Saiduo Liu1, Wei Chen2, Jichan Shi1, Xinchun Ye1, Hongye Ning1, Ning Pan1 and Xiangao Jiang1.
1 Departments of Infectious Disease, Wenzhou Central Hospital, 32 West Jiangbin Road, Wenzhou, Zhejiang 325000, PR China.
2
Department of Radiology, The Second Affiliated Hospital and Yuying
Children's Hospital of Wenzhou Medical University, Xueyuanxi Road, No
109, Wenzhou 325027, Zhejiang, PR China.
Correspondence to:
Xiangao Jiang, Department of Infectious Disease, Wenzhou Central
Hospital, 32 West Jiangbin Road, Wenzhou Zhejiang 325000, China. Tel:
+86 13676788085. Fax: +86 10 8316 1294. Email:
jxgjxg22@163.com
Published: September 1, 2022
Received: March 16, 2022
Accepted: August 6, 2022
Mediterr J Hematol Infect Dis 2022, 14(1): e2022063 DOI
10.4084/MJHID.2022.063
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
To
understand the clinical and imaging manifestations and the treatment
and follow-up of hepatic tuberculosis (HTB), we retrospectively
analysed the clinical and imaging data of 29 patients with HTB who had
been diagnosed clinically or by biopsy, and the clinical and imaging
data had been summarised. Patient characteristics were followed up
after anti-TB drug treatment. The median age of the 29 patients with
HTB was 37 years, and most were male (58.6%). The patient's symptoms
included fever (48.2%), respiratory symptoms (27.5%), abdominal pain
(24.1%), and abdominal distension (10.3%). Elevated erythrocyte
sedimentation rate (79.3%), elevated serum C-reactive protein (75.8%)
and hypoalbuminemia (62.0%) were common features. Three patients were
serologically positive for acquired human immunodeficiency syndrome,
and two were serologically positive for hepatitis B surface antigen
with normal tumour markers. The 29 patients with HTB included 17 with
serous HTB, 9 with parenchymal HTB (8 with parenchymal nodular HTB and
1 with parenchymal miliary HTB), 1 with intrahepatic abscess type HTB,
and 2 with hilar HTB. Approximately 86% of the patients also had
pulmonary TB. Most of the serous HTB patients also had tuberculous
peritonitis. Enhanced computerized tomography scans of the serous and
parenchymal HTB cases showed the progressive development of lesions.
Abnormal blood perfusion was observed in the hepatic artery, and the
clearest evidence of TB was observed in the hepatic portal vein.
Magnetic resonance imaging indicated that the lesions returned a high
signal in the diffusion-weighted imaging sequence. However, the
lesions' apparent diffusion coefficient values reflected high signals.
The Xpert MTB/RIF test detected Mycobacterium TB complex in the liver
biopsy fluid from 10 patients.
Regarding histopathology, one
patient showed granulomatous inflammation, and one patient's acid-fast
bacillus (AFB) stain was positive. The treatment of two patients was
stopped due to their adverse reactions to the drugs and the risk of
creating drug-resistant TB. The remaining patients received anti-TB
treatment, but one subsequently died, and two were unavailable for
follow-up.
The clinical symptoms of HTB are difficult to detect,
and it has diverse manifestations by imaging, with no obvious
specificity in terms of pathological results. Therefore, follow-up of
liver lesions for checking anti-TB therapy is another method for
diagnosing HTB. In addition, early active anti-TB treatment can achieve
good curative results.
|
Introduction
Hepatic
tuberculosis (HTB) refers to TB resulting from a liver infection by
Mycobacterium tuberculosis, a rare extrapulmonary TB that accounts for
less than 1% of TB cases.[1] Bristowe reported the first documented case of HTB in 1858.[2]
More than 20 years after Koch's discovery of Mycobacterium
tuberculosis, Ileston and McNee classified HTB into miliary
(disseminated) and local (isolated) types in 1905, as reported by Chien
et al.[3] Miliary HTB is more common than local HTB in literature reports, where it represents 79% of HTB cases.[4]
The
main clinical manifestations of HTB are fever, cyanosis, jaundice,
shortness of breath, cough, moist lung rale, hepatomegaly,
splenomegaly, and abdominal distension. These indicators lack
characteristic clinical symptoms and specific imaging manifestations,
which can easily lead to a missed or misdiagnosis.[5] A retrospective study by Longxin et al. found a misdiagnosis rate of up to 91% for HTB.[6]
Several clinical studies have found that HTB can easily be misdiagnosed
as liver cancer, cholangiocarcinoma, or other malignant diseases.[5,7-8]
The
diagnosis of HTB is challenging, even in areas where TB is endemic, due
to the non-specificity and diversity of its clinical symptoms.
Statistically, HTB accounts for 1.5%-4.2% of digestive system diseases
manifested by fever and approximately 2.7% of active TB autopsies.[9] Garmpis et al.[10]
found that HTB was characterized by central caseous necrotic granuloma
with or without anti-acid bacteria, indicating that a liver biopsy of
suspected cases of HTB may be useful for the timely diagnosis and
treatment of the disease. A study conducted by Freitas et al.[11]
also indicated the important role of a liver biopsy in correctly
diagnosing HTB in patients with significant liver injury factors but
atypical clinical manifestations. Simultaneously Mycobacterium
tuberculosis complex detection and testing for rifampicin resistance
could be performed on this specimen using the Xpert MTB/RIF assay.[12]
The
current paper explores the clinical and imaging manifestations,
treatment, and follow-up of HTB to provide a practical basis for
suspecting and then diagnosing HTB.
Data and Methods
General data. Twenty-nine
HTB patients, 17 males and 12 females, who each underwent a biopsy with
effective diagnostic and anti-TB treatment in our hospital between
April 2012 and May 2021, were selected for this study. The age of the
patients ranged from 16 to 81 years, with a median age of 37 years.
There was no significant difference in the patients' geographical
distribution. Among the participants, 14 (48.2%) had a fever as the
first clinical manifestation, 7 (24.1%) had abdominal pain, and 8
(27.5%) had cough and sputum symptoms. The total number of patients
with TB at additional sites other than the lungs was 25 (86.2%). Among
these, seven had TB peritonitis, three had splenic TB, six had lymph
TB, three had spinal TB, and six had tuberculous pleuritis. In
addition, three patients had acquired immunodeficiency syndrome, two
had type 2 diabetes, two presented with fatty livers, and two had
chronic viral hepatitis B. Additionally, 23 patients (79.3%) had
elevated erythrocyte sedimentation rates (ESRs), 22 (75.8%) had
elevated C-reactive protein (CRP) levels and 18 (62.0%) had
hypoalbuminemia (see Tables 1 and 2). The disease course ranged from 6 to 24 months.
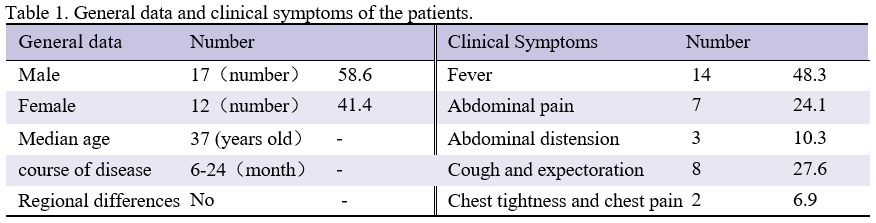 |
Table
1. General data and clinical symptoms of the patients. |
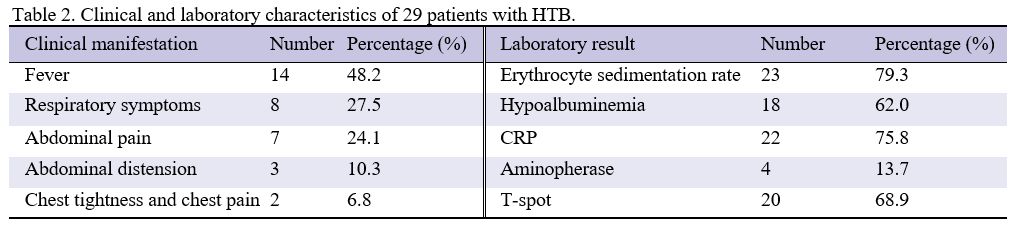 |
Table
2. Clinical and laboratory characteristics of 29 patients with HTB.
|
Methods. The
demographic data, clinical, laboratory, and imaging examinations, and
the pathological and microbiological outcomes of 29 patients with HTB
were analyzed. All patients were examined by computerized tomography
(CT) of the liver; some also had a magnetic resonance imaging (MRI)
scan for comparison. Plain and three-phase dynamic contrast enhancement
scans were performed using a LightSpeed or a Bright Speed Pro-16-layer
scanner (GE Healthcare, USA). The CT scanning requirements were set as
follows: 120 kV, 200-250 mA, and a pitch of 1.375. Some patients
underwent concurrent CT and MRI examinations. The MRI was performed
using a Philips Achieva 1.5 T superconducting-type MR instrument and an
abdominal surface coil for routine MRI examinations. In addition,
multiphase dynamic enhancement scanning was performed using the THRIVE
circum phase gradient echo sequence.
Statistical methods. Descriptive
statistics (mean, standard deviation, percentage, frequency count) were
calculated using the SPSS Statistics 17.0 software program. Categorical
data were examined using the chi-square test, and continuous data were
analysed using the analysis of variance.
Results
General data and clinical results.
Between April 2012 and May 2021, 29 patients in our hospital were
diagnosed with HTB. The median age in this cohort was 37 years (range =
16-81 years), and most patients were male (58.6%). Among the 29
patients, three had a history of close contact with pulmonary TB
patients, six (20.6%) had previously developed pulmonary TB, three were
seropositive for HIV, and two were positive for hepatitis B surface
antigen. Fever (48.2%), respiratory symptoms (27.5%), abdominal pain
(24.1%) and abdominal distension (10.3%) were common clinical features.
Common laboratory abnormalities included elevated ESR (79.3%), elevated
blood CRP (75.8%) and hypoalbuminemia (62.0%). Furthermore, 20 patients
had positive r-interferon tests (68.9%). All patients had normal tumour
markers (alpha foetoprotein [AFP] and carcinoembryonic antigen).
Initially, all patients received anti-TB therapy, but two patients
discontinued this due to their inability to tolerate the side effects.
Subsequently, one patient died of other causes, and two were
unavailable for follow-up (see Table 2).
The manifestations of hepatic tuberculosis seen by imaging.
The CT scans of the 29 patients showed primarily low-density foci,
including the 17 patients with serohepatic HTB showing mostly single
but sometimes multiple nodular lesions or hypertrophy on the liver
envelope.[13] Abnormal perfusion can occur in the
liver tissue adjacent to a lesion, which may be associated with altered
local vascular compression. The lesions observed during the enhanced
scanning of the delayed arterial phase showed progressive
intensification. The perihepatic parenchyma was compressed. The nine
patients with parenchymal HTB included eight with parenchymal nodular
HTB and one with parenchymal miliary HTB (Figure 1).
The images of these two types of HTB are the same, except that the
miliary lesions are approximately 0.5-1 cm and the nodular lesions are
about 2-3 cm.[14-15] In plain CT scan images, the
lesions showed low or equal density shadows, blurred margins, and
low-density changes due to liquefaction or caseous necrosis at the
centre of the lesion. Abnormal peripheral blood perfusion or irregular
enhancement of blood pressure was observed during the enhanced scanning
arterial stage, and clearer lesions were indicated during the portal
phase. Two patients with enlarged lymph nodes in the hilar area (Figure 2)
had no lesions in the liver parenchyma or subcapsular region. The
patients in this group included one with parenchymal abscess type TB
for whom the lesion was distributed through the right lobe of the
liver.
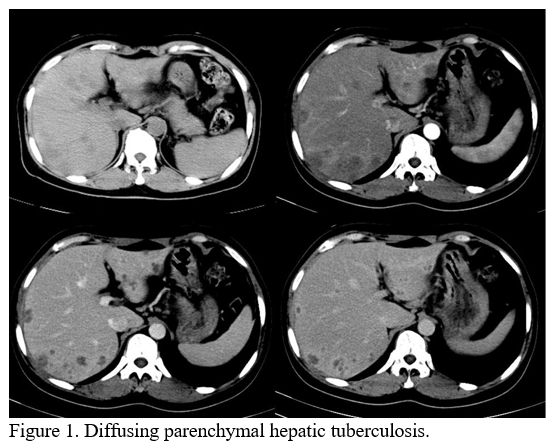 |
Figure 1. Diffusing parenchymal hepatic tuberculosis. |
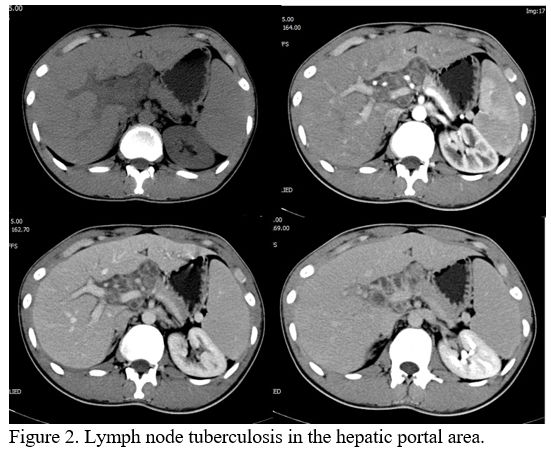 |
Figure
2. Lymph node tuberculosis in the hepatic portal area.
|
There
was no enhancement in the central liquefied necrosis area of the
lesion, and a mild annular enhancement was observed around the lesion
during the delayed phase of the CT scan. The lesion showed a low signal
in the T1 weighted image (T1WI) and a high signal in that T2 weighted
(T2WI). Restricted focal diffusion on the diffusion-weighted (DW)
sequence produced a high alert, but the apparent diffusion coefficient
(ADC) value was also increased, suggesting a benign condition.
Pathological diagnosis of hepatic tuberculosis.
Of the 29 patients included in the study, 10 underwent an
ultrasound-guided liver biopsy for suspected HTB. The tissue (liquid)
samples were submitted for the Xpert MBT/RIF assay. Seven patients
tested positive. One had rifampicin-resistant TB, and one had TB that
was resistant to isoniazid and streptomycin. Two patients each
underwent a liver biopsy for pathology. One of these was found to have
granulomatous lesions; the other had no specific lesions. One patient
tested positive for AFB staining. Finally, ten patients underwent
biopsies (liquid) for Mycobacterium TB culture. Two patients tested
positive, and the remaining patients tested negative.
Treatment and follow-up.
Among the participants, 27 patients underwent 4-5 anti-TB drug
treatments (ATT) for 6-9 months. Two became resistant to anti-TB drugs
after 18 months of treatment. Six patients with a previous history of
TB and three with HIV received ATT for 12 months. One died of organ
failure before follow-up (not associated with TB). Two patients were
removed from the controls. Two patients refused further ATT due to drug
intolerance. At the end of the treatment phase, a low-dose CT scan was
performed on 25 patients, and the results indicated absorption of
lesions in all patients and improvement in their symptoms. No
significant adverse events related to treatment were observed in these
25 patients.
Discussion
Immunosuppressed
patients are more susceptible to Mycobacterium tuberculosis infection,
and in recent years, TB, particularly in the hepatic manifestation, has
become more common in HIV-infected patients,[16-17]
indicating a close link between cellular immunity and HTB. There were
three cases of HIV infection among the participants in the current
study. The HTB patients were mostly aged 30-50 with a median age of 37,
and there were more men than women.[18-20] According to the literature, HTB patients with TB and TB contact history are uncommon.[20-21] In our study, five patients had a TB history, and three had close contact with TB patients.
Many
cases of HTB start slowly. In most cases, HTB patients have TB at
additional sites, but the primary tuberculosis focus has been absorbed
or overcome by fibrosis and calcification when HTB is found. Most
reports suggest that fever, abdominal pain, weight loss, and a loss of
appetite are common symptoms of HTB,[23-24] and a few patients present with jaundice.[25-26] In
our data, fever was the most common clinical symptom (48.2%), followed
by respiratory symptoms (27.5%) and abdominal pain (24.1%). A higher
number of respiratory symptoms than digestive tract symptoms reported
at the initial hospital visit may be related to the department the
patient attended, which is considered a referral bias. However, lung
localization, present in 86% of HTB patients, may cause respiratory
symptoms. Hepatosplenomegaly was the most common symptom of HTB.[27]
Unfortunately, hepatomegaly was not recorded in our data, and three
patients were thought to have splenic TB (one of whom had this
confirmed by a routine PET-CT examination). In our study, more than
half of the patients had elevated ESR and CRP and reduced albumin.
Tumour markers were within the normal range. Tuberculin tests showed
nine patients were strongly positive, six were moderately positive, and
five were negative. The r-interferon release test showed 20 patients
were positive and 9 negative (including 3 HIV patients). Therefore, a
negative tuberculin test result may not rule out TB in clinical
practice. In contrast, a positive r-interferon test should be
considered an alert for the possible presence of a TB infection, either
internal or external to the lungs.
All study patients showed
low-density liver lesions on abdominal CT scans. Several cases of mild
enhancement of the lesions' peripheral edges after contrast injection
have been reported in the literature.[28-29] The
study's CT and MRI results revealed that low-density shadows of
elliptical nodules were observed on CT scans in cases of serous HTB. A
progressive enhancement was observed between the artery and delayed
periods in enhanced scanning. The focal margin was blunt at the liver
parenchyma margin. The clinical findings for TB peritonitis and ovarian
cancer can overlap, leading to potential misdiagnoses.[30]
The
parenchymal HTB lesion was often surrounded by abnormal triangular
high-density shadows due to abnormal blood perfusion caused by an
inflammatory response. All TB nodules in the liver were clearer during
the portal phase, and enhancement was still visible around the lesion.
Their MRI often showed low T1WI and high T2WI and DW signals. However,
the ADC map showed a high signal. The enhanced scanning lesion had no
enhancement in the centre and a mild circular enhancement around it.
Intrahepatic bile duct TB is rare among HTB patients and was not found
in our study. Atypical HTB is characterized by irregular bile duct wall
thickening and expansion, enhanced scanning enhancement and delayed
enhancement, and diffuse punctate calcification of the bile duct wall.[31]
In some cases, the imaging sign appeared in liver abscesses, tumours,
and other lesions. In contrast, non-specific inflammation is not
evident in tumour lesions and could be considered an indirect sign of
HTB progression.[32] The enhanced scan portal stage
showed increased clarity around the lesions, and the delayed period
showed mild enhancement around the lesions, which are common
manifestations of the disease detected by MRI.
Study Limitations
The
present study was limited by the lack of Xpert MTB/RIF assay results,
cultures, and pathological specimens for some patients from the
clinical work used in this research. This omission may have led to some
patients with HTB remaining undiagnosed. Symptoms of the digestive
tract are frequently misdiagnosed as tumours or liver abscesses. Since
most patients refused a second biopsy procedure, imaging served as the
primary means of follow-up. Our experiments also have statistical
issues caused by small sample sizes due to the rarity of HTB.
Conclusion
In
conclusion, HTB is a rare disease with hidden clinical symptoms and
diverse imaging manifestations. Patients with pulmonary TB and an
existing history of TB or HIV infection should be made aware of the
possibility of HTB. Histopathology (diagnostic examination) showed
granuloma necrosis with giant cells. In all suspected cases diagnosed
by liver biopsy, Xpert MTB/RIF assay, and mycobacterium culture. AFP is
normal, so this test should be carried out whenever possible to
distinguish primary malignant liver tumours from HTB.
Ethics Approval and Consent to Participate
Ethics
approval and consent to participate. This study was conducted according
to the Declaration of Helsinki and approved by Wenzhou Central
Hospital. All participants signed an informed consent form for
inclusion in the study.
Availability of Data and Materials
All data generated or analyzed during this study are included in this published article.
Competing Interests
The authors had no personal, financial, commercial, or academic conflicts of interest.
Funding
Supported by basic medical and health science and technology project in Wenzhou, Zhejiang Province in 2021 (No. Y20210844).
References
- Al Umairi R, Al Abri A, Kamona A. Tuberculosis (TB)
of the Porta Hepatis Presenting with Obstructive Jaundice Mimicking a
Malignant Biliary Tumor: A Case Report and Review of the Literature.
Case Rep Radiol. 2018;2018:5318197. https://doi.org/10.1155/2018/5318197 PMid:30631628 PMCid:PMC6304509
- Bristowe
JS. On the connection between abscess of the liver and gastrointestinal
ulceration. Transac Pathol Soc London. 1858;9:241-252.
- Chien RN, Lin PY, Liaw YF. Hepatic tuberculosis: comparison of miliary and local form. Infection. 1995;23:9-12. https://doi.org/10.1007/BF01710049 PMid:7744492
- Hickey
AJ, Gounder L, Moosa MY, Drain PK. A systematic review of hepatic
tuberculosis with considerations in human immunodeficiency virus
co-infection. BMC Infect Dis. 2015; 15:209. https://doi.org/10.1186/s12879-015-0944-6 PMid:25943103 PMCid:PMC4425874
- Li
W, Tang YF, Yang XF, Huang XY. Misidentification of hepatic
tuberculosis as cholangiocarcinoma: A case report. World J Clin Cases.
2021;9(31):9662-9669. https://doi.org/10.12998/wjcc.v9.i31.9662 PMid:34877304 PMCid:PMC8610856
- Long
X, Zhang L, Zhao JP, Cheng Q, Zhu P, Zhang BX, Chen XP. Surgical
treatment of primary hepatic tuberculosis. Journal of Abdominal
Surgery, 2020, 33(04): 278-281.
- Yang C,
Liu X, Ling W, Song B, Liu F. Primary isolated hepatic tuberculosis
mimicking small hepatocellular carcinoma: A case report. Medicine
(Baltimore). 2020;99(41):e22580. https://doi.org/10.1097/MD.0000000000022580 PMid:33031307 PMCid:PMC7544287
- Kale
A, Patil PS, Chhanchure U, Deodhar K, Kulkarni S, Mehta S, Tandon S.
Hepatic tuberculosis masquerading as malignancy. Hepatol Int. 2021 Oct
23. doi: 10.1007/s12072-021-10257-9. Online ahead of print. https://doi.org/10.1007/s12072-021-10257-9 PMid:34687434
- Tang SJ, Gao W. Clinical tuberculosis. Beijing: The People's Health Press, 2011.
- Garmpis
N, Damaskos C, Garmpi A, Liakea A, Mantas D. The Unexpected Diagnosis
of Hepatic Tuberculosis in an Immunocompetent Patient. Case Rep Surg.
2020;2020:7915084. https://doi.org/10.1155/2020/7915084 PMid:33083083 PMCid:PMC7557907
- Freitas
M, Magalhães J, Marinho C, Cotter J. Looking beyond appearances: when
liver biopsy is the key for hepatic tuberculosis diagnosis. BMJ Case
Rep. 2020 May 5;13(5):e234491. https://doi.org/10.1136/bcr-2020-234491 PMid:32376662 PMCid:PMC7228446
- Kohli
M, Schiller I, Dendukuri N, Yao M, Dheda K, Denkinger CM, Schumacher
SG, Steingart KR. Xpert MTB/RIF Ultra and Xpert MTB/RIF assays for
extrapulmonary tuberculosis and rifampicin resistance in adults.
Cochrane Database Syst Rev. 2021 Jan 15;1(1):CD012768. doi:
10.1002/14651858. https://doi.org/10.1002/14651858
- Liu S. Analysis of diagnostic value of CT on serous hepatic tuberculosis. Guide of China Medicine, 2020,18(5):65.
- Wang QY. CT manifestations and diagnostic points of hepatic tuberculosis. Chinese Hepatology, 2016, 21(9):746-748.
- Lu
LQ, Li XW CT manifestations of parenchymal hepatic tuberculosis.
Chinese Imaging Journal of Integrated Traditional and Western Medicine,
2019, 17(3): 292-294.
- Hickey AJ, Gounder
L, Moosa MY, Drain PK. A systematic review of hepatic tuberculosis with
considerations in human immunodeficiency virus co-infection. BMC Infect
Dis. 2015;15:209. https://doi.org/10.1186/s12879-015-0944-6 PMid:25943103 PMCid:PMC4425874
- Zhang
L, Chen YP, Li Q. Research on the results of HIV infection and HBV,HCV,
TP and TB testing study. The Medical Forum, 2021, 25(26): 3792-3794.
- Forgione
A, Tovoli F, Ravaioli M, Renzulli M, Vasuri F, Piscaglia F, Granito A.
Contrast-Enhanced Ultrasound LI-RADS LR-5 in Hepatic Tuberculosis: Case
Report and Literature Review of Imaging Features. Gastroenterology
Insights. 2021; 12(1):1-9. https://doi.org/10.3390/gastroent12010001
- Park
J. Primary hepatic tuberculosis mimicking intrahepatic
cholangiocarcinoma: report of two cases. Ann Surg Treat Res.
2015;89(2):98-101. https://doi.org/10.4174/astr.2015.89.2.98 PMid:26236700 PMCid:PMC4518037
- Bandyopadhyay S, Maity P. Hepatobiliary Tuberculosis. J Assoc Physicians India. 2013;61(6):404-7.
- Hung
Y-M, Huang N-C, Wang J-S, Wann S-R. Isolated hepatic tuberculosis
mimicking liver tumors in a dialysis patient. Hemodial Int.
2015;19:330-351. https://doi.org/10.1111/hdi.12205 PMid:25123829
- Wu
Z, Wang WL, Zhu Y, Cheng JW, Dong J, Li MX, Yu L, Lv Y, Wang B.
Diagnosis and treatment of hepatic tuberculosis: report of fve cases
and review of literature. Int J Clin Exp Med. 2013;6(9):845-850.
- Chong VH. Hepatobiliary tuberculosis: a review of presentations and outcomes. South Med J. 2008;101:356-361. https://doi.org/10.1097/SMJ.0b013e318164ddbb PMid:18360350
- Alvarez SZ. Hepatobilary tuberculosis. J Gastroenterol Hepatol. 1998;13(8):833-839. https://doi.org/10.1111/j.1440-1746.1998.tb00743.x PMid:9736180
- Desai
CS, Joshi AG, Abraham P, Desai DC, Deshpande RB, Bhaduri A, et al.
Hepatic tuberculosis in t absence of dissiminated abdominal
tuberculosis. Ann Hepatol. 2006;5(1):41-43. https://doi.org/10.1016/S1665-2681(19)32038-1
- Park
J. Primary hepatic tuberculosis mimicking intrahepatic
cholangiocarcinoma: report of two cases. Ann Surg Treat Res.
2015;89(2):98-101. https://doi.org/10.4174/astr.2015.89.2.98 PMid:26236700 PMCid:PMC4518037
- Bandyopadhyay S, Maity P. Hepatobiliary Tuberculosis. J Assoc Physicians India. 2013;61(6):404-7.
- Saha
SK, Zeng C, Li X, Mishra AK, Singh A, Silin D, et al. CT
characterization of hepatic tuberculosis. Radiol Infect Dis.
2017;4:143-149. https://doi.org/10.1016/j.jrid.2017.09.001
- Levine C. Primary macronodular hepatic tuberculosis: US and CT appearances. Gastrointest Radiol. 1990;15:307-309. https://doi.org/10.1007/BF01888805 PMid:2210202
- Arezzo
F, Cazzato G, Loizzi V, Ingravallo G, Chiang NJ. Peritoneal
Tuberculosis Mimicking Ovarian Cancer: Gynecologic Ultrasound
Evaluation with Histopathological Confirmation. Gastroenterology
Insights, 2021, 12(2): 278-282. https://doi.org/10.3390/gastroent12020024
- Abascal
J, Martin F, Abreu L, Pereira F, Herrera J, Ratia T, Menendez J.
Atypical hepatic tuberculosis presenting as obstructive jaundice. Am J
Gastroenterol, 1988, 83(10):1183-1186.
- Kang
Suhai, Xie Wei, Zhang Hui, Liu QW, Zhang Z. Imaging Diagnosis of
Hepatic Tuberculosis. Chinese Computed Medical Imaging, 2013, 19(3):
227-230.
[TOP]



