Based on known biological functions of the Breg in health and autoimmune diseases, we hypothesized that the frequency of these cells might be impaired in Hodgkin lymphoma (HL), a clonal hematological disorder characterized by the presence of Hodgkin and Reed-Sternberg (HRS) cells, the neoplastic counterpart of germinal center B cells, surrounded by an inflammatory infiltrate.[7] In this retrospective case series of 24 consecutive HL patients, we investigated perturbations of circulating Breg and other immune cell subsets to provide additional evidence of the role of B cells in HL pathogenesis. Patients were diagnosed with HL based on 2008 World Health Organization criteria[8] at the Hematology and Transplant Center, University Hospital "San Giovanni di Dio e Ruggi d'Aragona", Salerno, Italy, from June 2013 to July 2014. Patients' characteristics are summarized in Table 1. Flow cytometry immunophenotyping was performed on 200 µL of fresh heparinized whole peripheral blood (PB) or BM specimens obtained at diagnosis, at the end of the second cycle of ABVD (interim PET evaluation, iPET), at the end of treatment, and at follow-up (+3 or +12 months). Briefly, five-color staining cytofluorimetry was carried out with the following antibodies according to manufacturers' instructions: CD3-phycoerythrin (PE)-Cyanine 5 (PC5), PE-Texas-Red (ECD), or PC5.5; CD4-PE; CD8-ECD; CD19-ECD; CD24-PE; CD25-PC5; CD27-PE-Cyanine 7 (PC7); CD38-PC7; CD45-fluorescein isothiocyanate (FITC); and CD56-PE (all from Beckman Coulter, Brea, CA, US). Sample acquisition was performed on a five-color FC500 cell analyzer cytometer equipped with blue (488 nm) and red (633 nm) lasers and with a CXP (Beckman Coulter) or FlowJo (BD Biosciences, Franklin Lakes, NJ, USA) software for data analysis. The presence of CD4+ and CD8+ T cells, CD19+ B lymphocytes, and FoxP3+ T regulatory cells (Treg) in lymph nodes from HL patients was confirmed by immunohistochemistry.
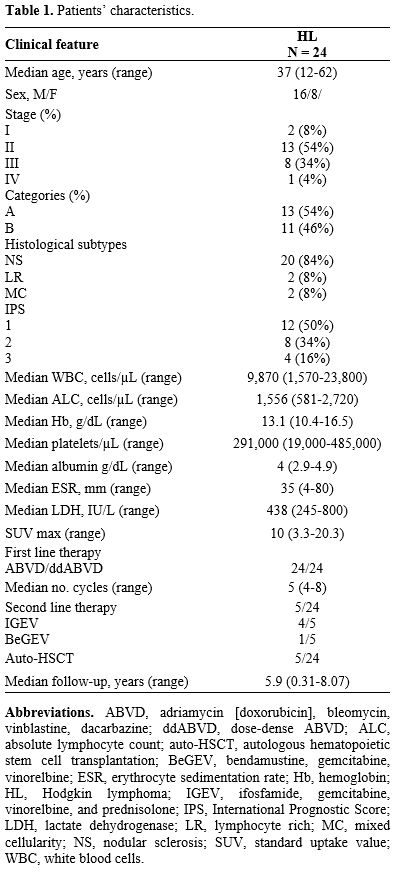 |
Table 1. Patients’ characteristics. |
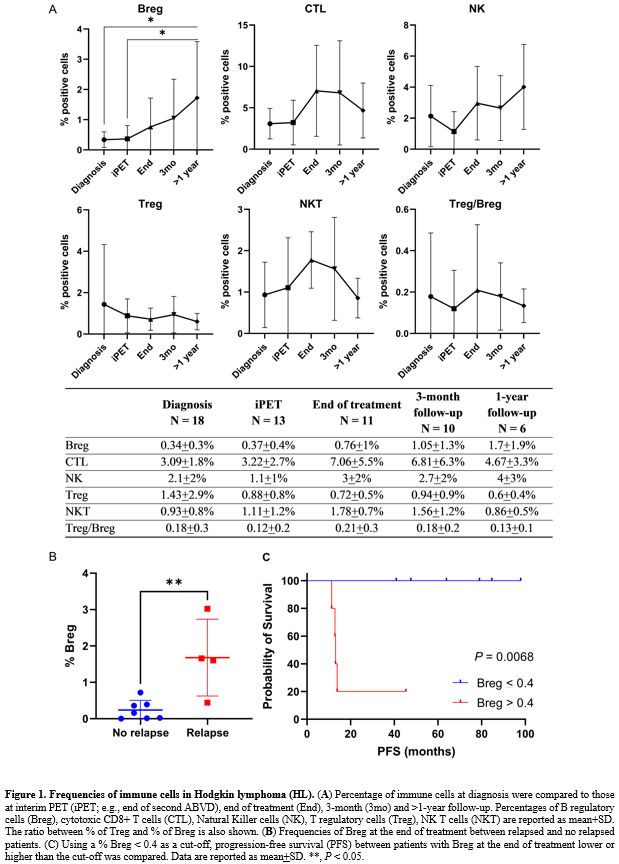
Frequencies of immune cells were compared between HL patients at diagnosis (N = 18) and patients in complete remission (CR) 3 months (N = 10) or more than one year (N = 6) after chemotherapy completion (HL follow-up). Breg frequencies were significantly decreased at diagnosis compared to follow-up (mean+SD, 0.34+0.3% vs 1.3+1.5%, newly diagnosed HL vs HL follow-up; P = 0.0117; unpaired t-test performed), as well as CTLs (mean+SD, 3.09+1.8% vs 5.96+5.3%, newly diagnosed HL vs HL follow-up; P = 0.0384). No differences were found for Treg (P = 0.4380), NK (P = 0.1765), and NKT cells (P = 0.8226). Moreover, HL patients at follow-up displayed a significantly lower Treg/Breg ratio (mean+SD, 3.92+3.4 vs 1.31+1.3, newly diagnosed HL vs HL follow-up; P = 0.0072). Differences in immune cell frequencies from diagnosis to >1-year follow-up were explored by one-way analysis of variance (ANOVA) with Tukey's test for multiple comparisons. Breg significantly increased after the end of chemotherapy (N = 11) and in long survivors compared to patients at diagnosis (P = 0.0163), as subjects at diagnosis and at iPET (N = 13) showed the lowest circulating Breg levels, especially compared to patients at 1-year follow up (P = 0.0194 or P = 0.0332, respectively) (Figure 1A). CTLs tended to increase at the end of treatment, reaching a plateau during the follow-up (diagnosis vs end of treatment, P = 0.0886). NK cell frequencies were at the lowest level after the second cycle of therapy and tended to normalize during follow-up, especially after 1-year (P = 0.0592). No significant differences were described for Treg (P = 0.7910), NKT cells (P = 0.1120), or Treg/Breg ratio (P = 0.9387) between time points. We showed that Breg were markedly decreased at diagnosis and after the second cycle of standard chemotherapy, while they started to increase at the end of treatment and normalized after at least one year from achieving a CR. CTLs and NK also displayed similar kinetics, while Treg and NKT cells did not show significant variations from diagnosis to follow-up. These preliminary results confirmed the different kinetics of B and T cell compartment perturbations during chemotherapy, as B and CD4+ T cells are rapidly depleted, while CD8+ T cells are not effectively removed by chemotherapy.[9] In autoimmune disorders, immunosuppressive therapies can cause a further reduction of circulating Breg, the B cell-depletion phase, that might interrupt a pathologic crosstalk between B and T regulatory cells, eventually blocking Treg expansion followed by reconstitution of functionally competent Breg.[1] Our case series confirmed that the chemotherapy induced the early B cell-depletion phase with a marked Treg/Breg ratio amplification that normalized during follow-up.
Breg variations are differently related to responsiveness to therapies; as in AA, Breg is higher in non-responders to immunosuppressive therapies than responders.[3] In our case series, five patients had a disease relapse with a median of 13.1 months from diagnosis (12.8-45.7 months) and were treated with a second-line therapy followed by autologous hematopoietic stem cell transplantation (HSCT). Three of them achieved a CR and are alive at the time of writing. The other two patients received a second auto-HSCT, and one of them died after nine days from transplant because of septic shock. The entire cohort's 5-year overall survival (OS) was 95.7%, 1-year progression-free survival (PFS) was 91%, and 5-year PFS was 77.7%. Circulating Breg levels at diagnosis were compared to those documented at the end of treatment between patients without disease relapse and patients who relapsed. Significant higher Breg levels were described in relapsed patients at the end of treatment compared to those who did not relapse (mean+SD, 0.24+0.3% vs. 1.68+1.1%, no relapse vs. relapse; P = 0.0062) (Figure 1B). The lowest value of circulating Breg in the relapse group (0.4%) was used as a cut-off for stratifying patients, and PFS was compared between groups by Log-rank (Mantel-Cox) test (Figure 1C). Subjects with higher Breg at the end of treatment (N = 5) had a 13.1 months PFS compared to those with lower Breg (N = 6; 1-year PFS, 100%; P = 0.0068; hazard ratio, 17.36; 95% confidential interval, 2.194-137.4); however, the number of censored subjects was small to draw conclusive assumptions. As reported in MS and AA,[3,5] relapsed HL patients had significantly higher circulating Breg levels at the end of treatment, suggesting that those patients might not have a complete B-cell depletion and an efficient subsequent immunological reset.
Our study has several limitations: the small number of patients retrospectively selected and the presence of rare variants; some heterogeneity of distribution in A/B categories, stages, or prognostic scores; and the lack of further Breg characterization or measurement of circulating interleukins not routinely performed in the diagnostic setting. Strengths of our study are: investigation of immune cell subset perturbations in HL at diagnosis and at short- and long-term (>1-year after the end of treatment) follow-up in a real-world study; patients homogeneously treated with ABVD as first-line therapy; simple staining for immunophenotyping of lymphocyte subsets with regulatory functions in a diagnostic setting; and we have reported for the first time Breg variations in HL.
In conclusion, we showed that Breg might be decreased at diagnosis in HL patients, and their normalization together with a normal immune reconstitution might indicate a restored immune tolerance and surveillance that might be related to long-last disease remission. However, our preliminary results need further validation in larger prospective studies, investigating frequencies and perturbations of immune cells in the site of inflammation (e.g., lymph nodes in HL) to support the hypothesis that the Breg migrate from peripheral blood to tissues and enrich at the site of disease.