalis S, McAuley DF, McDonald EG, McGlothlin A,
McGuinness SP, Middeldorp S, Montgomery SK, Mouncey PR, Murthy S, Nair
GB, Nair R, Nichol AD, Nicolau JC, Nunez-Garcia B, Park JJ, Park PK,
Parke RL, Parker JC, Parnia S, Paul JD, Pompilio M, Quigley JG,
Rosenson RS, Rost NS, Rowan K, Santos FO, Santos M, Santos MO,
Satterwhite L, Saunders CT, Schreiber J, Schutgens REG, Seymour CW,
Siegal DM, Silva DG, Singhal AB, Slutsky AS, Solvason D, Stanworth SJ,
Turner AM, van Bentum-Puijk W, van de Veerdonk FL, van Diepen S,
Vazquez-Grande G, Wahid L, Wareham V, Widmer RJ, Wilson JG, Yuriditsky
E, Zhong Y, Berry SM, McArthur CJ, Neal MD, Hochman JS, Webb SA,
Zarychanski R. Therapeutic Anticoagulation with Heparin in Critically
Ill Patients with Covid-19. N Engl J Med. 2021 Aug 26;385(9):777-89.
- ATTACC
Investigators, ACTIV-4a Investigators, REMAP-CAP Investigators, Lawler
PR, Goligher EC, Berger JS, Neal MD, McVerry BJ, Nicolau JC, Gong MN,
Carrier M, Rosenson RS, Reynolds HR, Turgeon AF, Escobedo J, Huang DT,
Bradbury CA, Houston BL, Kornblith LZ, Kumar A, Kahn SR, Cushman M,
McQuilten Z, Slutsky AS, Kim KS, Gordon AC, Kirwan B-A, Brooks MM,
Higgins AM, Lewis RJ, Lorenzi E, Berry SM, Berry LR, Aday AW, Al-Beidh
F, Annane D, Arabi YM, Aryal D, Baumann Kreuziger L, Beane A, Bhimani
Z, Bihari S, Billett HH, Bond L, Bonten M, Brunkhorst F, Buxton M,
Buzgau A, Castellucci LA, Chekuri S, Chen J-T, Cheng AC, Chkhikvadze T,
Coiffard B, Costantini TW, de Brouwer S, Derde LPG, Detry MA, Duggal A,
Džavík V, Effron MB, Estcourt LJ, Everett BM, Fergusson DA, Fitzgerald
M, Fowler RA, Galanaud JP, Galen BT, Gandotra S, García-Madrona S,
Girard TD, Godoy LC, Goodman AL, Goossens H, Green C, Greenstein YY,
Gross PL, Hamburg NM, Haniffa R, Hanna G, Hanna N, Hegde SM,
Hendrickson CM, Hite RD, Hindenburg AA, Hope AA, Horowitz JM, Horvat
CM, Hudock K, Hunt BJ, Husain M, Hyzy RC, Iyer VN, Jacobson JR,
Jayakumar D, Keller NM, Khan A, Kim Y, Kindzelski AL, King AJ, Knudson
MM, Kornblith AE, Krishnan V, Kutcher ME, Laffan MA, Lamontagne F, Le
Gal G, Leeper CM, Leifer ES, Lim G, Lima FG, Linstrum K, Litton E,
Lopez-Sendon J, Lopez-Sendon Moreno JL, Lother SA, Malhotra S, Marcos
M, Saud Marinez A, Marshall JC, Marten N, Matthay MA, McAuley DF,
McDonald EG, McGlothlin A, McGuinness SP, Middeldorp S, Montgomery SK,
Moore SC, Morillo Guerrero R, Mouncey PR, Murthy S, Nair GB, Nair R,
Nichol AD, Nunez-Garcia B, Pandey A, Park PK, Parke RL, Parker JC,
Parnia S, Paul JD, Pérez González YS, Pompilio M, Prekker ME, Quigley
JG, Rost NS, Rowan K, Santos FO, Santos M, Olombrada Santos M,
Satterwhite L, Saunders CT, Schutgens REG, Seymour CW, Siegal DM, Silva
DG, Shankar-Hari M, Sheehan JP, Singhal AB, Solvason D, Stanworth SJ,
Tritschler T, Turner AM, van Bentum-Puijk W, van de Veerdonk FL, van
Diepen S, Vazquez-Grande G, Wahid L, Wareham V, Wells BJ, Widmer RJ,
Wilson JG, Yuriditsky E, Zampieri FG, Angus DC, McArthur CJ, Webb SA,
Farkouh ME, Hochman JS, Zarychanski R. Therapeutic Anticoagulation with
Heparin in Noncritically Ill Patients with Covid-19. N Engl J Med. 2021
Aug 26;385(9):790-802.
- Spyropoulos AC,
Goldin M, Giannis D, Diab W, Wang J, Khanijo S, Mignatti A, Gianos E,
Cohen M, Sharifova G, Lund JM, Tafur A, Lewis PA, Cohoon KP, Rahman H,
Sison CP, Lesser ML, Ochani K, Agrawal N, Hsia J, Anderson VE, Bonaca
M, Halperin JL, Weitz JI, HEP-COVID Investigators. Efficacy and Safety
of Therapeutic-Dose Heparin vs Standard Prophylactic or
Intermediate-Dose Heparins for Thromboprophylaxis in High-risk
Hospitalized Patients With COVID-19: The HEP-COVID Randomized Clinical
Trial. JAMA Intern Med. 2021 Oct 7;
- Perepu
US, Chambers I, Wahab A, Ten Eyck P, Wu C, Dayal S, Sutamtewagul G,
Bailey SR, Rosenstein LJ, Lentz SR. Standard prophylactic versus
intermediate dose enoxaparin in adults with severe COVID-19: A
multi-center, open-label, randomized controlled trial. J Thromb Haemost
JTH. 2021 Sep;19(9):2225-34. https://doi.org/10.1111/jth.15450 PMid:34236768 PMCid:PMC8420176
- INSPIRATION
Investigators, Sadeghipour P, Talasaz AH, Rashidi F, Sharif-Kashani B,
Beigmohammadi MT, Farrokhpour M, Sezavar SH, Payandemehr P, Dabbagh A,
Moghadam KG, Jamalkhani S, Khalili H, Yadollahzadeh M, Riahi T,
Rezaeifar P, Tahamtan O, Matin S, Abedini A, Lookzadeh S, Rahmani H,
Zoghi E, Mohammadi K, Sadeghipour P, Abri H, Tabrizi S, Mousavian SM,
Shahmirzaei S, Bakhshandeh H, Amin A, Rafiee F, Baghizadeh E, Mohebbi
B, Parhizgar SE, Aliannejad R, Eslami V, Kashefizadeh A, Kakavand H,
Hosseini SH, Shafaghi S, Ghazi SF, Najafi A, Jimenez D, Gupta A,
Madhavan MV, Sethi SS, Parikh SA, Monreal M, Hadavand N, Hajighasemi A,
Maleki M, Sadeghian S, Piazza G, Kirtane AJ, Van Tassell BW, Dobesh PP,
Stone GW, Lip GYH, Krumholz HM, Goldhaber SZ, Bikdeli B. Effect of
Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation on
Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or
Mortality Among Patients With COVID-19 Admitted to the Intensive Care
Unit: The INSPIRATION Randomized Clinica Trial. JAMA. 2021 Apr
27;325(16):1620-30. https://doi.org/10.1001/jama.2021.4152 PMid:33734299 PMCid:PMC7974835
- Talasaz
AH, Sadeghipour P, Kakavand H, Aghakouchakzadeh M, Kordzadeh-Kermani E,
Van Tassell BW, Gheymati A, Ariannejad H, Hosseini SH, Jamalkhani S,
Sholzberg M, Monreal M, Jimenez D, Piazza G, Parikh SA, Kirtane AJ,
Eikelboom JW, Connors JM, Hunt BJ, Konstantinides SV, Cushman M, Weitz
JI, Stone GW, Krumholz HM, Lip GYH, Goldhaber SZ, Bikdeli B. Recent
Randomized Trials of Antithrombotic Therapy for Patients With COVID-19.
J Am Coll Cardiol. 2021 Apr 20;77(15):1903-21. https://doi.org/10.1016/j.jacc.2021.02.035 PMid:33741176 PMCid:PMC7963001
- Musoke
N, Lo KB, Albano J, Peterson E, Bhargav R, Gul F, DeJoy R, Salacup G,
Pelayo J, Tipparaju P, Azmaiparashvili Z, Patarroyo-Aponte G,
Rangaswami J. Anticoagulation and bleeding risk in patients with
COVID-19. Thromb Res. 2020 Dec;196:227-30. https://doi.org/10.1016/j.thromres.2020.08.035 PMid:32916565 PMCid:PMC7444469
- Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. 2008 Feb 15;167(4):492-9. https://doi.org/10.1093/aje/kwm324 PMid:18056625
Supplementary data
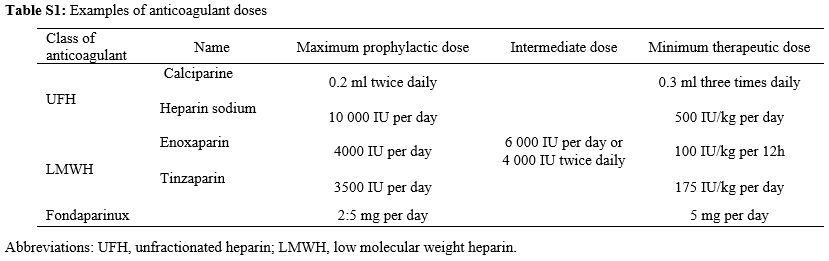 |
Table S1. Examples of anticoagulant doses |
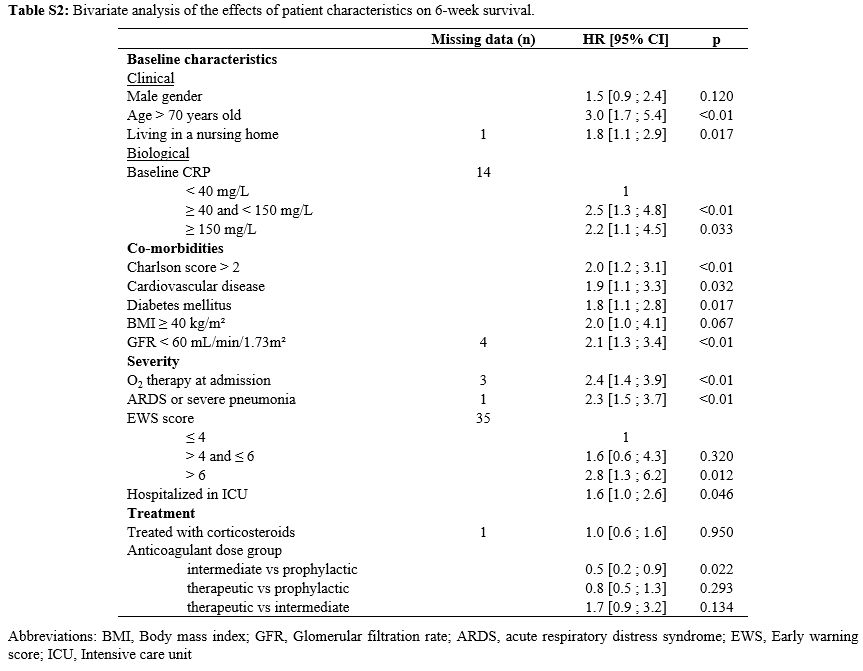 |
Table S2. Bivariate analysis of the effects of patient characteristics on 6-week survival. |
[TOP]





